Abstract
Background
There is still a lack of knowledge about the relationship between metabolic syndrome (MetS) and Parkinson’s disease (PD). This study aimed to determine whether MetS increases PD risk.
Methods
To identify relevant clinical studies, databases such as PubMed, Embase, and the Cochrane Library were searched in depth from the inception of databases until March 31, 2024. The study evaluated the correlation between MetS and the likelihood of developing PD through the computation of aggregated relative risks (RR) and their respective 95% confidence intervals (CIs) utilizing selnRR and lnRR.
Results
Seven studies were included in our systematic review. The meta-analysis revealed that patients with MetS have a 0.3-fold increased risk of developing PD (p = 0.001). Furthermore, the analysis revealed a positive correlation between central obesity and the incidence of PD, with an RR of 1.19 (95% CI, 1.16–1.22; p = 0.001), as well as a greater risk of PD in patients with elevated blood pressure, with an RR of 1.13 (95% CI, 1.07–1.19; p = 0.001); elevated serum triglyceride levels, with an RR of 1.09 (95% CI, 1.02–1.15; p = 0.001); lower serum HDL cholesterol levels, with an RR of 1.21 (95% CI, 1.15–1.28; p = 0.001); and elevated plasma fasting glucose levels, with an RR of 1.18 (95% CI, 1.11–1.26; p = 0.001).
Conclusion
MetS can contribute to the incidence of Parkinson’s disease, with individual components of MetS demonstrating comparable effects.
Keywords: Metabolic syndrome, Parkinson’s disease, Risk factors, Meta-analysis
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease in which dopamine-producing neurons in the brain gradually deteriorate, leading to various motor and nonmotor symptoms [1] and severely affecting quality of life. The rate of new PD cases ranges from 5 to over 35 per 100,000 people each year on the basis of healthcare data [2]. Several risk factors are associated with PD, including coffee consumption, hypertension, nonsteroidal anti-inflammatory drugs, calcium channel blockers, and alcohol consumption. [3].
Metabolic syndrome (MetS) is characterized by the presence of a minimum of three metabolic risk factors, such as central obesity, dyslipidemia, impaired glucose metabolism, elevated blood pressure, and low levels of high-density lipoprotein cholesterol [4]. The prevalence of MetS increased by 35% in the United States between 1980 and 2012 [5]. MetS plays a significant role in environmental factors impacting global human health, and several studies have shown that MetS might be associated with several neurodegenerative disorders [1, 6, 7]. There is growing interest in studying the link between MetS and PD, as both conditions are believed to share common pathophysiological mechanisms. Nevertheless, existing studies investigating the relationship between MetS and PD incidence have yielded conflicting findings. Recent studies have explored the link between MetS and PD, but a comprehensive assessment is lacking. In this study, we examined the relationship between MetS and PD risk.
Methods
This study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [8] and the MOOSE (Monitoring and Reporting of Observational Studies in Epidemiology) guidelines [9].
Search strategy and literature screening
A comprehensive search of the literature was performed via databases such as PubMed, Embase, and the Cochrane Library to find all relevant clinical research that examined the link between MetS and PD from the inception of the databases to March 31, 2024, with the terms “Parkinson’s disease”, “metabolic syndrome”, “PD”, and “MetS”. Discrepancies were resolved by a third reviewer after two reviewers independently reviewed titles, abstracts, and full-text articles. MetS was diagnosed if three or more of the following criteria were met: abdominal obesity, high triglycerides, low HDL cholesterol, high blood pressure, and high fasting blood glucose.
Eligibility
Inclusion criteria
A case‒control, cohort, or cross-sectional study was conducted to examine the link between MetS and the development of PD in humans. The study needed sufficient data, such as hazard ratios (HRs), odds ratios (ORs), or risk ratios (RRs).
Exclusion criteria
The exclusion criteria for this study included insufficient data; the exclusion of meta-analyses, reviews, letters, editorials, or case reports; and the removal of duplicate entries.
Data extraction and quality assessment
Two researchers independently collected data from qualified studies, including information on authorship, publication year, country of origin, research design, sample size, body mass index (BMI), and follow-up duration. The Newcastle‒Ottawa Scale (NOS) [10] was used to evaluate the quality of the included studies by two researchers to minimize bias and risk. In cases of disagreement, a third reviewer was consulted.
Statistical analysis
An examination of the association of MetS with PD risk was carried out by calculating relative risks (RRs) and 95% confidence intervals (CIs). To identify variability other than chance alone, the I2 statistic and the Q test were used to assess heterogeneity among the studies. I2 values (≥ 75%, 50% < I2 ≤ 75%, 25% < I2 ≤ 50%, and I2 ≤ 25%) were used to classify the level of heterogeneity as considerable, substantial, moderate, or slight. Heterogeneity was assessed using I² statistics. Studies with an I² value of 50% or greater were considered to exhibit significant heterogeneity, necessitating the use of a random-effects model for effect size pooling. In contrast, studies with an I² value less than 50% were deemed to have acceptable heterogeneity, thereby justifying the use of a fixed-effects model. Sensitivity analyses were performed on each individual study to ensure the robustness of the findings. Publication bias is evaluated through the application of a funnel plot, Egger’s test, and Begg’s test.
Results
Study selection and basic characteristics
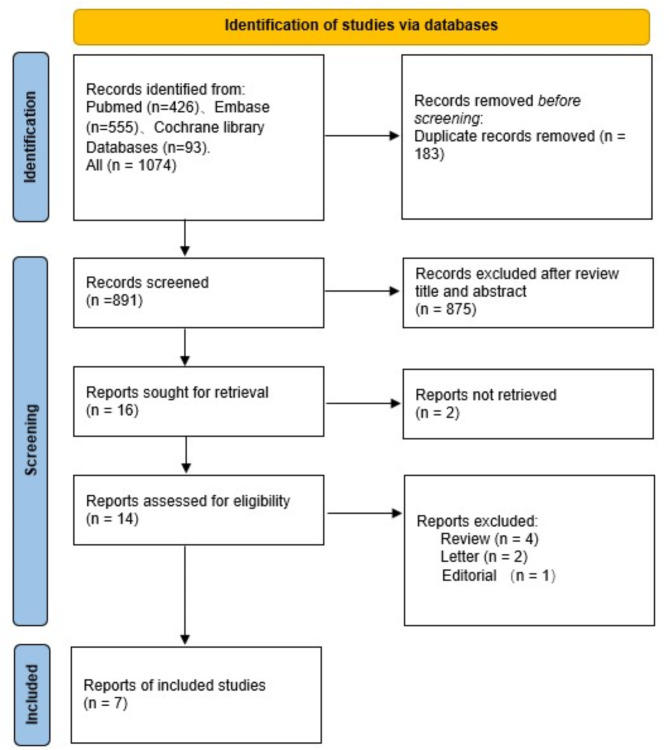
We found 1074 studies, and 891 studies were screened after removing duplicates. A total of 875 studies were excluded on the basis of the title and abstract. Sixteen full manuscripts were assessed, and 7 [11–17] were included in the analysis. See Fig. 1 for the selection process. Among the included studies, one originated from Finland, two from China, and three from Korea. All of the studies were retrospective studies. The meta-analysis included a sample size of 47,153,25 individuals, of whom approximately 17.1-35.2% exhibited MetS and 148,835 individuals were diagnosed with PD. The participants were followed up for five to thirty years and had a BMI ranging from 23.4 to 26.8. Detailed information can be found in Table 1.
Fig. 1.
The selection process of included studies
Table 1.
The basic characteristics of included studies of meta-analysis
| Author | Year | Country | Study design | N(case) | PD (case) | M (%) | Age | Mets (%) | BMI | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Katri | 2015 | Finland | retrospective | 6,641 | 89 | 54.6 | mean: 56.5 | 17.1 | mean: 26.2 | 30-year |
| Penga | 2018 | China | retrospective | NA | 255 | 58 | mean: 67.7 | 32.2 | mean: 25.8 | 5-year |
| Pengb | 2018 | China | retrospective | NA | 105 | 58.1 | mean: 68.5 | 35.2 | mean: 25.9 | 5-year |
| Dallaha | 2020 | Korea | retrospective | 6,098,405 | 20,895 | 100 | mean: 54.6 | 26.9 | mean:23.9 | 6.4-year |
| Dallahb | 2020 | Korea | retrospective | 6,098,405 | 20,895 | 0 | mean: 54.5 | 27.8 | mean:23.4 | 6.4-year |
| Jeong | 2021 | Korea | retrospective | 903,063 | NA | 53.81 | mean: 55.47 | NA | mean:24.08 | 5-year |
| Sanga | 2021 | Korea | retrospective | 5,522,813 | 20,524 | 55.40 | mean: 55.7 | NA | mean:24.8 | 7-year |
| Sangb | 2021 | Korea | retrospective | 5,522,814 | 20,524 | 54 | mean: 55.3 | NA | mean:24.5 | 7-year |
| Sangc | 2021 | Korea | retrospective | 5,522,815 | 20,524 | 48.4 | mean: 58.7 | NA | mean:25.9 | 7-year |
| Ga | 2018 | Korea | retrospective | 17,163,560 | 44,205 | 47.9 | mean: 58.1 | 34.1 | mean:25.6 | 5.3-year |
| Roh | 2020 | Korea | retrospective | 314,737 | 819 | 56.1 | NA | 27.2 | NA | 7.23-year |
Abbreviations: NA: not available; N: number; PD: Parkinson’s disease; Mets: Metabolic syndrome
Quality assessment
Four studies received a score of 9 points, two studies received a score of 8 points, and one study received a score of 7 points (Table 2). These scores indicate that the quality of the included studies ranged from moderate to high.
Table 2.
The quality assessment of included studies of meta-analysis
| Study | Selection (0–4) | Comparability (0–2) | Outcome (0–3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REC | SNEC | AE | DO | SC | AF | AO | FU | AFU | Total | |
| Katri | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Peng | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| Dallah | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Jeong | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Sang | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Ga | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Roh | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
REC: representativeness of exposed cohort; SNEC: selection of nonexposed cohort; AE: ascertainment of exposure; DO: outcome not present at the start of the study; SC: control for important factors; AF: additional factors; AO: assessment of outcome; FU: length of follow-up; AFU: adequacy of follow-up
Risk of PD associated with MetS
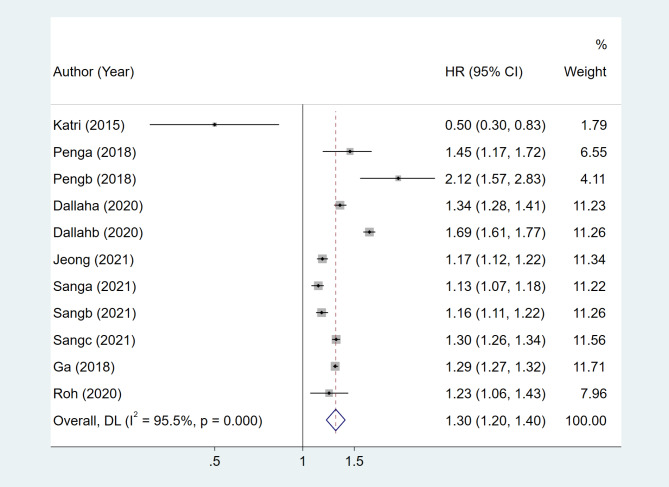
A pooled analysis of seven studies revealed that patients with MetS were more likely to develop Parkinson’s disease (RR, 1.30; 95% CI, 1.2–1.4; p = 0.001) (Fig. 2).
Fig. 2.
The pooled results of the relationship between patients with MetS and PD
Risk of PD associated with MetS components
Central obesity was utilized as the outcome measure in three studies, with findings indicating a positive association between central obesity and increased incidence of PD (RR, 1.19; 95% CI, 1.16–1.22; p = 0.001), according to a fixed-effects model (Table 3). Five studies have documented the correlation between blood pressure levels and the likelihood of developing PD. The pooled analysis revealed that patients diagnosed with higher blood pressure had a 0.13-fold increased risk of developing PD (95% CI, 1.07–1.19; p = 0.001) via a random effects model (Table 3). Researchers have examined the correlation between serum triglyceride levels and PD risk in five studies. A pooled analysis revealed that individuals with elevated serum triglyceride levels were at a greater risk of developing PD (RR, 1.09; 95% CI 1.02–1.15; p = 0.001) via a random effects model (Table 3). Five studies were conducted to investigate the relationship between HDL cholesterol levels and the development of PD. A pooled analysis indicated that individuals with lower serum HDL cholesterol levels had a 0.21-fold increased risk of developing PD (95% CI 1.15–1.28; p = 0.001), according to a random effects model (Table 3). Five studies were performed to examine the association between plasma fasting glucose levels and the probability of developing PD. According to a meta-analysis, individuals with elevated plasma fasting glucose levels have an increased risk of developing PD (RR, 1.18; 95% CI 1.11–1.26; p = 0.001), according to a random effects model (Table 3).
Table 3.
The results of the clinical outcomes
| Number | RR | LC | UC | P | I2 | P | |
|---|---|---|---|---|---|---|---|
| MetS | 7 | 1.3 | 1.2 | 1.4 | < 0.001 | 95.50% | < 0.001 |
| Plasma fasting glucose | 5 | 1.18 | 1.11 | 1.26 | < 0.001 | 90.20% | < 0.001 |
| Serum HDL | 5 | 1.21 | 1.15 | 1.28 | < 0.001 | 85.40% | < 0.001 |
| Serum triglycerides | 5 | 1.09 | 1.02 | 1.15 | 0.007 | 89.50% | < 0.001 |
| Bloodpressure | 5 | 1.13 | 1.07 | 1.19 | < 0.001 | 82.10% | < 0.001 |
| Central obesity | 3 | 1.19 | 1.16 | 1.22 | < 0.001 | 0.00% | 0.492 |
Abbreviations: RR: relative risk; LC: lower confidence; UC: Upper confidence; Mets: Metabolic Syndrome
Sensitivity analysis and publication bias
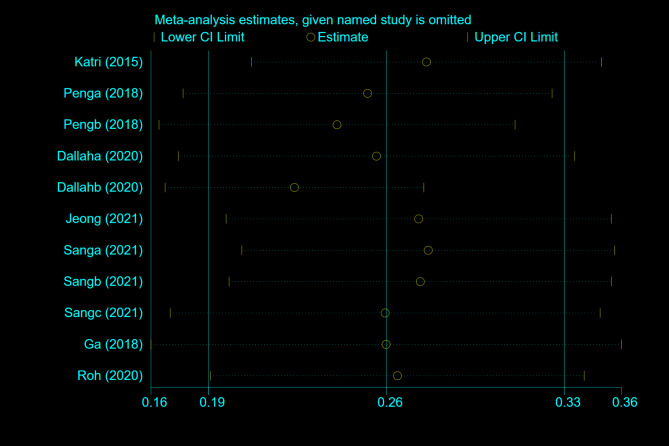
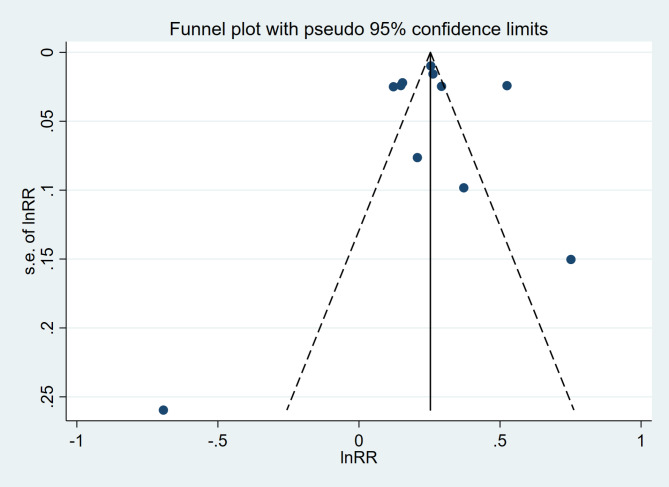
A sensitivity analysis was conducted through the systematic exclusion of individual studies from the pooled analysis, which demonstrated that no single study exerted undue influence on the overall results (Fig. 3). These findings suggest the stability and reliability of our results. Funnel plots were utilized to assess publication bias, revealing a symmetrical distribution in MetS patients (Fig. 4). The Begg and Egger tests produced values of 0.755 and 0.980, respectively, thereby suggesting the absence of significant publication bias within the study.
Fig. 3.
The results of sensitivity analysis
Fig. 4.
The funnel plot of the relationship between patients with MetS and PD
Discussion
The aim of this meta-analysis was to examine the relationships between MetS and its components and the likelihood that some individuals would develop PD. In our study, individuals diagnosed with MetS or its components, such as excessive central obesity, hypertension, low HDL cholesterol, high triglycerides, and elevated fasting glucose, had an increased risk of PD. A study utilizing data from the Mini-Finland Health Survey, which involved 6641 participants, revealed that after 30 years of follow-up, people without MetS were less likely to develop PD [12]. However, a separate study involving 787 patients revealed a significant correlation between MetS and PD in individuals exhibiting mild cognitive impairment or dementia [13]. Our study initially compiled and analyzed published articles examining the relationship between MetS and the likelihood of developing PD. We found that people with MetS are more likely to develop PD, as evidenced by a substantial sample size. In addition, we also assessed the components of MetS and the risk of PD. An analysis of a Korean retrospective study, encompassing a sample size of 5,522,813 individuals, revealed that the presence of abdominal obesity, low HDL-C, hypertriglyceridemia, and hyperglycemia, whether incident or persistent, were correlated with an increased likelihood of developing PD compared with individuals lacking these factors [16]. However, Katri et al. reported that increased levels of serum triglycerides and plasma fasting glucose are predictive of a lower incidence of Parkinson’s disease, whereas a higher BMI appears to be associated with an elevated risk of developing the disease [12]. Our study revealed that individuals with central obesity, hypertension, high triglyceride levels, low HDL cholesterol levels, and elevated fasting glucose levels were at increased risk of developing PD. The findings of Peng and colleagues indicated significant associations between elevated blood pressure and fasting plasma glucose levels in all primary cognitive domains, as well as a link between hypertriglyceridemia and executive function, language, memory, and visuospatial abilities. Furthermore, low levels of HDL cholesterol were found to be associated with deficits in memory and visuospatial function [13].
The link between MetS and PD is not fully understood, but insulin resistance plays a key role in both conditions. Studies suggest that nondiabetic individuals with PD may also have insulin resistance in their brains. In PD patients, insulin signaling pathways in the brain are dysregulated, with increased levels of phosphorylated serine in the IRS, decreased levels of phosphorylated and total AKT, and elevated GSK-3b expression [18, 19]. Oxidative stress plays a crucial role in the pathophysiology of MetS, with studies showing higher levels of oxidative stress markers and lower antioxidant defenses in individuals with MetS. It is evident that oxidative stress undoubtedly contributes to intricate and progressive neurodegenerative processes. Moreover, oxidative stress is widely acknowledged to play a significant role in intricate and progressive neurodegenerative processes. Increasing evidence suggests that oxidative stress is a primary factor in the degeneration of dopaminergic neurons in different manifestations of PD [20–22]. Furthermore, the development of MetS is intricately linked to a chronic state of inflammation, resulting in heightened levels of inflammatory markers in individuals afflicted with MetS. Patients with PD exhibit elevated levels of proinflammatory cytokines, which may be directly correlated with decreased levels of neurotrophic factors [11, 23]. Additional investigations into the correlation between MetS and PD are warranted. The study was subject to several limitations. All included studies were retrospective in nature, potentially introducing bias that could have influenced the outcomes. Therefore, additional prospective, multicenter, high-quality studies are needed to validate the findings. Furthermore, this study cohort was limited to individuals of Korean, Chinese, and Finnish descent, and additional validation is needed in diverse ethnic cohorts. Additionally, it is important to consider the potential for bias in the estimates of the strength of associations due to the uncertainty surrounding the definitive diagnosis of PD in all patients. However, it was determined that this risk was minimal.
Conclusion
This meta-analysis revealed that the presence of MetS and its individual components may act as potential risk factors for the development of PD. Consequently, the implementation of suitable interventions focused on the prevention and management of MetS could significantly contribute to the reduction in PD incidence.
Acknowledgements
None.
Author contributions
Yuan Zhong, Yu-Si Hua contribute to design and conception of the study. Yuan Zhong, Tian-Hong Wang, Li-Jiang Huang, and Yu-Si Hua contribute to the methodology. Yuan Zhong, and Tian-Hong Wang supported the software and Yuan Zhong, and Li-Jiang Huang conducted the statistical analysis. Yuan Zhong writed the article and all authors reviewed the manuscript.
Funding
None.
Data availability
The published article contains all of the data collected or analyzed during this investigation.
Declarations
Ethics approval and consent to participate
As all analyses were based on existing published research, patient consent and ethical approval were deemed unnecessary.
Competing interests
The authors declare no competing interests.
Conflict of interest
None.
Consent to publish
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li LY, et al. Recent research progress on metabolic syndrome and risk of Parkinson’s disease. Rev Neurosci. 2023;34(7):719–35. 10.1515/revneuro-2022-0093 [DOI] [PubMed] [Google Scholar]
- 2.Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord. 2003;18(1):19–31. 10.1002/mds.10305 [DOI] [PubMed] [Google Scholar]
- 3.Noyce AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72(6):893–901. 10.1002/ana.23687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. 10.1196/annals.1427.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira PI, et al. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J Neurol Sci. 2007;257(1–2):206–14. 10.1016/j.jns.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, Tian B. Metabolic syndrome: an important risk factor for Parkinson’s disease. Oxid Med Cell Longev, 2014. 2014: p. 729194. [DOI] [PMC free article] [PubMed]
- 8.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 11.Roh JH, Lee S, Yoon JH. Metabolic syndrome and Parkinson’s Disease incidence: a nationwide study using propensity score matching. Metab Syndr Relat Disord. 2021;19(1):1–7. 10.1089/met.2020.0060 [DOI] [PubMed] [Google Scholar]
- 12.Sääksjärvi K, et al. Prospective study on the components of metabolic syndrome and the incidence of Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(10):1148–55. 10.1016/j.parkreldis.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 13.Peng Z, et al. Metabolic syndrome contributes to cognitive impairment in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2018;55:68–74. 10.1016/j.parkreldis.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 14.Jeong E, Park JB, Park YG. Evaluation of the association between periodontitis and risk of Parkinson’s disease: a nationwide retrospective cohort study. Sci Rep. 2021;11(1):16594. 10.1038/s41598-021-96147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo D, et al. Serum gamma-glutamyltransferase activity and Parkinson’s disease risk in men and women. Sci Rep. 2020;10(1):1258. 10.1038/s41598-020-58306-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SH, et al. Association of dynamic changes in metabolic syndrome status with the risk of Parkinson’s Disease: a Nationwide Cohort Study. J Parkinsons Dis. 2021;11(4):1751–9. 10.3233/JPD-212589 [DOI] [PubMed] [Google Scholar]
- 17.Nam GE, et al. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med. 2018;15(8):e1002640. 10.1371/journal.pmed.1002640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mottillo S, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32. 10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 19.Naderali EK, Ratcliffe SH, Dale MC. Obesity and Alzheimer’s disease: a link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen. 2009;24(6):445–9. 10.1177/1533317509348208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armutcu F, et al. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin Chem Lab Med. 2008;46(6):785–90. 10.1515/CCLM.2008.166 [DOI] [PubMed] [Google Scholar]
- 21.Blesa J, et al. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91. 10.3389/fnana.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei M et al. Sphingolipid metabolism in brain insulin resistance and neurological diseases. Front Endocrinol, 2023. 14. [DOI] [PMC free article] [PubMed]
- 23.Ridker PM, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The published article contains all of the data collected or analyzed during this investigation.