Abstract
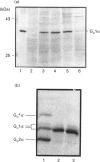
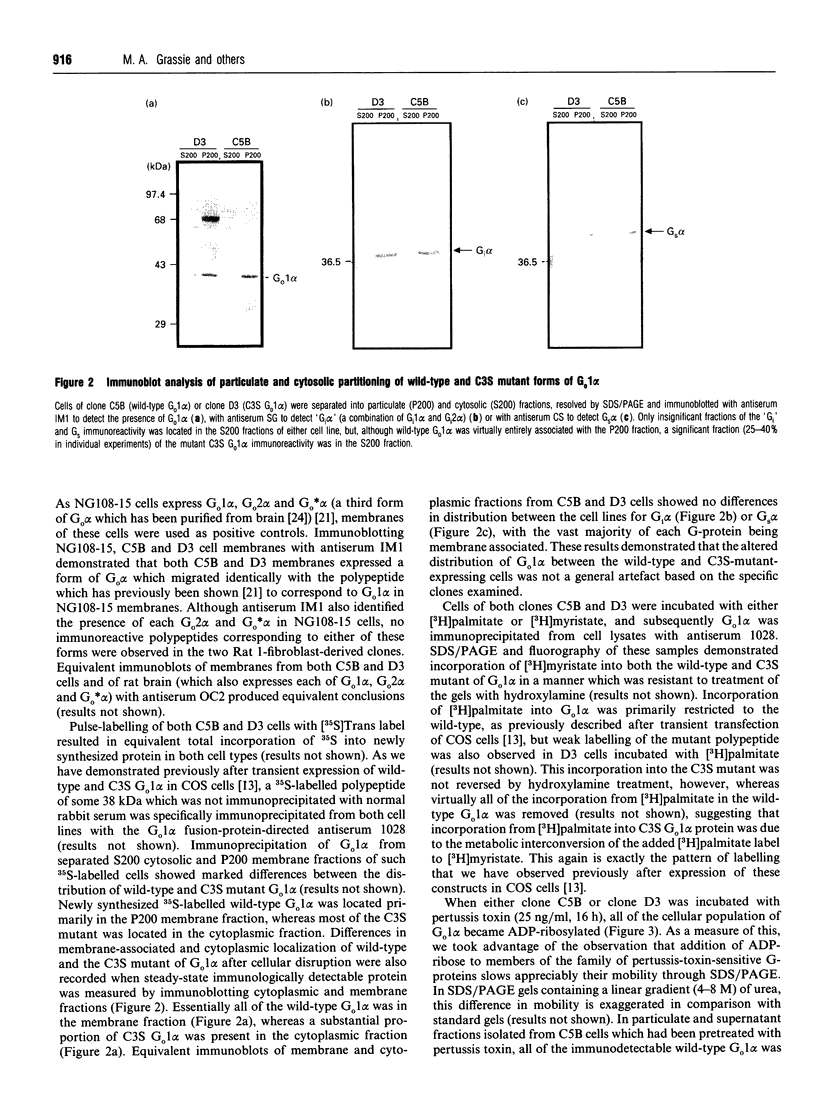
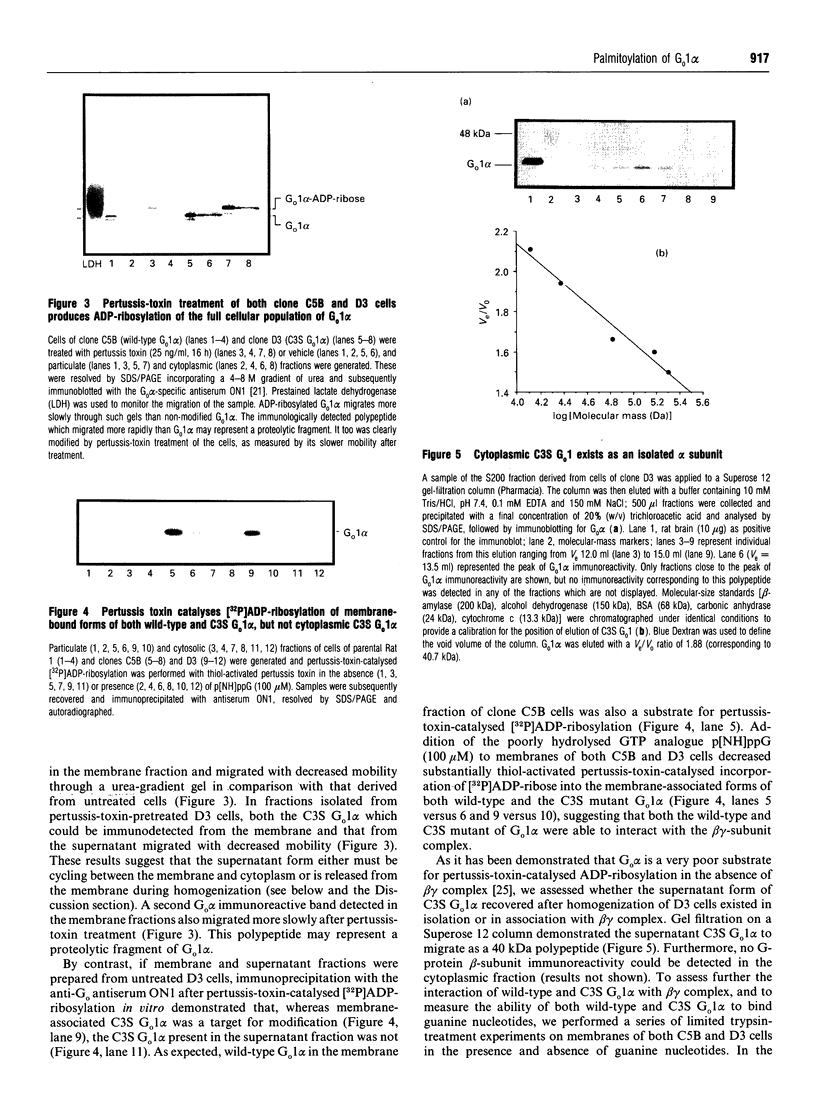
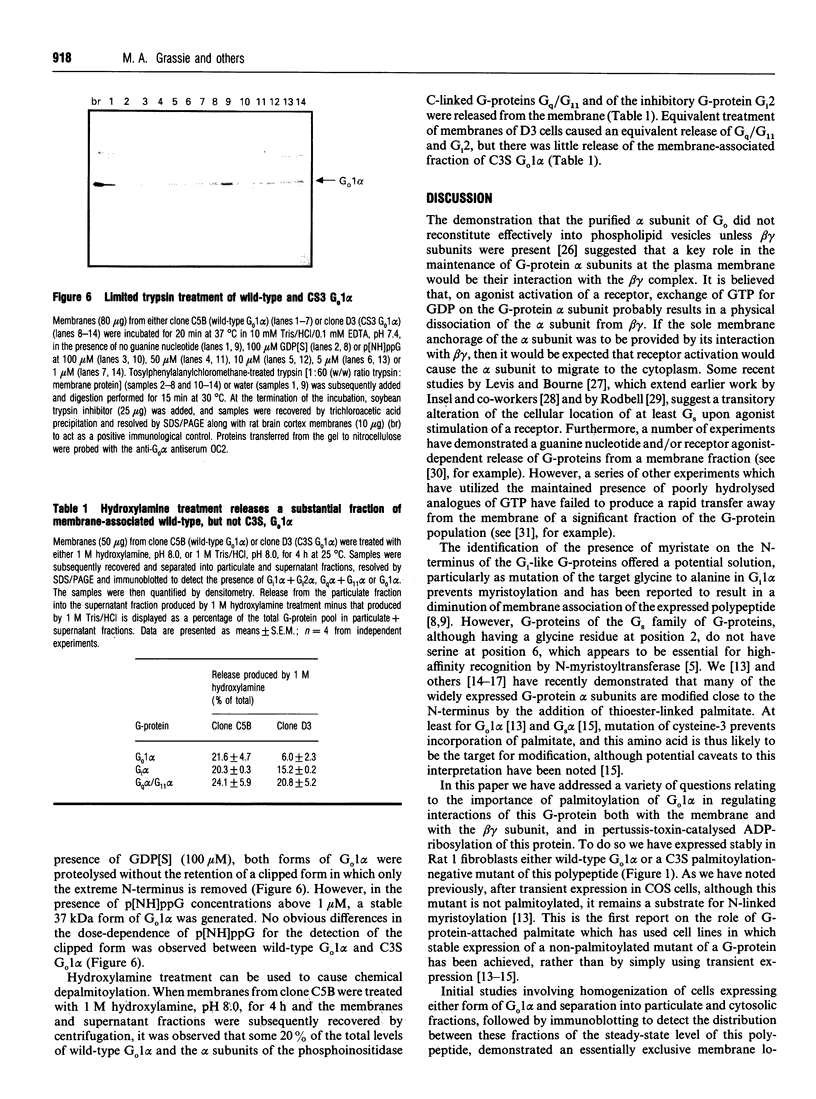
Plasmids containing cDNAs encoding either the wild-type guanine-nucleotide-binding protein G(o)1 alpha or the palmitoylation-negative cysteine-3-to-serine (C3S) mutant of G(o)1 alpha were transfected into Rat 1 cells, and clones stably expressing immunoreactivity corresponding to these polypeptides were isolated. Clones C5B (expressing wild-type G(o)1 alpha) and D3 (expressing the mutant form) were selected for detailed study. Immunoprecipitation of whole cell lysates of each clone labelled with either [3H]palmitate or [3H]myristate demonstrated incorporation of [3H]myristate into both wild-type and the C3S mutant of G(o)1 alpha, but that incorporation of hydroxylamine-sensitive [3H]palmitate was restricted to the wild type. When membrane and cytoplasmic fractions were prepared from cells of either the C5B or D3 clones, although immunodetection of wild-type G(o)1 alpha was observed only in the membrane fraction, the C3S mutant was present in both membrane and cytoplasmic fractions. Furthermore, a significant proportion of the C3S G(o)1 alpha immunoreactivity was also detected in the cytoplasmic fraction if immunoprecipitation of recently synthesized G(o)1 alpha was performed from fractions derived from cells pulse-labelled with [35S]Trans label. Pretreatment of cells of both clones C5B and D3 with pertussis toxin led to complete ADP-ribosylation of the cellular population of G(o)1 alpha in both cell types, irrespective of whether the polypeptide was subsequently found in the membrane or cytoplasmic fraction following cellular disruption. By contrast, separation of membrane and cytoplasmic fractions before pertussis-toxin-catalysed [32P]ADP-ribosylation allowed modification only of the membrane-associated G(o)1 alpha (whether wild-type or the C3S mutant). This labelling was decreased substantially by incubation of the membranes with guanosine 5'-[beta gamma-imido]triphosphate. No cytoplasmic G-protein beta subunit was detected immunologically, and the non-membrane-associated C3S G(o)1 alpha from D3 cells migrated as an apparently monomeric 40 kDa protein on a Superose 12 gel-filtration column. Membrane-associated wild-type and C3S G(o)1 alpha appeared to interact with guanine nucleotides with similar affinity, as no alteration in the dose-response curves for guanine-nucleotide-induced maintenance of a stable 37 kDa tryptic fragment was noted for the two forms of G(o)1 alpha. Chemical depalmitoylation of membranes of clone C5B with neutral 1 M hydroxylamine caused a release of some 25-30% of each of G(o)1 alpha, Gi2 alpha and Gq alpha/G11 alpha from the membranes. Equivalent treatment of D3 cells caused an equivalent release of Gi2 alpha and Gq alpha/G11 alpha, but was unable to cause any appreciable release of the CS3 form of G(o)1 alpha, which was membrane-bound.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Degtyarev M. Y., Spiegel A. M., Jones T. L. The G protein alpha s subunit incorporates [3H]palmitic acid and mutation of cysteine-3 prevents this modification. Biochemistry. 1993 Aug 17;32(32):8057–8061. doi: 10.1021/bi00083a001. [DOI] [PubMed] [Google Scholar]
- Eide B., Gierschik P., Milligan G., Mullaney I., Unson C., Goldsmith P., Spiegel A. GTP-binding proteins in brain and neutrophil are tethered to the plasma membrane via their amino termini. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1398–1405. doi: 10.1016/s0006-291x(87)80287-5. [DOI] [PubMed] [Google Scholar]
- Gallego C., Gupta S. K., Winitz S., Eisfelder B. J., Johnson G. L. Myristoylation of the G alpha i2 polypeptide, a G protein alpha subunit, is required for its signaling and transformation functions. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9695–9699. doi: 10.1073/pnas.89.20.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith P., Backlund P. S., Jr, Rossiter K., Carter A., Milligan G., Unson C. G., Spiegel A. Purification of heterotrimeric GTP-binding proteins from brain: identification of a novel form of Go. Biochemistry. 1988 Sep 6;27(18):7085–7090. doi: 10.1021/bi00418a062. [DOI] [PubMed] [Google Scholar]
- Grand R. J. Acylation of viral and eukaryotic proteins. Biochem J. 1989 Mar 15;258(3):625–638. doi: 10.1042/bj2580625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A., Johnson J. L., Milligan G. Down-regulation of Gi sub-types by prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1990 Mar 25;265(9):5206–5210. [PubMed] [Google Scholar]
- Iismaa T. P., Shine J. G protein-coupled receptors. Curr Opin Cell Biol. 1992 Apr;4(2):195–202. doi: 10.1016/0955-0674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Jones T. L., Simonds W. F., Merendino J. J., Jr, Brann M. R., Spiegel A. M. Myristoylation of an inhibitory GTP-binding protein alpha subunit is essential for its membrane attachment. Proc Natl Acad Sci U S A. 1990 Jan;87(2):568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiber D., Jasper J. R., Alousi A. A., Martin J., Bernstein D., Insel P. A. Alteration in Gs-mediated signal transduction in S49 lymphoma cells treated with inhibitors of microtubules. J Biol Chem. 1993 Feb 25;268(6):3833–3837. [PubMed] [Google Scholar]
- Levis M. J., Bourne H. R. Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992 Dec;119(5):1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M. E., Middleton P., Hepler J. R., Taussig R., Gilman A. G., Mumby S. M. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Delta-opioid-receptor-mediated inhibition of adenylate cyclase is transduced specifically by the guanine-nucleotide-binding protein Gi2. Biochem J. 1990 Apr 15;267(2):391–398. doi: 10.1042/bj2670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Carr C., Gould G. W., Mullaney I., Lavan B. E. Agonist-dependent, cholera toxin-catalyzed ADP-ribosylation of pertussis toxin-sensitive G-proteins following transfection of the human alpha 2-C10 adrenergic receptor into rat 1 fibroblasts. Evidence for the direct interaction of a single receptor with two pertussis toxin-sensitive G-proteins, Gi2 and Gi3. J Biol Chem. 1991 Apr 5;266(10):6447–6455. [PubMed] [Google Scholar]
- Milligan G. Foetal-calf serum stimulates a pertussis-toxin-sensitive high-affinity GTPase activity in rat glioma C6 BU1 cells. Biochem J. 1987 Jul 15;245(2):501–505. doi: 10.1042/bj2450501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Guanine nucleotide regulation of the pertussis and cholera toxin substrates of rat glioma C6 BU1 cells. Biochim Biophys Acta. 1987 Jul 6;929(2):197–202. doi: 10.1016/0167-4889(87)90176-5. [DOI] [PubMed] [Google Scholar]
- Mullaney I., Milligan G. Identification of two distinct isoforms of the guanine nucleotide binding protein G0 in neuroblastoma X glioma hybrid cells: independent regulation during cyclic AMP-induced differentiation. J Neurochem. 1990 Dec;55(6):1890–1898. doi: 10.1111/j.1471-4159.1990.tb05773.x. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Heukeroth R. O., Gordon J. I., Gilman A. G. G-protein alpha-subunit expression, myristoylation, and membrane association in COS cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):728–732. doi: 10.1073/pnas.87.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Negishi M., Hashimoto H., Ichikawa A. Translocation of alpha subunits of stimulatory guanine nucleotide-binding proteins through stimulation of the prostacyclin receptor in mouse mastocytoma cells. J Biol Chem. 1992 Feb 5;267(4):2364–2369. [PubMed] [Google Scholar]
- Newman C. M., Giannakouros T., Hancock J. F., Fawell E. H., Armstrong J., Magee A. I. Post-translational processing of Schizosaccharomyces pombe YPT proteins. J Biol Chem. 1992 Jun 5;267(16):11329–11336. [PubMed] [Google Scholar]
- Parenti M., Viganó M. A., Newman C. M., Milligan G., Magee A. I. A novel N-terminal motif for palmitoylation of G-protein alpha subunits. Biochem J. 1993 Apr 15;291(Pt 2):349–353. doi: 10.1042/bj2910349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransnäs L. A., Svoboda P., Jasper J. R., Insel P. A. Stimulation of beta-adrenergic receptors of S49 lymphoma cells redistributes the alpha subunit of the stimulatory G protein between cytosol and membranes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7900–7903. doi: 10.1073/pnas.86.20.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A. M., Backlund P. S., Jr, Butrynski J. E., Jones T. L., Simonds W. F. The G protein connection: molecular basis of membrane association. Trends Biochem Sci. 1991 Sep;16(9):338–341. doi: 10.1016/0968-0004(91)90139-m. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M. G proteins in cellular control. Curr Opin Cell Biol. 1992 Apr;4(2):203–211. doi: 10.1016/0955-0674(92)90034-a. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Adenylyl cyclases. Cell. 1992 Sep 18;70(6):869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- Taussig R., Iñiguez-Lluhi J. A., Gilman A. G. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993 Jul 9;261(5118):218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- Veit M., Nürnberg B., Spicher K., Harteneck C., Ponimaskin E., Schultz G., Schmidt M. F. The alpha-subunits of G-proteins G12 and G13 are palmitoylated, but not amidically myristoylated. FEBS Lett. 1994 Feb 14;339(1-2):160–164. doi: 10.1016/0014-5793(94)80406-0. [DOI] [PubMed] [Google Scholar]
- Wang N., Yan K., Rasenick M. M. Tubulin binds specifically to the signal-transducing proteins, Gs alpha and Gi alpha 1. J Biol Chem. 1990 Jan 25;265(3):1239–1242. [PubMed] [Google Scholar]
- Wedegaertner P. B., Chu D. H., Wilson P. T., Levis M. J., Bourne H. R. Palmitoylation is required for signaling functions and membrane attachment of Gq alpha and Gs alpha. J Biol Chem. 1993 Nov 25;268(33):25001–25008. [PubMed] [Google Scholar]
- Winslow J. W., Van Amsterdam J. R., Neer E. J. Conformations of the alpha 39, alpha 41, and beta.gamma components of brain guanine nucleotide-binding proteins. Analysis by limited proteolysis. J Biol Chem. 1986 Jun 5;261(16):7571–7579. [PubMed] [Google Scholar]