Abstract
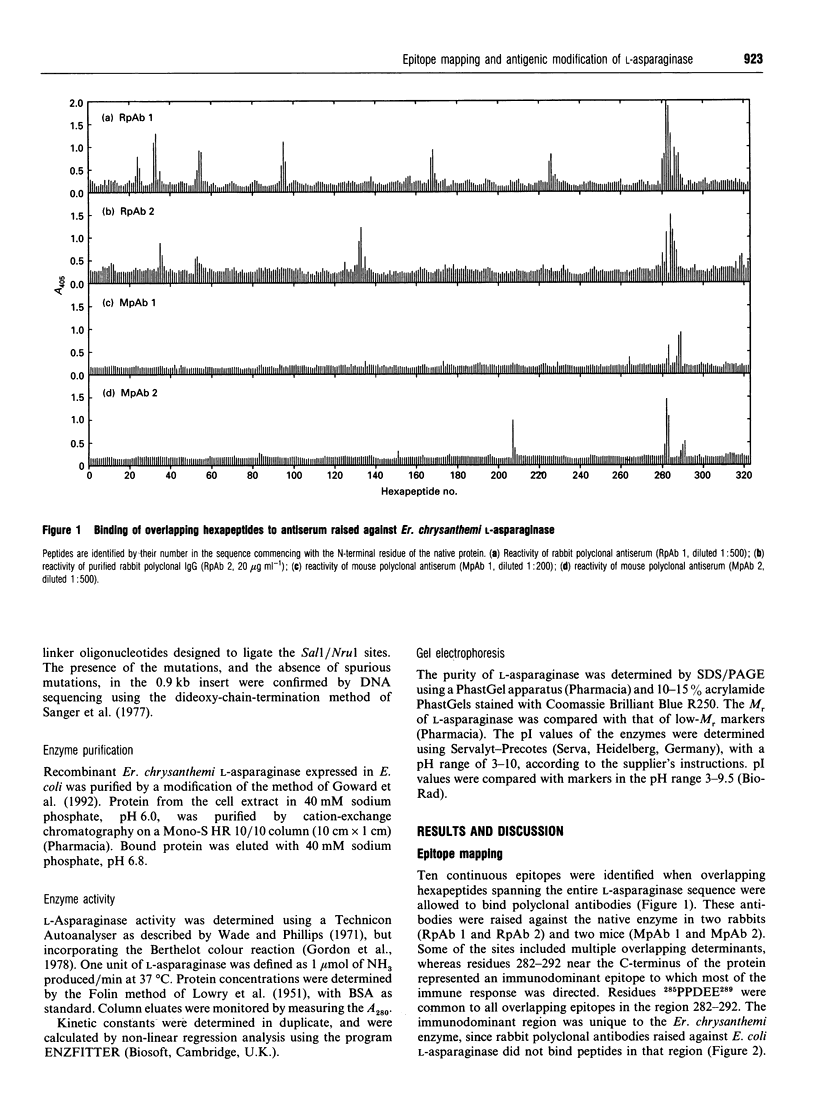
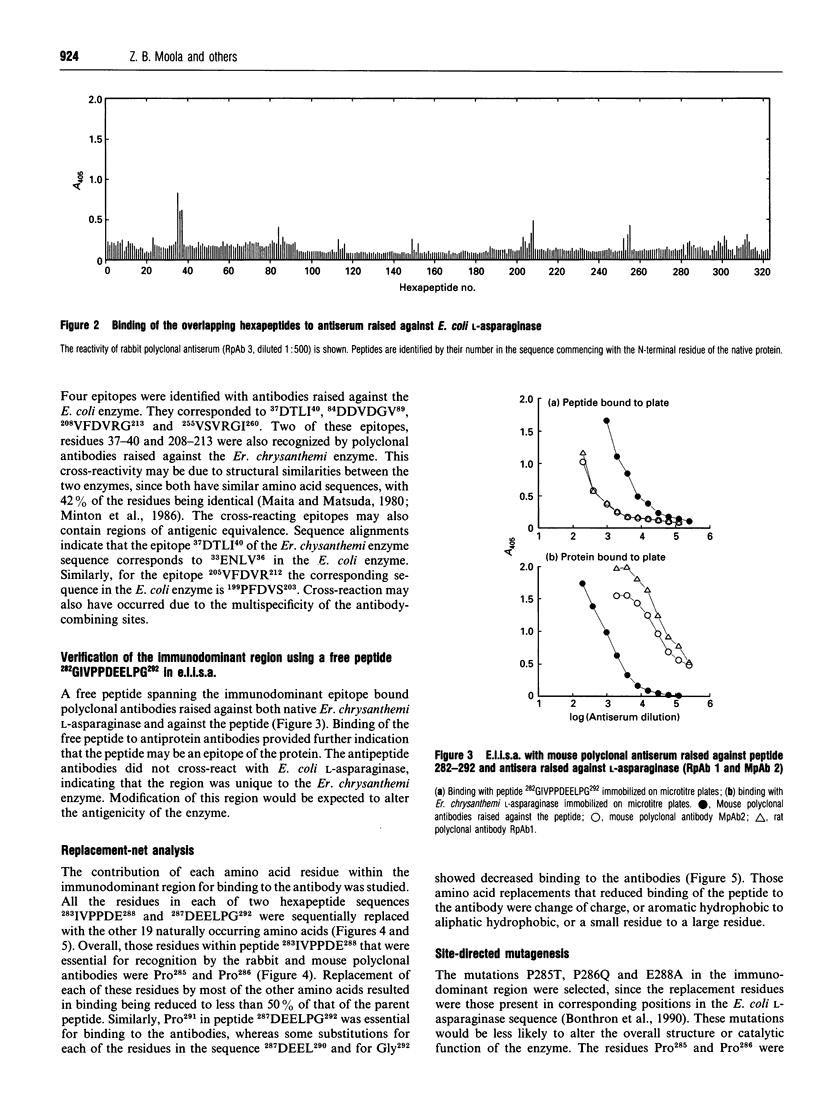
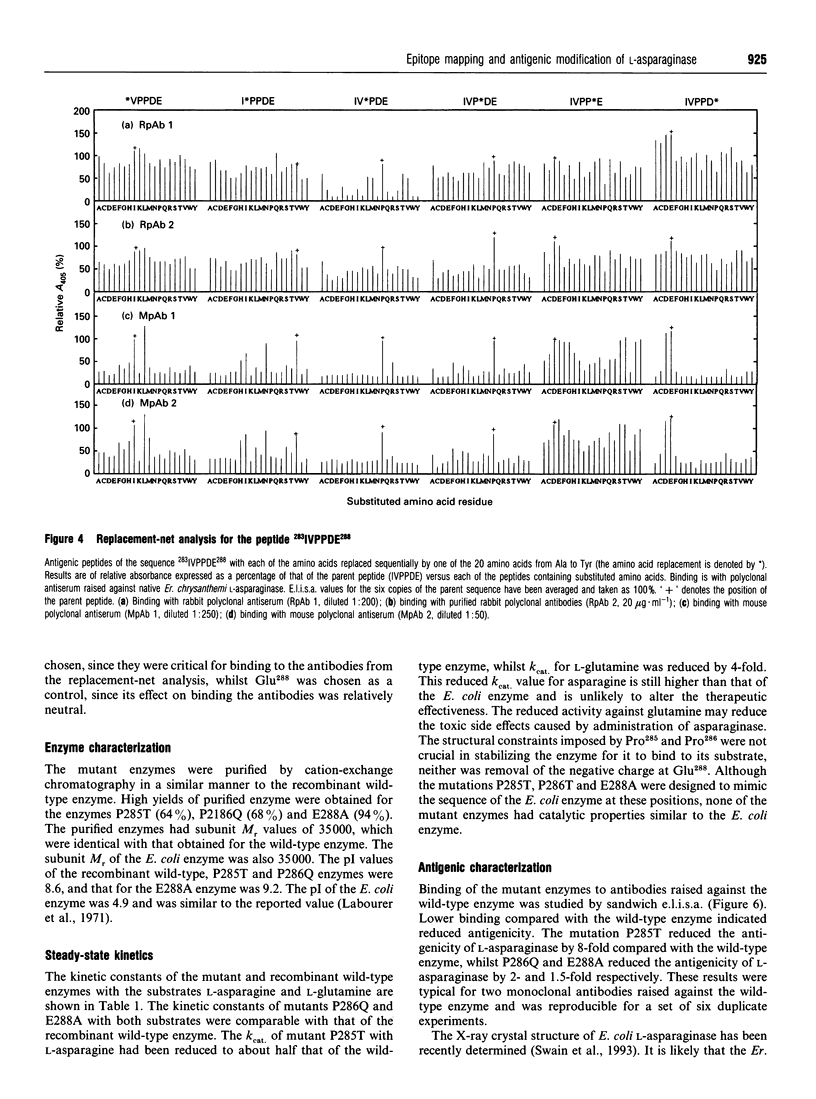
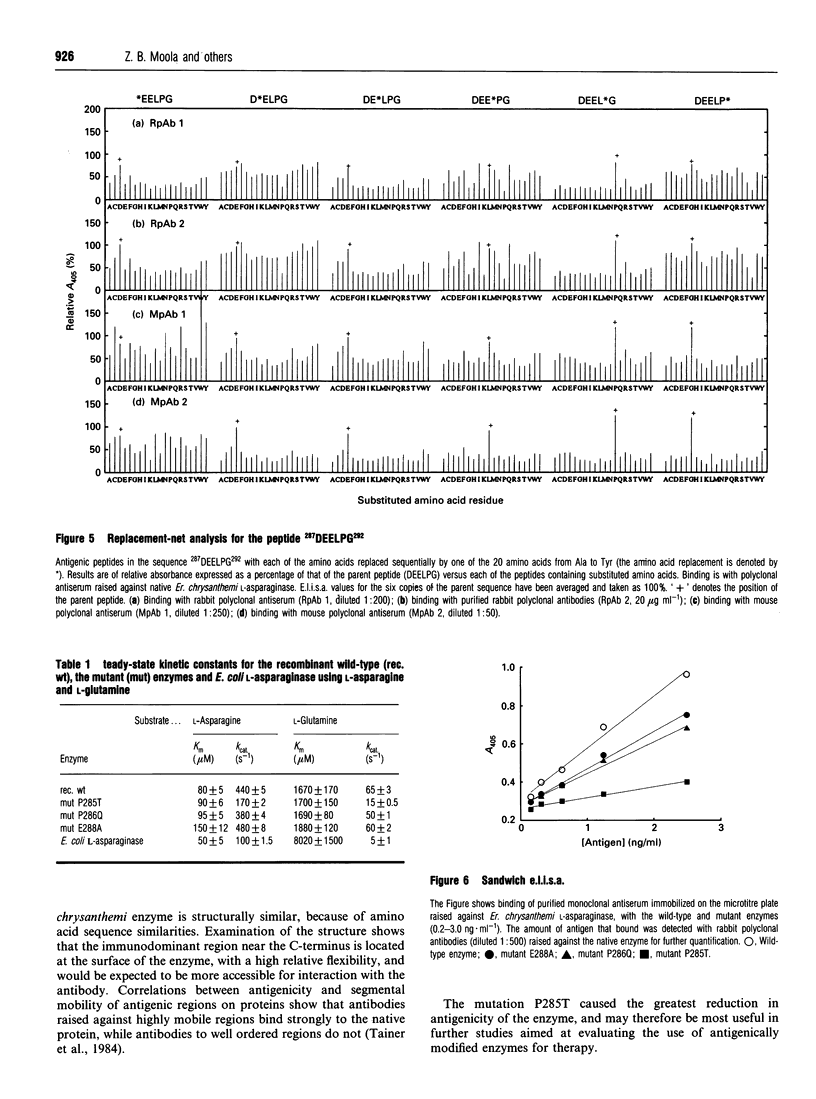
This study shows that the antigenicity of Erwinia chrysanthemi L-asparaginase can be reduced by site-directed mutagenesis. Ten B-cell epitopes of the enzyme were identified using synthetic hexapeptides and polyclonal antisera from rabbits and mice. The region 282GIVPPDEELP292 near the C-terminus was an immunodominant epitope. Binding of two hexapeptides (283IVPPDE288 and 287DEELPG292) to the antibodies was dependent on Pro285, and Pro286, since their replacement by almost any other amino acid resulted in reduced binding. The other residues were less important for binding the antibodies, as binding was relatively unaffected by amino acid substitutions. Three site-directed mutant enzymes, P285T (proline-285-->threonine etc.), P286Q and E288A, were expressed in Escherichia coli. The purified enzymes had subunit M(r) values of 35,000. The pI values of P285T, P286Q and the wild-type enzymes were 8.6, and that for the mutant E288A was 9.2. The kcat. and Km values for the mutants P286Q and E288A with L-asparagine and L-glutamine were comparable with those of the wild-type enzyme. The Km values for the mutant P285T with both substrates was similar to that of the wild-type enzyme, whereas the kcat. was reduced by 2-fold with L-asparagine and by 4-fold with L-glutamine. The change proline-->threonine reduced the antigenicity of the enzyme by 8-fold, as shown in sandwich e.l.i.s.a.s. using monoclonal antibodies raised against the wild-type enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., Kazo G. M., Verhoest C. R., Jr, Van Es T., Kafkewitz D., Nucci M. L., Viau A. T., Davis F. F. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys. 1984 Jun;7(2):175–186. [PubMed] [Google Scholar]
- Alexander H., Alexander S., Getzoff E. D., Tainer J. A., Geysen H. M., Lerner R. A. Altering the antigenicity of proteins. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3352–3356. doi: 10.1073/pnas.89.8.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOME J. D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963 Jul;118:99–120. doi: 10.1084/jem.118.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T. L-asparaginase II of Escherichia coli K-12: cloning, mapping and sequencing of the ansB gene. Gene. 1990 Jul 2;91(1):101–105. doi: 10.1016/0378-1119(90)90168-q. [DOI] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. P., Prior S. E., Barstow D. A., Minton N. P. The pMTL nic- cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988 Aug 15;68(1):139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Distasio J. A., Salazar A. M., Nadji M., Durden D. L. Glutaminase-free asparaginase from vibrio succinogenes: an antilymphoma enzyme lacking hepatotoxicity. Int J Cancer. 1982 Sep 15;30(3):343–347. doi: 10.1002/ijc.2910300314. [DOI] [PubMed] [Google Scholar]
- Eden O. B., Shaw M. P., Lilleyman J. S., Richards S. Non-randomised study comparing toxicity of Escherichia coli and Erwinia asparaginase in children with leukaemia. Med Pediatr Oncol. 1990;18(6):497–502. doi: 10.1002/mpo.2950180612. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Mason T. J., Rodda S. J. Cognitive features of continuous antigenic determinants. J Mol Recognit. 1988 Feb;1(1):32–41. doi: 10.1002/jmr.300010107. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Gilbert H. J., Blazek R., Bullman H. M., Minton N. P. Cloning and expression of the Erwinia chrysanthemi asparaginase gene in Escherichia coli and Erwinia carotovora. J Gen Microbiol. 1986 Jan;132(1):151–160. doi: 10.1099/00221287-132-1-151. [DOI] [PubMed] [Google Scholar]
- Goodman J. W. Modelling determinants for recognition by B cells and T cells. Chem Immunol. 1989;46:1–22. doi: 10.1159/000318820. [DOI] [PubMed] [Google Scholar]
- Gordon S. A., Fleck A., Bell J. Optimal conditions for the estimation of ammonium by the Berthelot reaction. Ann Clin Biochem. 1978 Sep;15(5):270–275. doi: 10.1177/000456327801500164. [DOI] [PubMed] [Google Scholar]
- Goward C. R., Stevens G. B., Tattersall R., Atkinson T. Rapid large-scale preparation of recombinant Erwinia chrysanthemi L-asparaginase. Bioseparation. 1992;2(6):335–341. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Hrushesky W. J., Slavik M., Penta J., Muggia F. The "other" asparaginase. Med Pediatr Oncol. 1976;2(4):441–442. doi: 10.1002/mpo.2950020411. [DOI] [PubMed] [Google Scholar]
- KIDD J. G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. II. Studies on the nature of the active serum constituent: histological mechanism of the regression: tests for effects of guinea pig serum on lymphoma cells in vitro: discussion. J Exp Med. 1953 Dec;98(6):583–606. doi: 10.1084/jem.98.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M. J., Holmes R., Lerner S., Ho D. H. L-asparaginase and PEG asparaginase--past, present, and future. Leuk Lymphoma. 1993;10 (Suppl):153–157. doi: 10.3109/10428199309149129. [DOI] [PubMed] [Google Scholar]
- Kodera Y., Tanaka H., Matsushima A., Inada Y. Chemical modification of L-asparaginase with a comb-shaped copolymer of polyethylene glycol derivative and maleic anhydride. Biochem Biophys Res Commun. 1992 Apr 15;184(1):144–148. doi: 10.1016/0006-291x(92)91170-u. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laboureur P., Langlois C., Labrousse M., Boudon M., Emeraud J., Samain J. F., Ageron M., Dumesnil Y. L-asparaginases d'Escherichia coli. II. Pluralité et origine des formes moléculaires. Relations avec l'activité biologique. Biochimie. 1971;53(11):1157–1165. [PubMed] [Google Scholar]
- Maita T., Matsuda G. The primary structure of L-asparaginase from Escherichia coli. Hoppe Seylers Z Physiol Chem. 1980;361(2):105–117. doi: 10.1515/bchm2.1980.361.1.105. [DOI] [PubMed] [Google Scholar]
- Minton N. P., Bullman H. M., Scawen M. D., Atkinson T., Gilbert H. J. Nucleotide sequence of the Erwinia chrysanthemi NCPPB 1066 L-asparaginase gene. Gene. 1986;46(1):25–35. doi: 10.1016/0378-1119(86)90163-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P. S. Predicting antigenic sites on proteins. Trends Biotechnol. 1991 May;9(5):163–169. doi: 10.1016/0167-7799(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Swain A. L., Jaskólski M., Housset D., Rao J. K., Wlodawer A. Crystal structure of Escherichia coli L-asparaginase, an enzyme used in cancer therapy. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1474–1478. doi: 10.1073/pnas.90.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Wada H., Imamura I., Sako M., Katagiri S., Tarui S., Nishimura H., Inada Y. Antitumor enzyme: polyethylene glycol-modified asparaginase. Ann N Y Acad Sci. 1990;613:95–108. doi: 10.1111/j.1749-6632.1990.tb18151.x. [DOI] [PubMed] [Google Scholar]
- Wade H. E., Robinson H. K., Phillips B. W. Asparaginase and glutaminase activities of bacteria. J Gen Microbiol. 1971 Dec;69(3):299–312. doi: 10.1099/00221287-69-3-299. [DOI] [PubMed] [Google Scholar]


