Summary
Background:
Rotavirus is a leading cause of morbidity and mortality in young children worldwide. In China, the universal immunization of children with the rotavirus vaccine has not been introduced, and the two globally distributed vaccines (RotaTeq and Rotarix) are not licensed in the country. We aim to determine the prevalence and strain diversity of rotavirus in children with diarrhea aged ≤ five years across China.
Materials and methods:
Sentinel-based surveillance of acute diarrhea was conducted at 213 participating hospitals in China from January 1, 2009, through December 31, 2015. Group A rotavirus (RVA) was tested by using enzyme-linked immunosorbent assays, and G- and P-genotype of RVA were tested by RT-PCR methods.
Results:
Of 33,616 children with diarrhea, 10,089 (30%) were positive for RVA; RVA-associated diarrhea was identified in 2247 (39.5%, n = 2247/5685) inpatients and 7842 (28.1%, n = 7842/27931) outpatients. Children living in low-middle-income regions suffered from the highest burden of rotavirus, with 40.7% of diarrhea cases attributed to rotavirus infection, followed by 31.3% in upper-middle-income and 11.2% in high-income regions. The majority of children (88.9%, n = 8976/10089) who tested positive for RVA were children aged ≤ 2 years. The seasonal peak of RVA was in the winter. Among all 2533 RVA strains genotyped, five strain combinations, G9P[8], G3P[8], G1P[8], G2P[4] and G3P[4], contributed to 71.3% (1807/2533) of the RVA-associated diarrhea cases. The predominant strain of RVA has rapidly evolved from G3P[8] and G1P[8] to G9P[8] in the recent years, with the proportion of G9P[8] having increased remarkably from 3.4% in 2009 to 60.9% in 2015.
Conclusions:
The burden of diarrhea attributed to rotavirus is high in China, highlighting the potential value of vaccination. The rapid shift of RVA strains highlights the importance of conducting rotavirus surveillance to ensure that currently marketed vaccines provide protective efficacy against the circulating strains.
Keywords: Rotavirus, Children, Prevalence, Diarrhea, Sentinel Surveillance
Introduction
Diarrhea is the second leading infectious cause of fatal childhood disease worldwide, causing half a million deaths among children aged < 5 years in 2015.1 Rotavirus is the single most common cause of severe and fatal diarrhea in this age group, estimated at ~10 million severe episodes and 118,000–183,000 deaths each year.1–4 Nearly half of all rotavirus deaths occur in Asia.3 Currently, two safe and effective rotavirus vaccines (RotaTeq, Merck & Co, Inc, USA and Rotarix, GlaxoSmithKline Biologicals SA, Belgium) are available globally,5 and the World Health Organization (WHO) recommends that rotavirus vaccination for infants should be included in all national immunization programs as a comprehensive strategy to control diarrheal diseases.6 Over 90 countries have since added rotavirus vaccine into their national immunization programs,7 and a substantial decrease in childhood mortality and hospitalization due to severe diarrhea has been documented following rotavirus vaccine introduction.8,9 However, fewer than 20% of the countries in Asia have introduced rotavirus vaccines into their national immunization programs.10,11
China is both one of the high-burden countries for diarrhea12 and one of the countries that delayed the introduction of rotavirus vaccination into the national immunization program. The two international rotavirus vaccines (RotaTeq and Rotarix) have not been licensed for use in China. However, a domestic Jennerain vaccine (the Lanzhou lamb rotavirus vaccine [LLR], the Lanzhou Institute of Biological Products, China) has been licensed since 20 0 0. At the end of 2014, over 60 million doses of LLR had been distributed to children, and a moderate-to-low efficacy of 30-50% and a coverage rate of 15%–30% were reported for the vaccine by several postmarketing observational studies.13–16 Unfortunately, LLR has not been included in the Expanded Program on Immunization (EPI) since its licensing, partly due to the lack of reliable data on childhood rotavirus infections at a national level to support vaccination activities.
To inform policy makers on rotavirus diarrhea prevention, a nationwide, multicenter, prospective study, the National Key Science and Technology Project on Infectious Disease Surveillance Technique Platform (IDSTP), has been ongoing since 2009,17 in which the activity and strain diversity of rotavirus have been continuously monitored. The primary objectives of the IDSTP are (i) to monitor the prevalence of rotavirus over time to identify priorities for intervention; (ii) to assist timely identification of antigenically distinct novel or rare strains, which emerged rapidly and spread widely in the community; and (iii) to inform policy makers on future vaccine development, enhance recommendations and improve implementation in national vaccination activities. In this study, we determined the prevalence and strain diversity of rotavirus among diarrheal children aged < 5 years in China using data generated by the IDSTP from 2009 to 2015.
Materials and methods
Sentinel-based surveillance
IDSTP is a nationwide sentinel-based surveillance system that has been conducting syndromic surveillance at 213 participating hospitals across the country since January 1, 2009 (Fig. 1 & supplementary table 1). These hospitals were selected based on three principles: (i) each of the 31 provinces of mainland China would include a minimum of one hospital; (ii) various types of hospitals (e.g., children’s, general, urban and rural community health service centers) would be included; and (iii) hospitals would have the capability and resources to conduct ongoing surveillance.
Fig. 1.
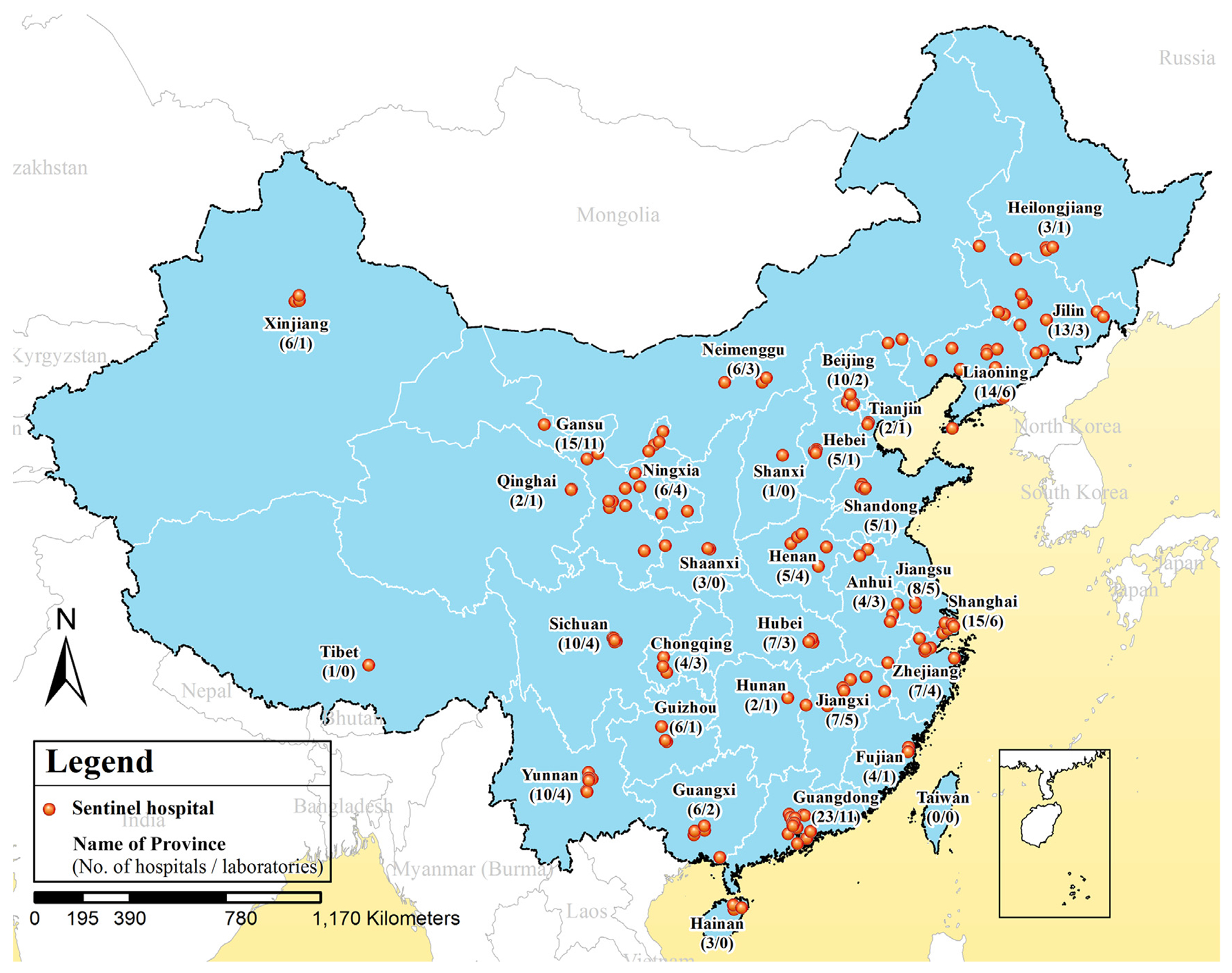
Locations of the 213 hospitals and 92 network laboratories participating in the diarrheal illnesses surveillance of China, 2009–2015.
Patients visiting these hospitals’ outpatient (e.g., emergency room and clinics) and/or inpatient (e.g., wards and intensive care unit) departments were registered and screened for eligibility of inclusion year-round by an attending practitioner. Diarrhea was defined as ≥ 3 passages of watery, loose, mucoid, or bloody-stools within a 24-h period. Patients meeting the case definition of diarrhea were eligible for inclusion. A total sample size of ~10,000 persons per year was selected for the whole country. The total sample size was then allocated and assigned to each sentinel hospital (median = 54 cases/hospital/year) after weighting using the recorded outpatient numbers from the previous year. Convenience sampling was used to recruit cases among eligible patients. Attending doctors in the hospital would decide when, whom, in what hospital setting and how many patients to enroll. To account for seasonal variations of diarrheal illness onset, it was determined that each hospital was to recruit ≥ 5% of the allocated number on a monthly basis.17 Between 2009 and 2011, both inpatients and outpatients were included. Since 2012, the enrollment of inpatients has been suspended because of the overlap of the study with another national surveillance program conducting surveillance for diarrhea among hospitalized children. Verbal consents were obtained from parents/guardians before enrollment. The study protocol was approved by the ethical review committee at the Chinese Center for Disease Control and Prevention (China CDC, Beijing, China).
Data and specimen collection
Patients or children’s guardians were interviewed with the use of a standardized case reporting form at the time of recruitment, and additional medical information was abstracted from medical charts by trained staff. The collected information included demographics (e.g., sex, date of birth, and address) and clinical characteristics (e.g., signs/symptoms, date of symptom onset, date of diagnosis). After recruitment, fresh, whole stool was collected in a sterilized container without preservative and stored at −20 °C until rotavirus screening and strain characterization. All collected stool samples were packed and transported in iceboxes within 48 h to a network laboratory according to UN3373 transportation requirements.18,19 There were 92 network laboratories for this project in the country, and each of them served a specific number of hospitals in the same catchment area.
Laboratory testing
A standardized method and operation procedure were adopted by all laboratories for rotavirus testing and characterizing of strains and validated before initiating surveillance. Group A rotavirus (RVA) antigens were detected directly in stool samples by using enzyme-linked immunosorbent assay (ELISA, ProSpecTTM Rotavirus kit, Oxoid Ltd, Basingstoke, UK), according to the manufacturer’s instructions. For ELISA-positive samples, multiplex reverse-transcription polymerase chain reaction assays were used for further genotyping of strains that were received at 33 network laboratories, due to the capacity restriction.17
Viral nucleic acid was extracted from each specimen using the Viral RNA/DNA Kit (Geneaid Biotech, Taipei, Taiwan) or the QI-Aamp® Viral RNA Mini Kit (Qiagen, Valencia, CA), according to the manufacturers’ instructions. The primers used in G- and P- genotyping PCRs (Supplementary Table 2) and procedures have been described in previous studies.20–22
Statistical Analysis
For this paper, data from children under five years old were analyzed. Prevalence was calculated by dividing the number of children that tested positive for rotavirus by the total number of children tested. To study the seasonal pattern of rotavirus activity, we scaled the prevalence of rotavirus for different months and weeks from 0 to 1 according to percentile rank and further represented data as thermodynamic diagrams. To quantify seasonal characteristics, multiple linear regression models using a binomial distribution and a log link were also fitted to individual patient data collected from 2009 to 2015, including sine and cosine functions of the illness onset week to represent annual and semiannual periodic cycles as described previously.23 ,24 Since the efficacy of rotavirus vaccines was shown to be lower in low-resource regions than in high-resource regions in previous studies,5 we measured rotavirus prevalence by economic regions according to the World Bank’s classification of economies25 to help identify priorities for future intervention. We divided the country into three regions based on the Gross Regional Product (GRP) per capita of the province from 2009–2015, 26 namely, lower-middle-income regions (GRP $1,056–3,955), upper-middle-income regions (GRP $3,956–12,235) and high-income regions (GRP ≥$12,236). Cases were also classified into rural and urban, depending on the administrative classification of the residence. We used Chi-square tests or Fisher’s exact tests for categorical variables, and Wilcoxon rank-sum or Kruskal–Wallis tests for continuous variables, as appropriate. All analyses were conducted in R version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria),27 and the geographic map was produced using ArcGIS 10.2.2 (ESRI, Redlands, CA, USA).
Results
Characteristics of patients
Between January 1, 2009, and December 31, 2015, a total of 33,616 children with diarrhea aged < 5 years across the country were included in our analysis, of which 27,931 (83.1%) were recruited at outpatient settings and 5,685 (16.9%) at inpatient settings. No patients were recruited at inpatient settings from 2012 to 2015. The median age of children was 0.9 years (interquartile range: 0.5–1.4 years) and 21,683 (64.5%) were male ( Table 1 ). Among the three economic regions, upper-middle-income regions had the most number of patients (n = 24,110, 71.7%), followed by lower-middle-income regions (n = 5,002, 14.9%) and high-income regions (n = 4,504, 13.4%). Of the total enrolled, 17,930 (54.0%) children lived in urban areas and 15,276 (46.0%) lived in rural areas.
Table 1.
Patient characteristics of children with diarrhea in China, 2009–2015.
| Characteristics | All patients, (n = 33,616) | Hospital settings |
||
|---|---|---|---|---|
| Outpatient, (n = 27,931) | Inpatient, (n = 5,685) | p-value | ||
| Sex, male | 21683 (64.5) | 17845 (63.9) | 3838 (67.5) | <0.001 |
| Age, median (IQR), yr | 0.9 (0.5-1.4) | 0.9 (0.5-1.4) | 0.7 (0.4-1.0) | <0.001 |
| Age group | <0.001 | |||
| 1–5 mo | 8186 (24.4) | 6258 (22.4) | 1928 (33.9) | |
| 6–11 mo | 12249 (36.4) | 9822 (35.2) | 2427 (42.7) | |
| 12–23 mo | 8257 (24.6) | 7526 (26.9) | 731 (12.9) | |
| 24–59 mo | 4924 (14.6) | 4325 (15.5) | 599 (10.5) | |
| Residential Area* | <0.001 | |||
| Rural | 15276 (46%) | 11494 (41.4) | 3782 (69.3) | |
| Urban | 17930 (54%) | 16253 (58.6) | 1677 (30.7) | |
| Year of surveillance | <0.001 | |||
| 2009 | 4319 (12.8) | 2455 (8.8) | 1864 (32.8) | |
| 2010 | 5576 (16.6) | 3801 (13.6) | 1775 (31.2) | |
| 2011 | 4427 (13.2) | 2381 (8.5) | 2046 (36.0) | |
| 2012 | 4573 (13.6) | 4573 (16.4) | – | |
| 2013 | 5631 (16.8) | 5631 (20.2) | – | |
| 2014 | 4634 (13.8) | 4634 (16.6) | – | |
| 2015 | 4456 (13.3) | 4456 (16.0) | – | |
Note: Data are presented as no. (%) of patients unless otherwise indicated. No patients were recruited at inpatient settings during years 2012–2015.
The no. of patients in rural and urban areas did not equal the total no. of patients due to missing data.
Prevalence of rotavirus
Overall, RVA was identified in 10,089 children (30.0%). Children aged 6–23 mo had the highest positive percentage of RVA tests compared to other age groups (34.7%, n = 7124/20,506, p < 0.001). Moreover, RVA was more frequently identified among children attending an inpatient setting than children visiting an outpatient department (39.5% vs. 28.1%, p < 0.001), it was more common among children in lower-middle-income regions than in upper-middle and high-income regions (40.7%, 31.3% and 11.2%, respectively; p < 0.001), and it was more common among children living in rural areas than in those living in urban areas (34.9% vs. 26.0%, p < 0.001) ( Table 2 ). However, when stratified by economic region, RVA infection was more common among children living in urban areas than those living in rural areas in lower-middle-income regions (45.2% vs. 37.5%, p < 0.001). The seasonal peak of RVA activity was in winter months, i.e., in week 50 (mid-December) for outpatients and in week 46 (mid-November) for inpatients (Fig. 2 ).
Table 2.
Frequency of Group A rotavirus detected in children with diarrhea in China, 2009–2015, stratified by economic regions.
| Characteristics | All patients |
p-value | Lower-middle-income regions |
p-value | Upper-middle-income regions |
p-value | High-income regions |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. tested | No. identified, (%) | No. tested | No. identified, (%) | No. tested | No. identified, (%) | No. tested | No. identified, (%) | |||||
| Total | 33616 | 10089 (30.0) | 5002 | 2034(40.7) | 24110 | 7552(31.3) | 4504 | 503 (11.2) | ||||
| Hospital settings | <0.001 | <0.001 | <0.001 | – | ||||||||
| Outpatient | 27931 | 7842 (28.1) | 3682 | 1356 (36.8) | 19745 | 5983 (30.3) | 4504 | 503 (11.2) | ||||
| Inpatient* | 5685 | 2247 (39.5) | 1320 | 678 (51.4) | 4365 | 1569 (35.9) | 0 | – | ||||
| Sex | 0.006 | 0.153 | 0.104 | 0.808 | ||||||||
| Male | 21683 | 6618 (30.5) | 3298 | 1365 (41.4) | 15611 | 4946 (31.7) | 2774 | 307 (11.1) | ||||
| Female | 11933 | 3471 (29.1) | 1704 | 669 (39.3) | 8499 | 2606 (30.7) | 1730 | 196 (11.3) | ||||
| Age group | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| 1-5 mo | 8186 | 1843 (22.5) | 1001 | 381 (38.1) | 6238 | 1393 (22.3) | 947 | 69 (7.3) | ||||
| 6-11 mo | 12249 | 4329 (35.3) | 1899 | 931 (49.0) | 9244 | 3273 (35.4) | 1106 | 125 (11.3) | ||||
| 12-23 mo | 8257 | 2795 (33.9) | 1263 | 490 (38.8) | 5551 | 2095 (37.7) | 1443 | 210 (14.6) | ||||
| 24-59 mo | 4924 | 1122 (22.8) | 839 | 232 (27.7) | 3077 | 791 (25.7) | 1008 | 99 (9.8) | ||||
| Residential Area# | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Rural | 15276 | 5336 (34.9) | 2408 | 902 (37.5) | 11795 | 4272 (36.2) | 1073 | 162 (15.1) | ||||
| Urban | 17930 | 4653 (26.0) | 2426 | 1097 (45.2) | 12076 | 3215 (26.6) | 3428 | 341 (9.9) | ||||
Regions were classified according to the World Bank’s classification of economies: Lower-middle-income regions = Gross Regional Product (GRP) in the province $1006–3955 per capita per year; upper-middle-income regions = GRP $3956–12,235 per capita per year; and high-income regions = GRP ≥ $12,236 per capita per year.
No patients were recruited at inpatient settings during the years 2012–2015.
The no. of patients in rural and urban areas did not equal the total no. of patients due to missing data; an em dash denotes that data are currently unavailable.
Fig. 2.
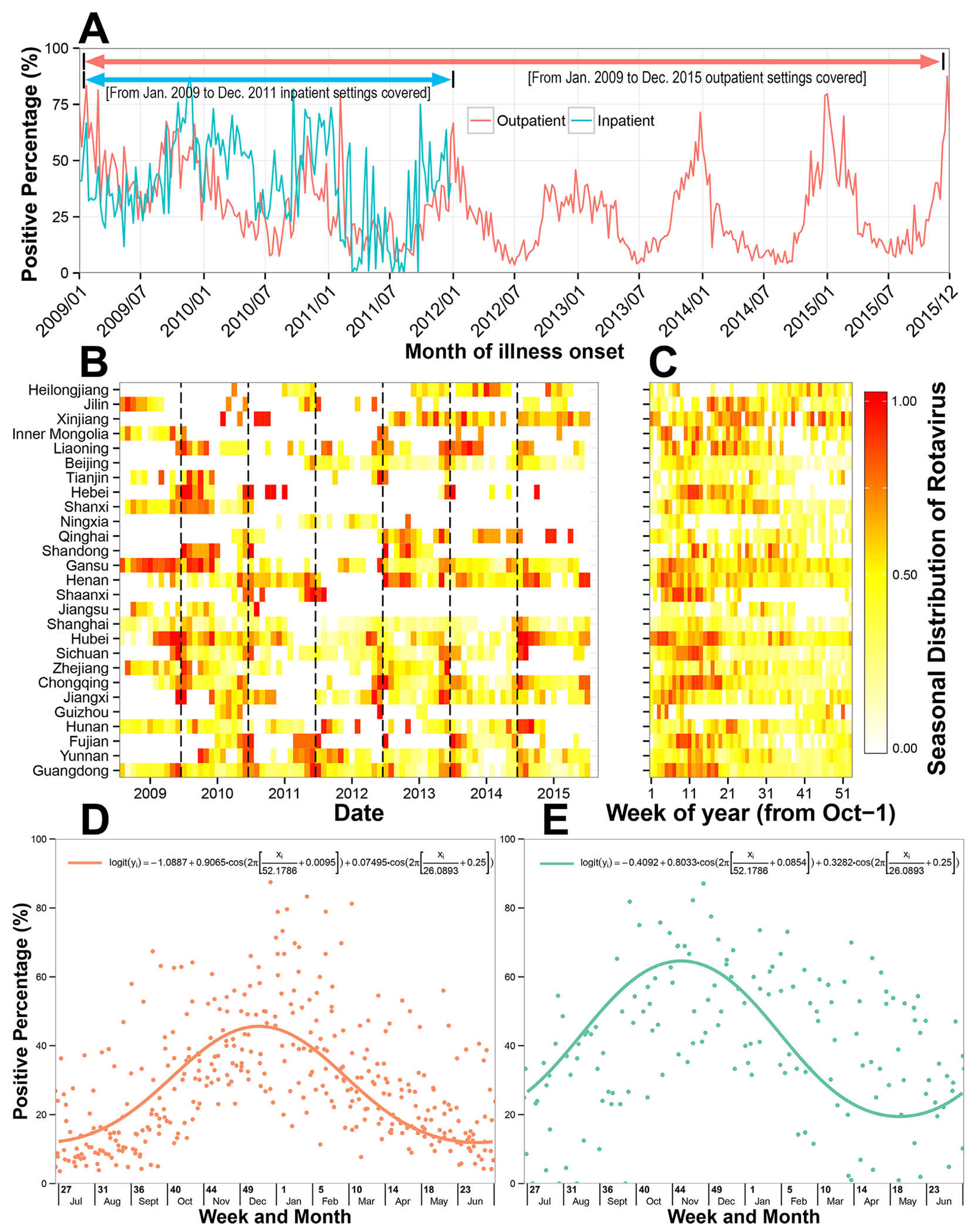
Temporal trends of rotavirus infection in children with diarrhea in China, 2009–2015. (A) Time series of weekly positive percentage of rotavirus, by hospital setting; (B) Heat map of monthly positive percentage of rotavirus, scaled from 0 to 1 according to percentile rank, by province; (C) Heat map of average weekly positive percentage of rotavirus, scaled from 0 to 1 according to percentile rank, by province; (D) The weekly positive percentage of rotavirus in outpatients, with a fitted seasonal curve superimposed; (E) The weekly positive percentage of rotavirus in inpatients, with a fitted seasonal curve superimposed.
Clinical presentations of rotavirus-associated diarrhea
The median age of RVA infection was 1.0 year (IQR = 0.6-1.3 years), and 88.9% (n = 8967) of RVA infection occurred among children aged ≥ 2 years. The median illness duration before seeing a doctor was 2 days (IQR = 1–3 days). Symptom/signs including fever, vomiting, dehydration, and watery-stool were more frequent among children who tested positive for RVA (28.6%, 39.4%, 4.1%, and 72.6%, respectively) than among children who tested negative for RVA (21.9 %, 21.8%, 1.9% and 60.1%, respectively, p < 0.001). Antibiotic usage before seeing a doctor was also more frequent among children with diarrhea associated with RVA (14.9% vs. 11.5%, p < 0.001) (Table 3). Among children with rotavirus-associated diarrhea, fever (41.9% vs. 20.3%, p < 0.001) and dehydration (20.5% vs. 0.9%, p < 0.001) were more common in inpatient than outpatient, while vomiting (51.2%% vs. 34.6%, p < 0.001) and respiratory symptom (13.2% vs.9.8%, p < 0.001) were more common in outpatient than inpatient (Supplementary Table 3).
Table 3.
Clinical presentations of children with and without rotavirus-associated diarrhea in China, 2009–2015.
| Clinical Characteristics | Children with rotavirus-associated diarrhea (n = 10,089) | Children without rotavirus-associated diarrhea (n = 23,527) | p-value |
|---|---|---|---|
| Illness duration before seeing a doctor, median days, (IQR) | 2 (1–3) | 2 (1–3) | <0.001 |
| Symptoms/signs, no. (%)* | |||
| Fever (body temperature > 38.0 °C) | 1414 (28.6) | 3148 (21.9) | <0.001 |
| Vomiting | 3773 (39.4) | 4924 (21.8) | <0.001 |
| Dehydration | 419 (8.5) | 470 (3.3) | <0.001 |
| Respiratory symptom | 1284 (12.7) | 3242 (13.8) | 0.01 |
| Antibiotic usage before visiting, no. (%)* | 1108 (14.9) | 2080 (11.5%) | <0.001 |
Note: No. of patients in the column did not sum up to equal the total no. of patients due to missing data. Respiratory symptoms included cough, sore throat, or rhinorrhea. Dehydration included thirst, decreased skin turgor, sunken eyes, or sunken fontanelle (in infants). IQR = interquartile range.
Distribution of rotavirus strains
A total of 2,533 isolates were genotyped during 2009-2015. For the G-types studied, 2,232 (88.1%) had a single G specificity, 151 (6.0%) had mixed G specificities and 150 (5.9%) were unable to be assigned any G-type specificity. Meanwhile, for P-types, 2,338 (92.3%) isolates had a single P specificity, 103 (4.1%) had mixed P specificities and 92 (3.6%) were unable to assign any P-type specificity.
Among the 2,232 isolates with a single G specificity, the most common G-type identified was the G9 strain (n = 922, 41.3%), while the four globally common strains, G1, G2, G3 and G4, constituted 57.3% (n = 1,278) of the single G-type identified isolates. Among 2,338 isolates with a single P specificity, P[8] (n = 1,785, 76.3%) was the most common P-type. Together, P[8] and P[4] constituted 89.5% (n = 2,093) of these single P-type identified isolates. Regardless of mixed infections, five strain combinations, G9P[8], G3P[8], G1P[8], G2P[4] and G3P[4], were associated with 71.3% (n = 1807/2533) of all RVA-associated diarrhea in children, with 794 (31.3%), 478 (18.9%), 365 (14.4%), 115 (4.5%), and 55 (2.2%) strains, respectively, during the whole period of 2009-2015 (Supplementary Table 4).
During the seven-year study period, RVA strains circulating in children changed from year to year, with no one single strain persistent in the population. G3P[8] was predominant in the first couple of years, whereas genotype P[11] gained predominance in 2011. In 2012, the G1P[8] strain dominated, but in the most recent years, G9P[8] became the predominant circulating strain across the whole country, raising from 3.4% (2009) to 60.9% (2015) of all strains isolated (Fig. 3).
Fig. 3.
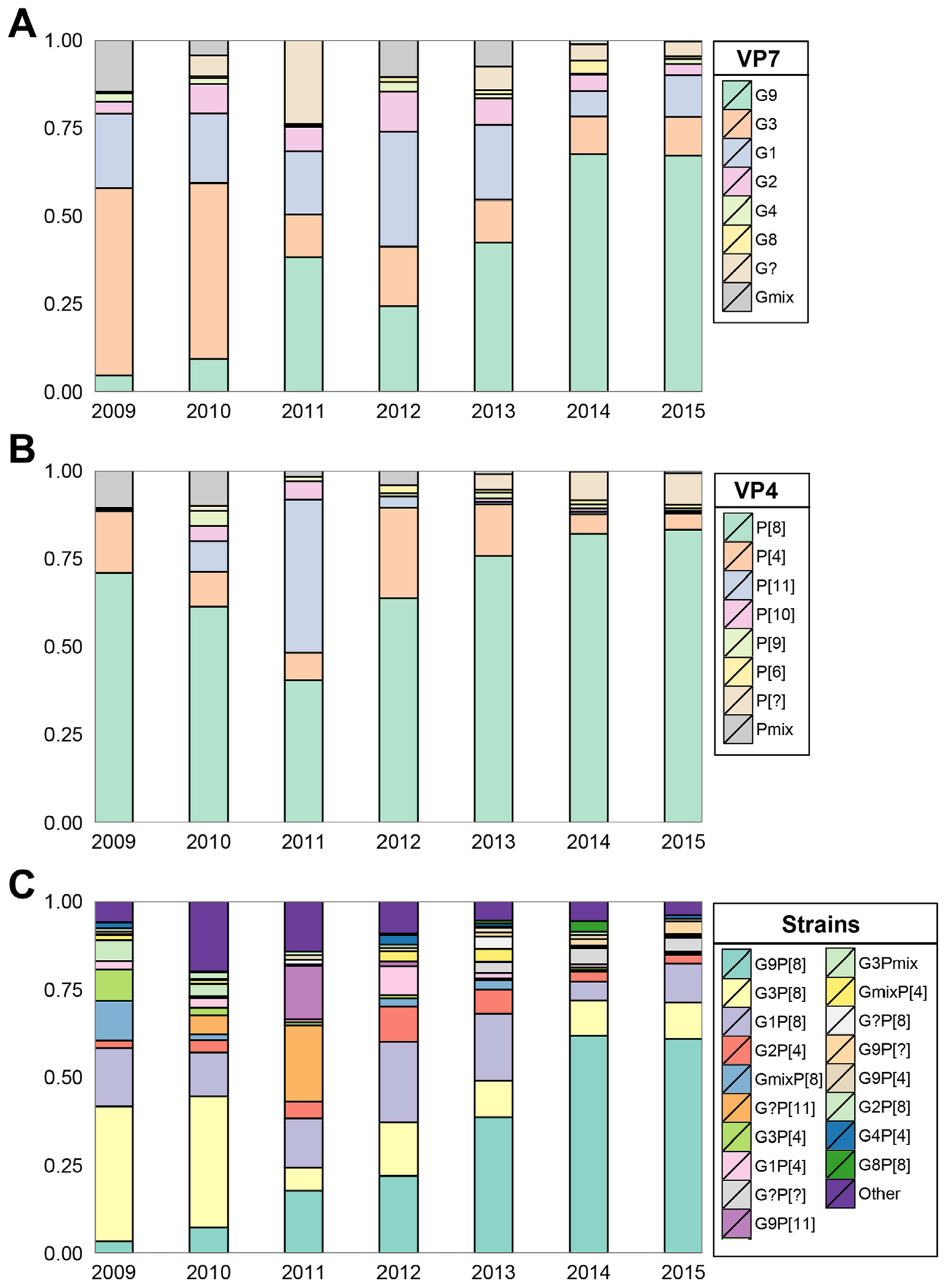
Rotavirus strains distribution in China, 2009–2015. (A) Strains classified by VP7 genotype; (B) Strains classified by VP4 genotype; (C) Strains classified by G-P genotype combinations. [?] = non-typeable; mix = two or more G-P-types identified.
Discussion
Using large-scale, up-to-date national surveillance data, this study examined rotavirus infection and strain diversity in China and highlighted the importance of using routine surveillance data to identify priorities for intervention and detect emergent or predominant strains to guide vaccination activity. Nationally, one-third of childhood diarrhea was associated with rotavirus infection, and the prevalence of rotavirus was higher in inpatients than in outpatients, accounting for 28% of outpatient visits and 40% of hospitalizations attributed to diarrhea in children. These numbers were very close to 32.5% of outpatients and 42.6% of inpatients reported in a 2011 review and to 30% of outpatients and 40% of inpatients reported in a 2016 review,16,28 suggesting that the burden of diarrhea attributed to rotavirus in the country has not changed very much over these years. To reduce the burden of diarrheal disease, the inclusion of rotavirus vaccine into the national immunization program should be seriously considered. Of the total rotavirus infections that occurred in children, 89% occurred in children aged ≤ 23 mo, and children aged 6-23 mo had the highest prevalence of rotavirus among all age groups in the study. The WHO recommends that the first dose of the rotavirus vaccine should be administered starting at age 6 weeks and the last dose at a maximum age of two years.6 Vaccination of children > 24 months of age is not recommended. In China, the one domestic available vaccine, LLR, is administrated to children aged between 2–35 mo, with one dose per year for three consecutive years,13 which is unfavorable for the vaccine to confer full protection for young children before their risk of infection is high. Our analysis of the relative frequency of rotavirus infection in children of different age groups suggested that the immunization procedure of LLR should be better optimized.
Our results showed that, as the region’s per capita income increased from less than $3,955 to $12,236 or more per year, the observed prevalence of rotavirus in children decreased from 40.7% to 11.2%. A region’s economic development seemed to have an impact on the burden of rotavirus disease Moreover, rotavirus was detected more commonly in children living in rural areas rather than in urban areas, yet children living in urban area of lower-middle-income regions had the highest burden of rotavirus infection, suggesting that population over-crowdedness had an effect on the burden of rotavirus illnesses when the population was stratified by region’s economic development. However, good hygiene and sanitation seemed unlikely to reduce the occurrence of rotavirus disease in previous studies29, and the prevalence of rotavirus was reportedly not associated with population density in previous studies.16,28 We postulated that people living in high-income regions might be more affluent and have more access to rotavirus vaccines that conferred protection to some of the children in these regions. The observed association between population density or economic development and rotavirus infection might have been biased to some extent by the vaccination status of children, which was not measured in our current study. Future studies measuring vaccine coverage and efficacy in this group of children with diarrhea are warranted.
Globally, five strains, G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8], have caused most of the severe diarrheal illness in children in most countries during the last 20 years.30 This was also true in our study; the top five strain combinations (G9P[8], G3P[8], G1P[8], G2P[4] and G3P[4]) were associated with 71.3% of rotavirus-associated diarrhea from 2009 to 2015, except G4P[8] was less common in our study (<1% of all isolates). Moreover, the dominant rotavirus strains circulating in the pediatric population changed unpredictably from year to year. No persistence of a single rotavirus strain was observed. From 2009 to 2015, the most dramatic change observed was that the previously predominant strains, G3P[8] and G1P[8], were rapidly replaced by genotype G9P[8] across all regions in the country in the most recent years, increasing from 3.4% in 2009 to 61.3% of all isolates in 2015. The newly emerged genotype G9P[8] has spread worldwide since its first emergency in the 1980s31 and has gained predominance in several countries, including Australia, Ghana and Thailand,32–34 as well as China.35 Although the two globally licensed vaccines, RotaTeq and Rotarix, both demonstrate protective efficacy against G9P[8] via their P[8] component contained in the vaccines,36–38 the one rotavirus vaccine in China, LLR, a lamb strain of rotavirus vaccine characterized as G10P[12], has no available data studying vaccine efficacy against the G9P[8] heterotypic strain. Previous studies conducted with other Jennerian vaccines reported inconsistent results,39,40 suggesting that animal strain vaccines might confer less heterotypic protection than homotypic protection to different G/P strains. The efficacy of this domestically available vaccine on other heterotypic strains needs to be assessed by well-designed clinical trials or observational studies in the future.
Our study had limitations. First, our surveillance protocol has undergone several modifications during the study period. Inclusion of children in inpatient settings was suspended since 2012. This affected comparison of our results between different study periods and between children subgroups. Second, the convenience sampling method used in our study can introduce unpredictable biases. For example, different health-seeking behaviors of patients, subjective judgment of clinicians on when and whom to sample, and a sampling scheme that was not pre-determined could have yielded patterns in the data that do not represent the true burden and epidemiological features of rotavirus. Nevertheless, the large sample size collected in our study might, to some extent, offer some relief to these concerns. Further improvement of the surveillance system attributes based on these aspects is welcomed. Third, we did not collect the historical information of vaccine usage for the diarrheal children in our study and were thus unable to evaluate the vaccine efficacy or to relate high prevalence of rotavirus in low-middle-income regions or rural areas to vaccine usage in the region. Fourth, population-based rates for rotavirus-associated diarrhea were not readily available, owing to the lack of population denominators in our study.
Conclusions
The burden of diarrhea attributed to rotavirus is high in China, especially in urban areas of lower-middle-income regions, where the per capita gross regional income is less than $4000 per year. The inclusion of the rotavirus vaccine in the national immunization program to mitigate rotavirus burden should be considered. No persistence of any single rotavirus strain was observed during 2009–2015, and the G9P[8] strain has shifted to gain dominance in recent years. Continuous surveillance of rotavirus strain evolution is needed to ensure that currently marketed vaccines provide protective efficacy against emergent or antigenically distinct novel strains in China and the global health community.
Supplementary Material
Acknowledgments
We wish to thank all the patients, nurses, and clinicians, as well as laboratory, research, and administrative staff, who took part in or contributed to this study. This work was supported by the Ministry of Science and Technology of China (2009ZX10004-201, 2009ZX10004-202, 2009ZX10004-203, 2009ZX10004-204, 2009ZX10004-205, 2009ZX10004-207, 2009ZX10004-208, 2009ZX10004-209, 2009ZX10004-210, 2009ZX10004-211, 2009ZX10004-212, 2009ZX10004-213, 2012ZX10004-201, 2013ZX10004-202, 2012ZX10004-206, 2012ZX10004-207, 2012ZX10004-208, 2012ZX10004-209, 2012ZX10004-210, 2012ZX10004-211, 2012ZX10004-212, 2012ZX10004-213).
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency or the official position of the China CDC.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2018.07.004.
References
- 1.GBD Diarrhoeal Diseases Collaborators Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global burden of disease study 2015. Lancet Infect Dis 2017;17(9):909–48 Jun 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C. et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016;388(10051):1291–301 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. World Health Organization–coordinated global rotavirus surveillance N. Global, regional, and national estimates of rotavirus mortality in children <5 Years of Age, 2000–2013. Clin Infect Dis 2016;62(Suppl 2):S96–S105 May 01. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1459–544 Oct 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis 2017;65(5):840–50 Sep 1. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Rotavirus vaccines. WHO position paper–January 2013. Week Epidemiol Rec 2013;88(5):49–64 Feb 1. [PubMed] [Google Scholar]
- 7.PATH. rotavirus vaccine advocacy resources; 2017. [cited October 25, 2017]; Available from: https://www.defeatdd.org/article/rotavirus-vaccine-advocacy-resources.
- 8.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010;362(4):299–305 Jan 28. [DOI] [PubMed] [Google Scholar]
- 9.do Carmo GM, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med 2011;8(4):e1001024 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PATH. National and regional rotavirus vaccine introductions. [cited 2017 August 31]; Available from: http://rotacouncil.org/vaccine-introduction/global-introduction-status/.
- 11.Kirkwood CD, Steele AD. Rotavirus vaccine will have an impact in Asia. PLoS Med 2017;14(5):e1002298 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA. et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405–16 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhen SS, Li Y, Wang SM, Zhang XJ, Hao ZY, Chen Y. et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg Microbes Infect 2015;4(10):e64 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu C, He Q, Xu J, Xie H, Ding P, Hu W, et al. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine 2012;31(1):154–8 Dec 17. [DOI] [PubMed] [Google Scholar]
- 15.Yu JX, Zhu WP, Ye CC, Xue CY, Lai SJ, Zhang HL. et al. A cross-sectional study of acute diarrhea in Pudong, Shanghai, China: prevalence, risk factors, and healthcare-seeking practices. Epidemiol Infect 2017;145(13):2735–44 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D Yen C, Yin ZD, Li YX, Liu N, Liu YM. et al. The public health burden of rotavirus disease in children younger than five years and considerations for rotavirus vaccine introduction in China. Pediatr Infect Dis J 2016; 35(12):e392–8 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J Jing H, Lai S, Xu W, Li M, Wu J. et al. Etiology of diarrhea among children under the age five in China: results from a five-year surveillance. J Infect 2015;71(1):19–27 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinese Center for Disease Control and Prevention. Diarrheal Syndrome Surveillance Protocol (2012 version). Management Office of National Science and Technology Major Project of China, Chinese Center for Disease Control and Prevention; 2012. [in Chinese]. [Google Scholar]
- 19.International Civil Aviation Organizatio. ICAO Technical Instructions for the Safe Transport of Dangerous Goods by Air 2005-2006 (Doc 9284 AN/905). International Civil Aviation Organization (ICAO), 2005. [Google Scholar]
- 20.Chen Y, Li Z, Han D, Cui D, Chen X, Zheng S, et al. Viral agents associated with acute diarrhea among outpatient children in Southeastern China. Pediatr Infect Dis J 2013;32(7):e285–90. [DOI] [PubMed] [Google Scholar]
- 21.Simmonds MK, Armah G, Asmah R, Banerjee I, Damanka S, Esona M. et al. New oligonucleotide primers for P-typing of rotavirus strains: Strategies for typing previously untypeable strains. J Clin Virol 2008;42(4):368–73 Aug. [DOI] [PubMed] [Google Scholar]
- 22.Iturriza Gomara M, Kang G, Mammen A, Jana AK, Abraham M, Desselberger U, et al. Characterization of G10P[11]rotaviruses causing acute gastroenteritis in neonates and infants in Vellore, India. J Clin Microbiol 2004;42(6):2541–7 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolwijk AM, Straatman H, Zielhuis GA. Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Commun Health 1999;53(4):235–8 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Alonso WJ, Feng L, Tan Y, Shu Y, Yang W, et al. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: spatio-temporal modeling of surveillance data. PLoS Med 2013;10(11):e1001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The World Bank. World Bank country and lending groups: country classification. 2017. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. [Google Scholar]
- 26.National Bureau of Statistics of China. Gross Regional Product (GRP) per capita by province, 2009-2015. In: National Bureau of Statistics of China. [Cited 2017 November 1]; Available from: http://data.stats.gov.cn/easyquery.htm?cn=E0103. [in Chinese].
- 27.R Core Team. A language and environment for statistical computing. Vienna, Austria: R. Foundation for Statistical Computing; 2013. [Google Scholar]
- 28.Liu N, Xu Z ,Li D, Zhang Q, Wang H, Duan ZJ .Update on the disease burden and circulating strains of rotavirus in China: a systematic review and meta-analysis. Vaccine 2014;32(35):4369–75 Jul 31. [DOI] [PubMed] [Google Scholar]
- 29.Parashar UD, Bresee JS, Gentsch JR, Glass RI. Rotavirus. Emerg Infect Dis 1998;4(4):561–70 Oct–Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leshem E, Lopman B, Glass R, Gentsch J, Banyai K, Parashar U. et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14(9):847–56 Sep. [DOI] [PubMed] [Google Scholar]
- 31.Kirkwood CD. Genetic and antigenic diversity of human rotaviruses: potential impact on vaccination programs.J Infect Dis 2010;202(Suppl):S43–8 Sep 01. [DOI] [PubMed] [Google Scholar]
- 32.It WC. Chanta C. Emergence of G9P[8]rotaviruses in children with acute gastroenteritis in Thailand, 2015–2016. J Med Virol 2017. Oct 27. [DOI] [PubMed] [Google Scholar]
- 33.Armah GE, Steele AD, Binka FN, Esona MD, Asmah RH, Anto F, et al. Changing patterns of rotavirus genotypes in ghana: emergence of human rotavirus G9 as a major cause of diarrhea in children. J Clin Microbiol 2003;41(6):2317–22 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkwood C, Bogdanovic-Sakran N, Palombo E, Masendycz P, Bugg H, Barnes G. et al. Genetic and antigenic characterization of rotavirus serotype G9 strains isolated in Australia between 1997 and 2001. J Clin Microbiol 2003;41(8):3649–54 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Liu H, Jia L, Payne DC, Hall AJ , Xu Z, et al. Active, population-based surveillance for rotavirus gastroenteritis in Chinese children. Pediat Infect Dis J 2015;34(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel MM, Patzi M, Pastor D, Nina A, Roca Y, Alvarez L. et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 2013;346:f3726 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snelling TL, Schultz R, Graham J, Roseby R, Barnes GL, Andrews RM, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis 2009;49(3):428–31 Aug 01. [DOI] [PubMed] [Google Scholar]
- 38.Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R. et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007;370(9601):1757–63 Nov 24. [DOI] [PubMed] [Google Scholar]
- 39.Dennehy PH. Rotavirus vaccines: an overview. Clin Microbiol Rev 2008;21(1):198–208 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Mol P ZissisG, Butzler JP, Mutwewingabo A, Andre FE. Failure of live, attenuated oral rotavirus vaccine. Lancet 1986;2(8498):108 Jul 12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


