Abstract
Diabetic foot ulcers (DFUs) are chronic wounds and one of the most common complications of diabetes, imposing significant physical and mental burdens on patients due to their poor prognosis and treatment efficacy. Adipose-derived stem cells (ADSCs) have been proven to promote wound healing, with studies increasingly attributing these beneficial effects to their paracrine actions. Consequently, research on ADSC secretome as a novel and promising alternative for DFU treatment has been extensively conducted. This article provides a comprehensive review of the mechanisms underlying refractory DFU wounds, the secretome of ADSCs, and its role in promoting wound healing in diabetes foot ulcers. And the review aims to provide reliable evidence for the clinical application of ADSC secretome in the treatment of refractory DFU wounds.
Keywords: Diabetic foot ulcers, Adipose-derived stem cells, Secretome, Wound healing
Background
Diabetes mellitus (DM) is a severe metabolic condition affecting over 10.5% of the world’s adult population [1], with China currently having the highest number of diabetic patients globally [2]. DFUs, characterized by chronic and difficult-to-heal wounds, are one of the most common complications of DM, with high morbidity, recurrence, amputation, and mortality rates, as well as high treatment costs, imposing a substantial weight on patients, families and society [3]. The current clinical treatment for diabetic foot ulcer wounds focuses on debridement, anti-infection, improvement of blood supply to the lower limbs and foot decompression and braking [4], but these methods often have poor therapeutic efficacy. In recent years, new strategies, such as various wound debridement techniques, dressings, gas therapy, wound physical therapy, skin substitutes, cell products, medications, negative pressure wound therapy, have shown promise for the treatment of severe diabetic foot ulcers [5]. However, in 2023 the International Working Group on the Diabetic Foot (IWGDF) incorporated the feasibility and equity of intervention implementation into the assessment criteria and made 29 separate recommendations. They concluded that existing strategies do not provide a cost-effective solution to this persistent condition, highlighting the need for continuous exploration of new treatments [6].
Since the first clinical application of mesenchymal stem cells (MSCs) therapy in 1995, the therapeutic capacity of stem cell therapy for various diseases has gained significant attention [7]. Among these, ADSCs have consistently demonstrated the ability to enhance the process of wound healing [8–10], a finding supported by preliminary studies from our research team [11]. Moreover, the secretome of ADSCs has demonstrated significant therapeutic effects in wound healing [12], although the underlying mechanism remains unclear. To further investigate, we conducted a literature search on PubMed and Web of Science using keywords such as ‘adipose-derived stem cells,’ ‘secretome,’ and ‘diabetic foot ulcers’ to identify relevant cell studies, animal research, and clinical trials published in the past 10 years. This paper reviews the mechanisms of delayed healing in DFUs and the role of the ADSC secretome in promoting wound healing in diabetic foot ulcers, aiming to establish a reliable basis for the clinical application of ADSC secretome in treating difficult-to-heal DFUs.
Mechanisms of delayed healing in diabetic foot ulcer wounds
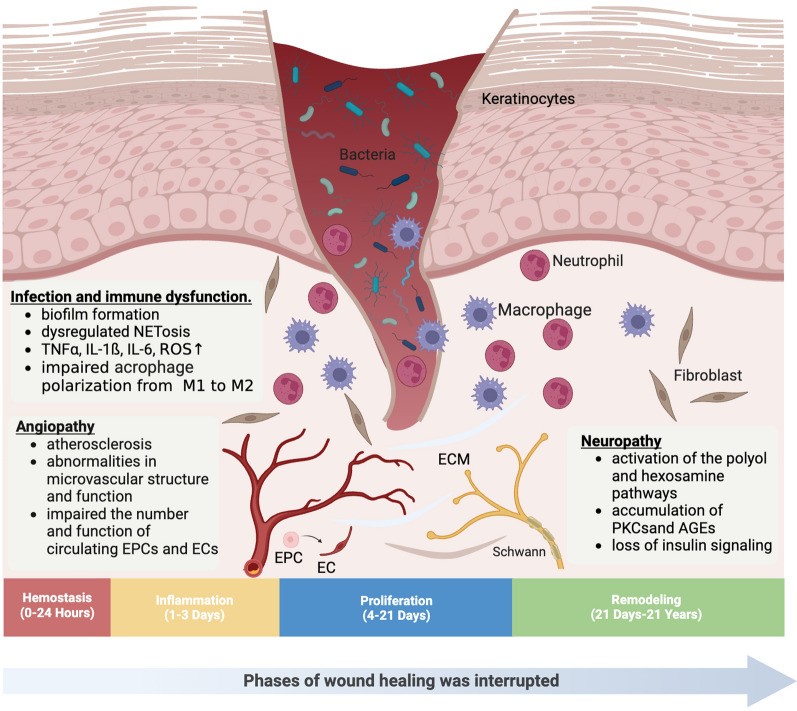
The process of skin wound healing typically involves four consecutive and overlapping stages: hemostasis, inflammation, proliferation, and remodelling [13]. However, this process is hindered by various factors in diabetic foot ulcer wounds, leading to delayed healing (Fig. 1).
Fig. 1.
Mechanisms of diabetes foot ulcers (Created with BioRender.com). PKC Protein kinase C, AGE Advanced glycation end product, TNFα Tumor Necrosis Factor-alpha, IL Interleukin, ROS Reactive oxygen specie, EPC Endothelial progenitor cell, EC Endothelial cell, ECM Extracellular matrix
Neuropathy
Diabetic neuropathy occurs in nearly 90% of diabetic foot ulcers [14]. It is a peripheral neurodegenerative disease primarily affecting sensory and autonomic nerves, and eventually, motor nerves [15]. Sensory neuropathy leads to neuropathic pain and/or sensory loss, while motor neuropathy causes muscle atrophy and functional deterioration, both of which result in uneven foot loading, increased plantar pressure, subcutaneous edema, and an 8 to 18-fold increased risk of falls and foot ulcers, and a 2 to 15-fold increased risk of lower limb amputation. Autonomic neuropathy impairs the function of sweat and sebaceous glands in the feet, coupled with vasomotor paralysis, leading to dry, cracked, and damaged skin. This impairs the skin’s natural barrier function, providing an entry point for bacteria and other microorganisms [16]. The above biological, behavioral, or combined effects have resulted in the elevated recurrence rate of DFUs [17].
Further exploration of its mechanism revealed that hyperglycemia, dyslipidemia, and/or insulin resistance promote the activation of the polyol and hexosamine pathways, the accumulation of protein kinase C (PKC) subtypes and advanced glycation end products (AGEs), as well as the loss of insulin signaling, collectively leading to imbalanced mitochondrial redox status and excessive formation of reactive oxygen species (ROS) [18]. Subsequently, Schwann cells and dorsal root ganglion (DRG) neurons undergo metabolic and oxidative damage, inducing axonal degeneration, loss of neurotrophic signaling, and local blood supply decreases, which ultimately causes irreversible damage to the nervous system [15]. Preventing neurodegeneration can improve healing, such as using the C-X-C chemokine receptor type 4 (CXCR4) antagonist AMD3100 to improve wound healing in db/db mice by selectively targeting the CXCR4 receptor, which is lacking in DRG neurons [14].
Angiopathy
Peripheral vascular dysfunction is one of the main causes of DFUs formation in most diabetic patients. Diabetic vasculopathy can be divided into macroangiopathy and microangiopathy. The former manifests primarily as atherosclerosis, with the formation of plaques that rupture to trigger peripheral arterial thrombosis, particularly in the diabetic setting, leading directly to arterial occlusion and lower limb ischemia, ultimately resulting in DFUs [19]. Research confirms hyperglycemia in diabetes can lead to glycated hemoglobin, vascular narrowing, red blood cell membrane changes, dyslipidemias, and foam cell formation from macrophages, triggering the formation of atherosclerosis, that causes vascular damage, and stimulating endothelial cells to generate ROS. And ROS utilizes the Nuclear Factor-kappa B (NF-κB) pathway to induce leukocyte recruitment and apoptosis, leading to inflammation, ischemia, and neuronal cell damage [20]. The latter manifests as abnormalities in microvascular structure and function, and important theories that can explain this phenomenon are the hemodynamic hypothesis and capillary steal syndrome [21]. Furthermore, additional research has confirmed that diabetic neuropathy can be caused by microvascular ischemia involving the nerve [22]. Therefore, vascular repair and reconstruction are crucial for wound healing.
However, vascular regeneration exists in two modes, one being angiogenesis, which denotes the growth of new blood vessels either by budding or non-budding forms, based on existing capillaries or venules through proliferation, differentiation, and migration of endothelial cells. The other mode is vasculogenesis, which involves endothelial progenitor cells (EPCs) from bone marrow that differentiate into endothelial cells to form new blood vessels [23]. Nevertheless, fully developed endothelial cells are specialized cells that have reached their final stage of differentiation and have a restricted ability to proliferate, especially when functioning in a diabetic environment, thus inhibiting angiogenesis [24]. Therefore, EPCs significantly contribute to angiogenesis by mobilizing, proliferating, nesting and differentiating into endothelial cells to promote angiogenesis and accelerate wound healing [25]. Nonetheless, diabetic patients experience reduced counts and functionality of circulating EPCs, leading to impaired neovascularization and delayed wound healing [26].
Infection and immune dysfunction
50% of diabetic foot ulcers will develop infections, and recurrent or chronic ulcers increase the risk of infection [27]. Inadequate blood flow to the lower extremities, along with various neuropathies, elevates the likelihood of Diabetic Foot Infections (DFIs) [19]. Diabetic infections result from the combined effects of pathogenic virulence and immune dysfunction.
A multi-omic study found that in the majority of mild to moderate DFIs, Staphylococcus aureus and Streptococcus species are abundant and highly active, with a significant enrichment of related virulence genes. In extreme DFIs, individuals demonstrate increased microbial diversity, encompassing Pseudomonas aeruginosa and other bacteria, along with Candida albicans and other fungi. The variance in gene expression of these organisms suggests an abundance of multi-species virulence genes [28]. When planktonic pathogenic bacteria or fungi, together with certain bacteria previously considered non-pathogenic or unable to sustain chronic infections, form a polymicrobial pathogenic biofilm, it can protect them from the immunological response of the host and the impact of antibiotics, leading to multidrug resistance and delayed wound healing [29].
Furthermore, persistent DFIs are closely associated with abnormal immune cell activity. In the diabetic microenvironment, neutrophil extracellular traps (NETosis) are dysregulated, resulting in an overproduction of pro-inflammatory cascades, cytokines, and ROS, thereby delaying wound healing [30]. Concurrently, sustained hyperglycemia levels upregulate pro-inflammatory factors such as Tumor Necrosis Factor-alpha (TNFα), Interleukin-1 beta (IL-1ß), and Interleukin-6 (IL-6), increasing macrophage sensitivity to these cytokines while downregulating the expression of Cluster of Differentiation 36 (CD36) and Class B Scavenger type I receptors required for phagocytic activity, hindering M1 macrophage phagocytic activity, and promoting a senescent-associated secretory profile (SASP) in macrophages, which ultimately leads to impaired polarization from pro-inflammatory (M1) to anti-inflammatory (M2) phenotype [31]. Additionally, macrophages can secrete large amounts of proteases, including matrix metalloproteinase-9 (MMP-9), which degrades newly synthesized extracellular matrix (ECM) and hinders cell migration, thus inhibiting wound healing [32, 33].
Adipose-derived stem cells secretome
Adipose-derived stem cells
In 2001, Zuk et al. discovered and isolated ADSCs from human adipose tissue using a negative pressure suction technique [34]. ADSCs provide benefits such as minimal invasiveness, easy accessibility, low immunogenicity, high regenerative potential, low carcinogenicity, and no ethical concerns, making them a prominent focus of research. Their therapeutic potential in treating a range of diseases, including wound healing, is well-established [35]. ADSCs fall into the mesenchymal stem cell category and display typical mesenchymal stem cell characteristics in vitro: (1) ADSCs adhere to plastic culture bottles under standard culture conditions; (2) they exhibit similar surface markers to bone marrow mesenchymal stem cells (BMSCs), such as CD13, CD29, CD49d, CD73, CD90, CD105, and Stro-1, but do not express CD45, CD14, CD133, and CD144 [36]; (3) they possess multipotent differentiation potential, capable of differentiating into adipocytes, osteocytes, chondrocytes, hepatocytes, endothelial cells, keratinocytes, Schwann cells, myocytes, and pancreatic acinar cells, and can be rewired into induced pluripotent stem cells (iPSCs) [37, 38]. Despite these advantages, challenges remain in standardized cell extraction, stable storage, and transportation. High costs further complicate the widespread use of traditional ADSC cell therapy [39].
Adipose-derived stem cells secretome
While recent studies have shown that ADSCs mediate wound repair and regeneration through paracrine signaling by secreting various bioactive factors, known as ADSC secretome [40]. In 2000, the concept of the secretome was first introduced by Tjalsma to describe all proteins released by bacteria out of cells and their secretion mechanisms [41]. Currently, the secretome is broadly defined as various soluble factors released directly from cells into the extracellular space, and the carriers including extracellular vesicles (EVs) and migrasomes that transport these factors [42]. Numerous studies use conditioned media to obtain the secretome, exploring its role in various diseases and tissue repair [43]. Gregorio et al. identified a total of 569 factors through proteomic analysis of ADSC-conditioned medium (ADSC-CM) [44]. Presently, EVs are the most extensively studied components of the secretome [45]. According to the latest international guidelines, “Minimal Information for Studies of Extracellular Vesicles 2023 (MISEV2023),” EVs are defined as particles released by cells, enclosed by a lipid bilayer, and incapable of self-replication due to the lack of a functional nucleus [46]. Evs are broadly classified into three subtypes based on their biogenesis, release pathways, size, content, and functions: apoptotic vesicles (apoVs), 50–5000 nm; microvesicles (MVs), 100–1000 nm; and exosomes (Exos), 30–200 nm [47]. In terms of molecular composition, EVs include abundant proteins, lipids, nucleic acids, polysaccharides, playing roles in intercellular communication and participating in physiological processes like cell proliferation, apoptosis, migration, and differentiation. They show great potential in wound repair and tissue regeneration [48]. Furthermore, in 2014, Ma et al. first observed ellipsoid membrane-bound structures secreted by migrating cells in vitro, defining them as migrasomes [49]. Migrasomes are rich in signaling molecules such as chemokines, cytokines, and angiogenic factors, fulfilling a crucial function in the spatially precise delivery of these signaling molecules, influencing key physiological processes including organ morphogenesis and angiogenesis [50]. Therefore, using ADSC secretome to enhance tissue regeneration may be a promising alternative to traditional ADSCs therapy. Table 1 summarizes the key components of the ADSC secretome.
Table 1.
The key components of the ADSC secretome
| Category | Component | References | |
|---|---|---|---|
| Soluble Protein | Growth factors | VEGF, PDGF, KGF, EGF, TGF-β, HGF, bFGF, IGF, BDNF, GDNF, NGF, IGFBP1, IGFBP2, CNTF, NT-3 and NT-4 | [52, 59, 87–89] |
| Cytokines | IL-1α, IL-1β, IL-2, IL-4, I-5, TL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-15, MCP-1, GM-CSF, IFN-γ, RANTES | [90] | |
| Chemokines | CXCL1, CXCL2, CXCL3, CXCL5, CXCL12, CCL27, CCL2, CCL5, CCL27, CX3CL1, XCL1 | [91–94] | |
| Carriers | EVs | apoVs, MVs, Exos | [47] |
| Migrasomes | [64] |
Mechanism of ADSC secretome in promoting diabetic foot ulcer wound healing
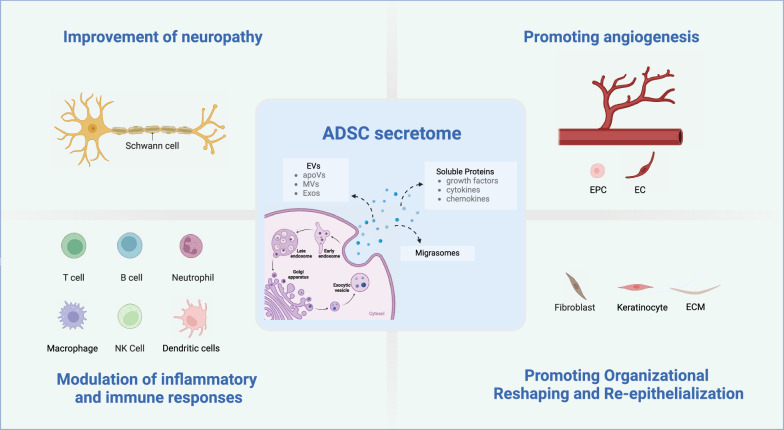
Research has demonstrated that the secretome of ADSCs promotes wound healing through multiple mechanisms. These include improving neuropathy, promoting angiogenesis, modulating inflammation and immune response, as well as facilitating tissue remodeling and re-epithelialization (Fig. 2). The following sections will elucidate these processes in detail.
Fig. 2.
Mechanism of ADSC secretome in promoting diabetic foot ulcers (Created with BioRender.com). ADSC Adipose-derived stem cell, EVs Extracellular Vesicles, apoVs Apoptotic Vesicles, MVs Microvesicles, Exos Exosomes, EPC Endothelial progenitor cell, EC Endothelial cell, ECM Extracellular Matrix, T cell T lymphocyte cell, B cell B lymphocyte cell, NK cell Natural Killer cell
Improvement of neuropathy
Neuropathic changes significantly contribute to the occurrence and development of diabetic foot. Research has demonstrated that the neuroprotective effects of the ADSC secretome are attributed to the inhibition of apoptosis, reduction of neuronal energy depletion, promotion of cerebral blood vessel formation, and reduction in astrocyte proliferation [51]. ADSCs secrete numerous neurotrophic factors that promote nerve regeneration, including brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), nerve growth factor (NGF), and neurotrophins-3 and -4 (NT-3 and NT-4) [52]. Chen et al. found that ADSC-EVs carrying miR-130a-3p promote Schwann cell proliferation and prevent diabetic peripheral neuropathy through the DNMT1/NRF2/HIF1α/ACTA1 axis [53]. Yin et al. found that ADSC-Exos enhance the autophagy of injured Schwann cells induced by nerve damage by reducing miRNA-26b targeting Kpna2, thereby promoting remyelination [54]. Additionally, studies have shown that the bioactive medium mixture present in ADSC-CM can transform a neurodestructive/pro-inflammatory microenvironment into a neuroprotective/anti-inflammatory one, improve thermal mechanical sensitivity, restore epidermal nerve fiber density, stimulate Schwann cell proliferation, inhibit neuronal autophagy and apoptosis, and promote remyelination to enhance nerve regeneration [44].
Promoting angiogenesis
Vascular regeneration is crucial for wound healing as it increases immune cell recruitment, provides oxygen and nutrients to metabolically active wounds, and removes toxic metabolites and waste. Numerous studies have shown that ADSCs upregulate the expression of miR-125a [55], miR-126 [56], miR-128 [57], miR-132 [58], increase the secretion of vascular growth factors such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF), regulate endothelial cell proliferation and migration, promote vascular regeneration, and improve wound healing efficiency [59]. In addition, the miR-21-5p-enriched secretion of ADSCs activates the PI3K/AKT/PTEN or mTOR signaling pathway, promoting the expression of HIF-1α and VEGF which contributes to vascular regeneration in the diabetic microenvironment [60]. Research has also shown that ADSC-Exos upregulate the expression of lncRNA-SENCR through EGR-1 to activate the DKC1/VEGF-A axis, promoting the proliferation and migration of human umbilical vein endothelial cells (HUVECs), thus improving vascular regeneration, and facilitating wound healing [61]. Similarly, Zhang et al. found that ADSC-exos can regulate the expression of SIRT3 and its downstream protein SOD2, improve oxidative stress and inflammatory microenvironment, improve endothelial cell dysfunction caused by hyperglycemia, promote vascular regeneration, and further facilitate the healing of chronic diabetic wounds [62]. Li et al. found that overexpression of Nrf2 in ADSC-Exos can increase granulation tissue formation, vascular regeneration, and growth factor expression levels, decrease levels of inflammation and oxidative stress-related proteins, and eventually promote wound healing in rat diabetic foot ulcers [63]. In addition, SDF-1 was detected in MSC migratory bodies by Deniz et al. [64], and Zhang et al. found that SDF-1 mediates the mobilization of endothelial progenitor cells and neovascularization, promoting wound healing in diabetic mice [65].
Modulation of inflammatory and immune responses
Diabetic foot ulcer non-healing is related to excessive oxidative stress, persistent inflammatory response, and imbalanced activation of signaling pathways, which lead to abnormal immune cell function, induce excessive inflammation, and ultimately disrupt the microenvironment for wound healing. ADSCs secrete pro-inflammatory cytokines (IL-7, IL-8, IL-9, IL-11, IL-12, IL-15, IL-17 and INF-γ) and anti-inflammatory cytokines (IL-1Ra, IL-4, IL-10, and IL-13) [35]. The release of these factors is enhanced under inflammatory conditions, resulting in inhibition of T cell function (proliferation, differentiation, and cytotoxicity), B cell function, NK cell cytotoxicity, decreased maturation and activation of dendritic cells, and increased regulatory T cells, effectively participating in human inflammation and immune response and playing a positive role through immune regulation [66]. In addition, the conversion of macrophages from a pro-inflammatory phenotype (M1) to an anti-inflammatory phenotype (M2) is crucial during the inflammatory and proliferation phase of wound healing. Multiple studies have found that the TLR4/NF-κB pathway is associated with enhanced M1 macrophage polarization promoting the production of ROS, TNF-α, IL-1β, and oxidative stress, significantly activating MAPKs and NF-κB pathways, while anti-inflammatory M2 macrophages inhibit NF-κB-mediated expression of inflammatory factors [67]. Other studies have shown that ADSC-Exos inhibit MIF and promote M1 to M2 macrophage polarization through miR-451a, thereby reducing inflammation and shortening the inflammatory phase of wound healing in diabetic wounds [68]. In addition, ADSCs release antimicrobial peptides (AMPs), which are effective and safe for combating bacterial, viral, and fungal infections, and can block the pro-inflammatory M1 macrophage response [69]. Furthermore, Yu et al. found that ADSC-CM can inhibit Propionibacterium acnes-induced NETosis [70].
Facilitating organizational reshaping and re-epithelialization
An essential indicator of wound healing is the remodeling of tissue and re-epithelialization, which forms a complete epidermal structure to restore the skin’s barrier function, thereby regulating the deposition and remodeling of ECM and improving late-stage scar formation. ADSC-EVs can promote the proliferation and migration of human dermal fibroblasts (HDF) and keratinocytes (HaCaT) via lncRNA MALAT-1 targeting miR-124 [71]. Furthermore, research indicates that ADSC-Exos can effectively inhibit ECM production in scar fibroblasts by suppressing the gene and protein expression of collagen I (COL-1), collagen III (COL-3), fibronectin (FN), and α-smooth muscle actin (α-SMA), as well as the TGF-β/Smad signaling pathway and Notch-1/Jagged-1 pathway, thereby promoting wound healing [72]. Additionally, the inhibition of scar proliferation can be significantly enhanced by the combination of ADSC-CM and in situ cross-linking of polysaccharide hydrogels [73].
The proper functioning of keratinocytes during re-epithelialization is crucial. The secreted proteome of PL-cultured ADSC contains more proteins, such as proliferative, differentiation or pro-angiogenic factors, which enhance keratinocyte movement and survival and improve wound healing [74]. In addition, epidermal stem cells from hair follicles can migrate to the wound site to differentiate into epidermal cells and contribute to re-epithelialization [75]. ADSC-EVs also contain VEGF and FGF, cytokines that stimulate follicle growth and activate the Wnt signaling pathway necessary for hair follicle induction [76]. Moreover, the stratum corneum (SC) is responsible for providing the epidermal barrier of the skin. It is composed of keratinocytes and a combination of intercellular lipids, including ceramides, free fatty acids, and cholesterol. ADSC-Exos effectively restore the function of the outermost layer of the skin in Atopic Dermatitis (AD) by stimulating the production of new ceramides [77].
Limitations and challenges in the clinical translation of ADSC secretome
The secretome of ADSCs has been well validated in the healing of DFU and other conditions, making it an ideal non-cellular therapy in regenerative medicine. According to data from ClinicalTrials.gov, the quantity of officially recorded clinical trials utilizing ADSC secretome as an intervention is increasing (see Table 2, available online at http://www.clinicaltrials.gov/, accessed July 8, 2024). However, the clinical translation of ADSC secretome continues to encounter significant challenges. First, the composition of secretome is highly variable due to differences in donors, tissue sources, culture conditions, extraction methods, and identification techniques. This variability complicates the precise determination of the secretome’s components and active ingredients. For instance, Kalinina et al. identified over 600 secreted proteins in the ADSC-CM using LC–MS [78], whereas Gregorio et al. identified 569 factors [44]. Additionally, Huang et al. demonstrated that varying isolation methods could alter the protein composition of exosomes [79]. Thus, accurately identifying the specific components of the secretome, efficiently extracting them, and eliminating irrelevant molecules are critical challenges that must be addressed. Moreover, successful clinical translation requires large-scale production while ensuring batch-to-batch consistency and reproducibility. Currently, there is no standardized protocol for the isolation and amplification of secretome components suitable for large-scale production. Furthermore, the method of administration, pharmacokinetics, and extension of the half-life of active ingredients present additional challenges for clinical translation. Lastly, Wang et al. discovered that ADSC-Exos could promote breast cancer cell growth by activating the Hippo signaling pathway [80]. The potential tumorigenic risks and immunogenicity associated with the use of biological products continue to be subjects of significant debate. In response to these concerns, a substantial number of in vivo and in vitro studies aim to address these challenges, focusing on pretreatment strategies and material phase aspects. It has been found that the biological activity of the secretome of ADSCs can be enhanced and wound healing can be promoted by physicochemical factors (e.g., hypoxia) [81], drugs (e.g., lipopolysaccharides, hydrogen peroxide) [82, 83], genetic engineering or inheritance (e.g., surface modification, genetic modification, and epigenetic reprogramming) [84], and by combining a variety of biomaterials (e.g., hydrogels,3D printed biomimetic scaffold) [85, 86]. Nevertheless, more systematic and extensive studies are needed to fully understand the specific components and mechanisms of action of the ADSC secretome and to facilitate its translation from the laboratory to clinical settings.
Table 2.
Clinical Trials of ADSC Secretome
| NCT no | Title | Status | Conditions/disease | Interventions | Results |
|---|---|---|---|---|---|
| NCT04544215 | A Clinical Study of Mesenchymal Progenitor Cell Exosomes Nebulizer for the Treatment of Pulmonary Infection | Unknown | Drug resistant pulmonary infection | 8 × 108 or 16 × 108 or no exosomes nano vesicles/3 mL per day for 7 days | N/A |
| NCT05296863 | Adipose-derived Stem Cell Conditioned Media as a Novel Approach for Hair Regrowth in Male Androgenetic Alopecia | Completed | Alopecia, Androgenetic Hair Loss/Baldness | 2 ml intradermal injection of non- or concentrated ADSC-CM or Placebo + 1 ml of 5% topical Minoxidil daily | N/A |
| NCT04276987 | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | Completed | Coronavirus | Conventional treatment or 2 × 108 MSCs-derived exosomes /3 mL per day for 5 days | N/A |
| NCT04223622 | Effects of ASC Secretome on Human Osteochondral Explants | Recruiting | Osteoarthritis | The osteochondral explants isolated from arthroplasty patients will be induced to an OA phenotype and treated with ASC secretome (either complete conditioned medium or extracellular vesicles) in order to investigate its therapeutic potential | N/A |
Conclusion
DFU is a prevalent condition associated with diabetes, resulting from the combined effects of neuropathy, vascular disease, and infection. Increasing research indicates that the beneficial effects of ADSCs are primarily due to their paracrine actions, which promote tissue repair by mediating intercellular communication. The secretome of ADSCs includes various components such as cytokines, growth factors, proteins, lipids, mRNAs, microRNAs, lncRNAs, and DNA. These components, delivered via EVs and Exos, create an anti-inflammatory microenvironment that enhances diabetic wound healing. They also promote the proliferation and migration of M2 macrophages, endothelial cells, Schwann cells, fibroblasts, and keratinocytes, regulating inflammation and immune responses, promoting angiogenesis, improving neuropathy, facilitating tissue remodeling and re-epithelialization, and ultimately accelerating wound healing. Although extensive research and clinical trials are needed for clinical translation, the ADSC secretome holds promise for significant breakthroughs not only in chronic wound repair but also in other areas of regenerative medicine.
Acknowledgements
Not applicable. (The authors declare that they have not used Artificial Intelligence in this study.)
Abbreviations
- DFUs
Diabetes foot ulcers
- ADSCs
Adipose-derived stem cells
- DM
Diabetes mellitus
- IWGDF
The international working group on the diabetic foot
- MSCs
Mesenchymal stem cells
- PKC
Protein kinase C
- AGEs
Advanced glycation end products
- ROS
Reactive oxygen species
- DRG
Dorsal root ganglion
- CXCR4
C-X-C chemokine receptor type 4
- NF-κB
Nuclear factor-kappa B
- EPCs
Endothelial progenitor cells
- DFIs
Diabetic foot infections
- NETosis
Neutrophil extracellular traps
- TNFα
Tumor necrosis factor-alpha
- IL
Interleukin
- CD
Cluster of differentiation
- SASP
Senescent-associated secretory profile
- MMP-9
Matrix metalloproteinase-9
- ECM
Extracellular matrix
- BMSCs
Bone marrow mesenchymal stem cells
- iPSCs
Induced pluripotent stem cells
- EVs
Extracellular vesicles
- ADSC-CM
Adipose-derived stem cell conditioned medium (ADSC-CM)
- MISEV2023
Minimal information for studies of extracellular vesicles 2023
- apoVs
Apoptotic vesicles
- MVs
Microvesicles
- Exos
Exosomes
- VEGF
Vascular endothelial growth factor
- PDGF
Platelet-derived growth factor
- KGF
Keratinocyte growth factor
- EGF
Epidermal growth factor
- HGF
Hepatocyte growth factor
- bFGF
Basic fibroblast growth factor
- IGF
Insulin-like growth factor
- BDNF
Brain-derived neurotrophic factor
- GDNF
Glial cell line-derived neurotrophic factor
- NGF
Nerve growth factor
- HUVECs
Human umbilical vein endothelial cells
- IGFBP
Insulin-like growth factor binding protein
- MCP-1
Monocyte chemoattractant protein-1
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IFN-γ
Interferon gamma
- RANTES
Regulated on activation, normal T cell expressed and secreted
- CXCL
C-X-C motif chemokine ligand
- CCL
CC Chemokine ligand
- CX3CL1
C-X3-C motif chemokine ligand 1
- XCL1
X-C motif chemokine ligand 1
- CNTF
Ciliary neurotrophic factor
- NT
Neurotrophin
- miR
MicroRNA
- DNMT1
DNA (cytosine-5)-methyltransferase 1
- NRF2
Nuclear factor erythroid 2-related factor 2
- ACTA1
Actin, alpha 1, skeletal muscle
- PI3K
Phosphoinositide 3-kinase
- AKT
RAC-alpha serine/threonine-protein kinase
- PTEN
Phosphatase and tensin homolog
- mTOR
Mechanistic target of rapamycin
- lncRNA
Long non-coding RNA
- SENCR
Smooth muscle and endothelial cell-enriched migration/differentiation-associated long non-coding RNA
- DKC1
Dyskerin pseudouridine synthase 1
- EGR-1
Early growth response 1
- SIRT3
Sirtuin 3
- SOD2
Superoxide dismutase 2, mitochondrial
- MAPKs
Mitogen-activated protein kinases
- AMPs
Antimicrobial peptides
- HDF
Human dermal fibroblasts
- HaCaT
Keratinocytes
- SC
Stratum corneum
- AD
Atopic dermatitis
- T cell
T lymphocyte cell
- B cell
B lymphocyte cell
- NK cell
Natural killer cell
Author contributions
XW and XN conducted the literature review, collected data, authored the manuscript, and drafted the figures. BW and XS conceived the project and revised the manuscript. YX and LC edited the manuscript, while BC, QL, RK and TH designed the outline and also revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study was funded by Young and Middle-aged Key Personnel Training Project of Fujian Provincial Health Commission (No.2020GGB029); Joint Funds for the Innovation of Science and Technology, Fujian Province (No.2020Y9124); Natural Science Fundation of Fujian Province (No.2021J01244).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaofen Wan and Xuejun Ni contributed equally to this work.
Contributor Information
Xiuying Shan, Email: xiuyingshan@fjmu.edu.cn.
Biao Wang, Email: biaowang@fjmu.edu.cn.
References
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji L. Current challenges of diabetes and metabolic disorders in China. Diabetes Obes Metab. 2023;25(Suppl 1):3–4. [DOI] [PubMed] [Google Scholar]
- 3.McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 2023;46(1):209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Favila A, Martinez-Fierro ML, Rodriguez-Lazalde JG, Cid-Baez MA, Zamudio-Osuna MJ, Martinez-Blanco MDR, et al. Current therapeutic strategies in diabetic foot ulcers. Med (Kaunas). 2019;55(11):714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oyebode OA, Jere SW, Houreld NN. Current therapeutic modalities for the management of chronic diabetic wounds of the foot. J Diabetes Res. 2023;2023:1359537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Vilorio NC, Dhatariya K, Jeffcoate W, Lobmann R, McIntosh C, et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. 2024;40(3):e3644. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–64. [PubMed] [Google Scholar]
- 8.Yuan T, Meijia L, Xinyao C, Xinyue C, Lijun H. Exosome derived from human adipose-derived stem cell improve wound healing quality: a systematic review and meta-analysis of preclinical animal studies. Int Wound J. 2023;20(6):2424–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huayllani MT, Sarabia-Estrada R, Restrepo DJ, Boczar D, Sisti A, Nguyen JH, et al. Adipose-derived stem cells in wound healing of full-thickness skin defects: a review of the literature(). J Plast Surg Hand Surg. 2020;54(5):263–79. [DOI] [PubMed] [Google Scholar]
- 10.Iacomi DM, Rosca AM, Tutuianu R, Neagu TP, Pruna V, Simionescu M, et al. Generation of an immortalized human adipose-derived mesenchymal stromal cell line suitable for wound healing therapy. Int J Mol Sci. 2022;23(16):8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni X, Shan X, Xu L, Yu W, Zhang M, Lei C, et al. Adipose-derived stem cells combined with platelet-rich plasma enhance wound healing in a rat model of full-thickness skin defects. Stem Cell Res Ther. 2021;12(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Zhao B, Zhang XL, Lu YJ, Lu ST, Cheng J, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer AJ. Healing mechanisms in cutaneous wounds: tipping the balance. Tissue Eng Part B Rev. 2022;28(5):1151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak NC, Menichella DM, Miller R, Paller AS. Cutaneous innervation in impaired diabetic wound healing. Transl Res. 2021;236:87–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers: a review. JAMA. 2023;330(1):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–75. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93(6):1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng H, Li B, Shen Q, Zhang C, Kuang L, Chen R, et al. Mechanisms of diabetic foot ulceration: a review. J Diabetes. 2023;15(4):299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen DT, Zaferanieh MH, Black AC Jr, Hamedi KR, Goodwin RL, Nathaniel TI. Obstetric neuropathy in diabetic patients: the “double hit hypothesis.” Int J Mol Sci. 2023;24(7):6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma S, Schaper N, Rayman G. Microangiopathy: is it relevant to wound healing in diabetic foot disease? Diabetes Metab Res Rev. 2020;36(Suppl 1): e3244. [DOI] [PubMed] [Google Scholar]
- 22.Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, et al. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest. 2001;107(9):1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23(7):1185–9. [DOI] [PubMed] [Google Scholar]
- 25.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. [DOI] [PubMed] [Google Scholar]
- 26.Pyšná A, Bém R, Němcová A, Fejfarová V, Jirkovská A, Hazdrová J, et al. Endothelial progenitor cells biology in diabetes mellitus and peripheral arterial disease and their therapeutic potential. Stem Cell Rev Rep. 2019;15(2):157–65. [DOI] [PubMed] [Google Scholar]
- 27.Holt RIG, Cockram CS, Ma RCW, Luk AOY. Diabetes and infection: review of the epidemiology, mechanisms and principles of treatment. Diabetologia. 2024;67(7):1168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radzieta M, Sadeghpour-Heravi F, Peters TJ, Hu H, Vickery K, Jeffries T, et al. A multiomics approach to identify host-microbe alterations associated with infection severity in diabetic foot infections: a pilot study. NPJ Biofilms Microbiomes. 2021;7(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pouget C, Dunyach-Remy C, Pantel A, Schuldiner S, Sotto A, Lavigne JP. Biofilms in diabetic foot ulcers: significance and clinical relevance. Microorganisms. 2020;8(10):1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raja JM, Maturana MA, Kayali S, Khouzam A, Efeovbokhan N. Diabetic foot ulcer: a comprehensive review of pathophysiology and management modalities. World J Clin Cases. 2023;11(8):1684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Y, Chen K, Liu C, Qu X. Harnessing strategies for enhancing diabetic wound healing from the perspective of spatial inflammation patterns. Bioact Mater. 2023;28:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastar I, Balukoff NC, Marjanovic J, Chen VY, Stone RC, Tomic-Canic M. Molecular pathophysiology of chronic wounds: current state and future directions. Cold Spring Harb Perspect Biol. 2023;15(4):a041243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Chen J, Sun H, Zhang Y, Zou D. New insights into fibrosis from the ECM degradation perspective: the macrophage-MMP-ECM interaction. Cell Biosci. 2022;12(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. [DOI] [PubMed] [Google Scholar]
- 35.Trzyna A, Banaś-Ząbczyk A. Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy.” Biomolecules. 2021;11(6):878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czerwiec K, Zawrzykraj M, Deptuła M, Skoniecka A, Tymińska A, Zieliński J, et al. Adipose-derived mesenchymal stromal cells in basic research and clinical applications. Int J Mol Sci. 2023;24(4):3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan X, Li L, Liu H, Luo J, Zhao Y, Pan C, et al. Strategies for improving adipose-derived stem cells for tissue regeneration. Burns Trauma. 2022;10:tkac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, Wu JC. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc. 2011;6(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flamant S, Loinard C, Tamarat R. MSC beneficial effects and limitations, and MSC-derived extracellular vesicles as a new cell-free therapy for tissue regeneration in irradiated condition. Environ Adv. 2023;13:100408. [Google Scholar]
- 40.An YH, Kim DH, Lee EJ, Lee D, Park MJ, Ko J, et al. High-efficient production of adipose-derived stem cell (ADSC) secretome through maturation process and its non-scarring wound healing applications. Front Bioeng Biotechnol. 2021;9:681501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tjalsma H, Bolhuis A, Jongbloed JD, Bron S, van Dijl JM. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev. 2000;64(3):515–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajit A, Ambika GI. Adipose-derived stem cell secretome as a cell-free product for cutaneous wound healing. 3 Biotech. 2021;11(9):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar L, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K, Verma RS. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. [DOI] [PubMed] [Google Scholar]
- 44.De Gregorio C, Contador D, Díaz D, Cárcamo C, Santapau D, Lobos-Gonzalez L, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bormann D, Gugerell A, Ankersmit HJ, Mildner M. Therapeutic application of cell secretomes in cutaneous wound healing. J Invest Dermatol. 2023;143(6):893–912. [DOI] [PubMed] [Google Scholar]
- 46.Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding JY, Chen MJ, Wu LF, Shu GF, Fang SJ, Li ZY, et al. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: roles, opportunities and challenges. Mil Med Res. 2023;10(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hade MD, Suire CN, Mossell J, Suo Z. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42(6):2102–25. [DOI] [PubMed] [Google Scholar]
- 49.Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25(1):24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiao H, Li X, Li Y, Guo Y, Hu X, Sho T, et al. Localized, highly efficient secretion of signaling proteins by migrasomes. Cell Res. 2024;34(8):572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rau CS, Kuo PJ, Wu SC, Huang LH, Lu TH, Wu YC, et al. Enhanced nerve regeneration by exosomes secreted by adipose-derived stem cells with or without FK506 stimulation. Int J Mol Sci. 2021;22(16):8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Li G, Liu X, Chen K, Wang Y, Qin J, et al. Delivery of miR-130a-3p through adipose-derived stem cell-secreted EVs protects against diabetic peripheral neuropathy via DNMT1/NRF2/HIF1α/ACTA1 Axis. Mol Neurobiol. 2023;60(7):3678–94. [DOI] [PubMed] [Google Scholar]
- 54.Yin G, Yu B, Liu C, Lin Y, Xie Z, Hu Y, et al. Exosomes produced by adipose-derived stem cells inhibit schwann cells autophagy and promote the regeneration of the myelin sheath. Int J Biochem Cell Biol. 2021;132:105921. [DOI] [PubMed] [Google Scholar]
- 55.Pi L, Yang L, Fang BR, Meng XX, Qian L. Exosomal microRNA-125a-3p from human adipose-derived mesenchymal stem cells promotes angiogenesis of wound healing through inhibiting PTEN. Mol Cell Biochem. 2022;477(1):115–27. [DOI] [PubMed] [Google Scholar]
- 56.Ma J, Zhang Z, Wang Y, Shen H. Investigation of miR-126-3p loaded on adipose stem cell-derived exosomes for wound healing of full-thickness skin defects. Exp Dermatol. 2022;31(3):362–74. [DOI] [PubMed] [Google Scholar]
- 57.Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318(5):C848–56. [DOI] [PubMed] [Google Scholar]
- 58.Zhu LL, Huang X, Yu W, Chen H, Chen Y, Dai YT. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50(2):e12871. [DOI] [PubMed] [Google Scholar]
- 59.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8. [DOI] [PubMed] [Google Scholar]
- 60.Sun D, Mou S, Chen L, Yang J, Wang R, Zhong A, et al. High yield engineered nanovesicles from ADSC with enriched miR-21-5p promote angiogenesis in adipose tissue regeneration. Biomater Res. 2022;26(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y, Ju Y, Fang B. Exosomes from human adipose-derived mesenchymal stromal/stem cells accelerate angiogenesis in wound healing: implication of the EGR-1/lncRNA-SENCR/DKC1/VEGF-A axis. Hum Cell. 2022;35(5):1375–90. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Bai X, Shen K, Luo L, Zhao M, Xu C, et al. Exosomes derived from adipose mesenchymal stem cells promote diabetic chronic wound healing through SIRT3/SOD2. Cells. 2022;11(16):2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deniz IA, Karbanová J, Wobus M, Bornhäuser M, Wimberger P, Kuhlmann JD, et al. Mesenchymal stromal cell-associated migrasomes: a new source of chemoattractant for cells of hematopoietic origin. Cell Commun Signal. 2023;21(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Zhang YY, Pan ZW, Li QQ, Sun LH, Li X, et al. GDF11 promotes wound healing in diabetic mice via stimulating HIF-1ɑ-VEGF/SDF-1ɑ-mediated endothelial progenitor cell mobilization and neovascularization. Acta Pharmacol Sin. 2023;44(5):999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franz S, Ertel A, Engel KM, Simon JC, Saalbach A. Overexpression of S100A9 in obesity impairs macrophage differentiation via TLR4-NFkB-signaling worsening inflammation and wound healing. Theranostics. 2022;12(4):1659–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li R, Li D, Wang H, Chen K, Wang S, Xu J, et al. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res Ther. 2022;13(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maguire G. The safe and efficacious use of secretome from fibroblasts and adipose-derived (but not bone marrow-derived) mesenchymal stem cells for skin therapeutics. J Clin Aesthet Dermatol. 2019;12(8):E57-e69. [PMC free article] [PubMed] [Google Scholar]
- 70.Yu H, Zhang B, Zhan Y, Yi Y, Jiang Q, Zhang Q, et al. Neutrophil extracellular trap-related mechanisms in acne vulgaris inspire a novel treatment strategy with adipose-derived stem cells. Sci Rep. 2024;14(1):1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He L, Zhu C, Jia J, Hao XY, Yu XY, Liu XY, et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. 2020. Biosci Rep. 10.1042/BSR20192549. [DOI] [PMC free article] [PubMed]
- 72.Li J, Li Z, Wang S, Bi J, Huo R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered. 2022;13(4):8515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang C, Wang T, Zhang L, Chen P, Tang S, Chen A, et al. Combination of lyophilized adipose-derived stem cell concentrated conditioned medium and polysaccharide hydrogel in the inhibition of hypertrophic scarring. Stem Cell Res Ther. 2021;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hermann M, Peddi A, Gerhards A, Schmid R, Schmitz D, Arkudas A, et al. Secretome of adipose-derived stem cells cultured in platelet lysate improves migration and viability of keratinocytes. Int J Mol Sci. 2023;24(4):3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nuutila K. Hair follicle transplantation for wound repair. Adv Wound Care (New Rochelle). 2021;10(3):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Cheng L, Zhao H, Li Z, Chen J, Cen Y, et al. The Therapeutic role of ADSC-EVs in skin regeneration. Front Med (Lausanne). 2022;9:858824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin KO, Ha DH, Kim JO, Crumrine DA, Meyer JM, Wakefield JS, et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells. 2020;9(3):680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalinina N, Kharlampieva D, Loguinova M, Butenko I, Pobeguts O, Efimenko A, et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res Ther. 2015;6:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang LH, Rau CS, Wu SC, Wu YC, Wu CJ, Tsai CW, et al. Identification and characterization of hADSC-derived exosome proteins from different isolation methods. J Cell Mol Med. 2021;25(15):7436–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S, Su X, Xu M, Xiao X, Li X, Li H, et al. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther. 2019;10(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qian L, Li B, Pi L, Fang B, Meng X. Hypoxic adipose stem cell-derived exosomes carrying high-abundant USP22 facilitate cutaneous wound healing through stabilizing HIF-1α and upregulating lncRNA H19. Faseb j. 2024;38(10):e23653. [DOI] [PubMed] [Google Scholar]
- 82.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bai Y, Han YD, Yan XL, Ren J, Zeng Q, Li XD, et al. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem Biophys Res Commun. 2018;500(2):310–7. [DOI] [PubMed] [Google Scholar]
- 84.Yu H, Wu Y, Zhang B, Xiong M, Yi Y, Zhang Q, et al. Exosomes derived from E2F1(-/-) adipose-derived stem cells promote skin wound healing via miR-130b-5p/TGFBR3 Axis. Int J Nanomedicine. 2023;18:6275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang W, Zhan Y, Zhang Y, Sun D, Zhang G, Wang Z, et al. Synergistic large segmental bone repair by 3D printed bionic scaffolds and engineered ADSC nanovesicles: Towards an optimized regenerative microenvironment. Biomaterials. 2024;308:122566. [DOI] [PubMed] [Google Scholar]
- 87.Park BS, Kim WS, Choi JS, Kim HK, Won JH, Ohkubo F, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31(1):27–34. [DOI] [PubMed] [Google Scholar]
- 88.Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24(6):737–64. [DOI] [PubMed] [Google Scholar]
- 89.Leone A, Nicolò A, Prevenzano I, Zatterale F, Longo M, Desiderio A, et al. Methylglyoxal impairs the pro-angiogenic ability of mouse adipose-derived stem cells (mADSCs) via a senescence-associated mechanism. Cells. 2023;12(13):1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiśniewska J, Słyszewska M, Stałanowska K, Walendzik K, Kopcewicz M, Machcińska S, et al. Effect of pig-adipose-derived stem cells’ conditioned media on skin wound-healing characteristics in vitro. Int J Mol Sci. 2021;22(11):5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L, Li H, Lin J, He R, Chen M, Zhang Y, et al. CCR2 improves homing and engraftment of adipose-derived stem cells in dystrophic mice. Stem Cell Res Ther. 2021;12(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H, Ning H, Banie L, Wang G, Lin G, Lue TF, et al. Adipose tissue-derived stem cells secrete CXCL5 cytokine with chemoattractant and angiogenic properties. Biochem Biophys Res Commun. 2010;402(3):560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang WT, Lee SS, Wang YC, Lai YW, Kuo YR, Tang Chen YB, et al. Impaired cutaneous T-cell attracting chemokine elevation and adipose-derived stromal cell migration in a high-glucose environment cause poor diabetic wound healing. Kaohsiung J Med Sci. 2018;34(10):539–46. [DOI] [PubMed] [Google Scholar]
- 94.Bhang SH, Lee S, Shin JY, Lee TJ, Jang HK, Kim BS. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther. 2014;22(4):862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- He L, Zhu C, Jia J, Hao XY, Yu XY, Liu XY, et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. 2020. Biosci Rep. 10.1042/BSR20192549. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Not applicable.