Abstract
Background
Binge eating (BE) is associated with a range of cognitive control deficits related to impulsivity, including lower response inhibition, preference for immediate gratification, and maladaptive decision-making. The aim was to investigate whether impulsivity and BE may interact with the decision process and underlying brain activity in outpatients with overweight or obesity who are starting a treatment to achieve weight loss.
Methods
A sample of 26 treatment-seeking outpatients with overweight or obesity was evaluated for impulsivity, BE, and temporal discounting rates. Impulsivity was measured with the Barratt Impulsiveness Scale (BIS-11), according to which two groups were composed: high BIS and low BIS; BE was assessed with the eating disorders module of the Structured Clinical Interview for DSM5—Research Version, according to which two groups were composed: with (BE group) or without BE (NBE group). Changes in subjective value of rewards were measured with the Temporal Discounting Task (TDt) where participants had to choice between sooner but smaller vs. later but larger monetary rewards. These choices were made in two differently delayed conditions (“Now” and “Not-now”). Brain rhythms were recorded through high-density electroencephalogram (hd-EEG) during the TDt.
Results
Patients with BE reported more impulsive tendencies and perceived sooner rewards as more gratifying when both options were delayed (Not-now condition, p = 0.02). The reward choice in the TDt was accompanied by a general EEG alpha band desynchronization in parietal areas observed without differences between experimental conditions and patients groups. No effects were observed within the Now condition or in the other EEG bands.
Conclusions
The tendency to favor immediate rewards may constitute an obstacle to adhering to treatment plans and achieving weight loss goals for outpatients with overweight or obesity. Clinicians are therefore encouraged to include psychological factors, such as impulsivity and dysfunctional eating behaviors, when designing weight loss programs. By addressing these psychological aspects, clinicians can better support patients in overcoming barriers to adherence and achieving sustainable weight loss.
Trial registration
This study was approved by the Ethics Committee of the Department of Psychological, Health, and Territorial Sciences of the University G. d’Annunzio of Chieti-Pescara (Prot. n. 254 of 03/14/2017).
Keywords: Impulsivity, Binge eating, Temporal discounting, Overweight, Obesity, Alpha rhythm
Although the health risk of overweight and obesity is widely acknowledged, weight loss treatments are often unsuccessful in the long term due to several factors. Binge eating (BE) might explain some of the reduced benefits from the treatments. In this study, we investigated whether impulsivity and BE may interact decision processes among outpatients with overweight or obesity, and we looked at their underlying brain activity. Patients with BE tended to be more impulsive and to prefer sooner rewards compared to later ones. In all patients, α oscillations in the parietal lobe have been observed to play a dominant role during reward choice processes. A preference for immediate rewards might help to explain the low long-term success of weight loss treatment plans. The study may also provide useful information for clinicians working with patients with overweight or obesity.
Introduction
The World Health Organization (WHO) [1] defines overweight and obesity as clinical conditions characterized by abnormal or excessive fat accumulation that may impair health. These clinical conditions are commonly evaluated through the body mass index (BMI, kg/m2), a simple index of weight-for-height. Overweight (BMI > 25) is considered a major risk factor for many non-fatal but disabling disorders (e.g. osteoarthritis) and is associated with some of the leading causes of death (e.g. diabetes, cardiovascular disease, and cancer [2–4]) whereas obesity (BMI ≥ 30) is thought to confer a 2- to 10-year decrease in life expectancy [5]. The prevalence of overweight and obesity has increased over the past 30 years. Currently, about half of adults in developed countries are living with overweight or obesity, with prevalence rates ranging from 30 to 66% [6, 7]. Obesity is a growing and serious public health issue, as well as a major economic and even environmental burden [8, 9]. Although the health risks associated with living with overweight and obesity are widely acknowledged in both the literature and public opinion, long-term success in weight loss treatments remains elusive. Several factors have been linked to challenges in maintaining weight loss over the long term [10]. These factors include behavioral aspects such as physical activity and self-monitoring of weight [10], cognitive aspects like poor self-image, high expectations from treatment, and dichotomous thinking [11], and weight-related components including baseline BMI and maximum lifetime weight [12]. This data has stimulated the development of multidisciplinary lifestyle modification teams aimed at providing patients with a comprehensive long-term management of obesity [13]. Although less frequently explored, the high prevalence of binge eating (BE) behavior among individuals seeking weight loss treatment [14] is often underestimated in lifestyle modification programs and may contribute to reduced treatment efficacy [15, 16]. Furthermore, BE is more common among those seeking weight loss interventions compared to those who are not, with 9–29% of individuals reporting binge episodes [17]. Over 25% of patients seeking treatment for obesity meet the diagnostic criteria for Binge Eating Disorder (BED) [18, 19]. BED is characterized by regular episodes of binge eating during which individuals consume large amounts of food and experience a loss of control over their eating behavior [20]. Patients with BED often fail to self-regulate their behavior when coping with negative emotions such as anxiety, depression, guilt, and shame, thus exposing them to a number of unhealthy risk factors including overweight and obesity [21–24]. Therefore, the evaluation of self-regulatory control difficulties in patients with either subclinical BE behaviors and full-blown BED in weight loss treatments is a relevant clinical factor when planning more effective clinical interventions [25].
Evidence suggests that BE is associated with a range of cognitive control deficits related to impulsivity, particularly lower response inhibition, preference for immediate gratification (i.e., increased temporal discounting), and maladaptive decision-making [26]. The emotional attraction to food items and consumption is related to the balance between impulsive immediate reward-sensitivity (the ‘doers’) with reflective self-control for delay of gratification (the ‘planners’) [27, 28]. It has been observed that bingeing behaviors can be driven by emotional states and impulsivity traits (i.e., looking for immediate need gratification), whereas eating restriction can be driven by disproportionate self-control (i.e., looking at negative consequences for self-image [26, 29]). Individuals who show a strong reward-related response to foods combined with low levels of self-control are particularly susceptible to overeating and overweight, whereas those with effective self-control appear to be protected [25, 30].
Delay or temporal discounting (TD) measures one's preference for smaller-sooner (the ‘doers’) versus larger-later rewards (the ‘planners’). The temporal discounting task (TDt) is considered a reliable behavioral measure of the maladaptive behavior pattern underlying overeating [22, 31–33]. Performance on the task is usually reported as the discounting parameter k (see [34]). It assesses the capacity to delay gratification/receipt of a reward which is a facet of impulsivity [34]. The general procedure involves participants completing a series of trials involving a selection between a larger reward provided following a variable delay versus a smaller reward provided immediately. Choosing the larger reward with a delay is associated with reduced temporal discounting, while picking the smaller reward with no delay is associated with increased temporal discounting. Including a "now" and "not now" condition in the TDt is useful because it directly measures impulsivity and mirrors real-life decision-making scenarios [34]. By presenting choices between immediate and delayed rewards, the TDt mirrors the decision-making processes individuals face in various contexts, such as health-related behaviors like dieting. Individuals who consistently choose smaller, immediate rewards over larger, delayed rewards demonstrate higher difficulties in delaying gratification, a relevant component to understanding the underlying mechanisms of binge eating and obesity [25, 30].
Monetary stimuli are commonly employed in the task though food-related stimuli have been used too [35–37]. However, presenting food-related items to BE subjects may bias the assessment of the underlying impulsive functioning because of the stimuli contents. In other words, subjects may be conditioned by their psychological status in being compulsively attracted by food items or feeling guilty in their avoidance of food contents. Therefore, using monetary stimuli may present the advantage of evaluating impulsivity as a cognitive functioning rather than a by-product of the subjects’ approach to food [38].
Even though TD has been recently proposed as a behavioral marker of obesity [39], data regarding TD in people with overweight or obesity are mixed. Several inconsistencies across studies of obesity, including sampling criteria and heterogeneity in TD tasks/analyses, are likely to contribute to such disparity in findings [40–43]. Furthermore, most obesity TD trials failed to specify whether BE subjects were excluded or whether BE behavior was controlled for. Given the high rates of co-morbidity between overweight/obesity and BED [44], the increased rates of TD found in obesity studies not accounting for BE conditions may not be attributable to obesity alone.
With bingeing behaviors, self-regulatory control difficulties are thought to be mainly underpinned by aberrant prefrontal neural circuitry, manifesting in impaired regulation of appetite, emotion, and self-control [45–48]. For example, [49] suggested that reduced connectivity between the ventromedial and dorsolateral prefrontal cortex is associated with increased rates of TD. Given that increased TD was found to be associated with poorer adherence to a calorie-restricted diet in that study, impaired functional connectivity between frontal and parietal brain areas, crucial in cognitive control mechanisms, could be a key contributor to the development and maintenance of obesity and, moreover, a predictor of treatment response [49]
To fill this gap, we tested obese patients divided in tow groups with and without BE. To take in account also a clean impulsivity dimension we presented the Barratt Impulsiveness Scale (BIS-11) and divided the sample also according to the relative data (low BIS and high BIS groups; see the Methods section for more details). Patients were engaged in a temporal discounting task [50] in which they had to choice between early small monetary rewards and delayed but larger ones. In the Now condition, the smaller rewards were immediate and the larger ones could be obtained after a variable delay (2–365 days). In the Not-now condition the smaller rewards could be obtained after 60 days and the larger ones variably from 62 to 425 days. There was also a control condition in which no delay differences between the small and larger reward options were present, added to ensure that participants were actually focused on the task and responded not randomly.
Brain rhythms were recorded during the task using high density electroencephalography (hd-EEG). Different previous studies in the field analyzed mainly event-related potentials and found a preponderant role of frontal areas [51–56] along with parietal areas. In particular when EEG source localization was performed, the main generators were anterior areas, such as the dorsolateral prefrontal cortex and the insula. Even the P300, an important brain response to salient stimuli considered in several studies in the field, has been shown to have also a frontal source [57, 58]. It should be also mentioned that deep brain areas important for reward behavior (ventral tegmental area, nucleus accumbens, septum) cannot be reliably studied with EEG due to their distance from the scalp.
Here we focus on whole spectrum brain oscillations as these were only partially investigated in the field and never with the present patients typology [59–61]. Furthermore, brain rhythms are informative about the psychological status of the subject [62], in particular in perceptual and decision-making conditions relevant in our study. The relatively large number of electrodes of our hd-EEG device allowed us to select regions of interest (ROIs) at the level of the Brodmann areas, an a priori methodology widely used in EEG studies (see e.g. [63, 64]).
The main goal was to investigate whether impulsivity and BE may interact with the TDt and to explore the underlying brain activity in outpatients with overweight or obesity who are starting treatment to achieve weight loss. In particular, the aims of the study were: (a) to investigate whether patients with overweight or obesity and higher impulsivity or binge eating (BE) behaviors show a preference for immediate gratification (i.e., decreased temporal discounting) in different conditions of rewards available sooner or later, compared to patients with lower impulsivity or without BE behaviors; (b) to evaluate whether the groups with different levels of impulsivity and BE behavior show different brain activity through hd-EEG recording during TDt. Based upon the previous literature, we expected that: (a) both groups of patients with higher impulsivity or with BE would exhibit more TD in Now and Not-now conditions than patients with lower levels of impulsivity and without BE behavior, (b) different levels of impulsivity and BE behavior would be accompanied by a modulation of frontal cerebral rhythms during TDt.
Methods
Participants
A sample of 40 treatment-seeking outpatients with overweight or obesity were enrolled at the Clinical Nutrition Centre of the University Clinical Hospital of Chieti (Italy), consecutively selected from referrals to a dietary control program for any medical reason during a 12 months-period (January to December 2019). Patients were evaluated during their first medical examination. All the participants were started on a non-surgical, non-medication weight loss program, which was aimed at dietary change, weight control, adequate daily food intake, paced eating, and healthy lifestyle maintenance, tailored on individual basis.
To maximize ecological validity, patients aged 18 to 65 and with a BMI ≥ 25 were included. Subjects were selected for inclusion only if their main reason for consultation was being overweight and had no significant medical comorbidity. Documented or self‐reported psychiatric disorders, cognitive impairment, pregnancy, severe medical comorbidity (e.g., thyroid dysfunction, diabetes, chronic liver disease, and any other physical diseases which could interfere with eating behavior), or inability to perform/understand the self‐rating scales were considered exclusion criteria. The sample included 40 outpatients and, after removing those who did not meet the inclusion criteria or provided incomplete data, 26 (65%) outpatients formed the final sample. A team of expert physicians and psychologists evaluated patients for their medical history and past or current psychopathology.
Participants were categorized as either with (BE group) or without BE (NBE group) based on BED or subthreshold BED status (less than 1-weekly episode and/or for less than 3 months) assessed with the eating disorders module of the Structured Clinical Interview for DSM5—Research Version (SCID-5-RV) [65]. Thirteen participants (BE group) met the criteria for BED or subthreshold BED, and thirteen (NBE group) had 0-monthly episodes. The same participants were also categorized either as having low impulsivity (Low BIS) or high impulsivity (High BIS) according to the outcome of the Barratt Impulsiveness Scale (BIS-11, see below). Handedness was evaluated with Edinburgh Handedness Inventory [66] and showed that all participants were right-handed except one (mean handedness score = 80.33 ± 41.36).
All patients gave written informed consent to participate. The study was designed and carried out in accordance with the World Medical Association Declaration of Helsinki and its subsequent revisions [67] and was approved by the Ethics Committee of the Department of Psychological, Health, and Territorial Sciences of the University G. d’Annunzio of Chieti-Pescara (Prot. n. 254 of 03/14/2017).
Procedures
Temporal discounting task
The experimental paradigm of Temporal Discounting Task (TDt) was adapted from a standard procedure previously used in literature (see [50, 68–70]) and completely automated by means of a homemade software written in Microsoft Visual Basic v6.0. Before starting the task, participants were informed that in each trial they had to press “Esc'' for the left-side option and “Enter” for the right-side option on the computer keyboard. The left-side “Esc” key was to be used if they chose the option tag located at the left side of the screen (corresponding to a lower amount of money available sooner) and the right-side “Enter” key if they chose the option tag located at the right side of the screen (corresponding to a larger amount of money available later). They were informed that there were no right or wrong choices and that all choices were fictitious, namely, that they will not receive the actual consequences of their choice.
The option stimuli consisted of 2 labels reporting a monetary amount and a temporal delay (e.g., 20 euros now/40 euros tomorrow). Each trial began with a 1 s fixation, followed by a screen depicting the two available options. The two options appeared on the left and on right side of the screen, indicating the amount (e.g., a lower amount on the left-side choice tag compared to the amount indicated on the right-side choice tag) and the delay of delivery of the reward (e.g., ‘now’ on the right, ‘later’ on the left). The positions of the labels reporting the amount of money (smaller amount vs larger amount) and the temporal delays (immediately, after a delay) were balanced across conditions.
In the TDt, two temporal ‘Now’ and ‘Not-now’ conditions were included:
In the “Now” condition, participants had to choose between a smaller amount of money available immediately (e.g., right-side choice) or a larger amount of money available after a variable delay (e.g., left-side choice). There were six possible delays: 2, 14, 30, 90, 180, and 365 days, which were presented in different blocks wherein for each block five choices had to be made.
In the “Not-now” condition, participants had to choose between a smaller amount of money that could be obtained in 60 days (e.g., right-side choice) and a greater amount of money that could be obtained after a variable delay of more than 60 days (e.g., left-side choice). As in the "Now" condition, there were six possible variable delays: 62, 74, 90, 150, 240, and 425 days, which were presented in different blocks wherein for each block five choices had to be made.
Within each block, the amount of the sooner reward was adjusted based on the participant’s previous choice, using a titration procedure that converged towards the amount of the sooner reward that was equal, in subjective value, to the later reward. For example, if participants were presented with a choice between either 40€ later (i.e., later reward or larger-later) or 20€ sooner (i.e., sooner reward or smaller-sooner), whenever the participant picked the sooner reward, the subsequent trial presented an amount that was smaller (e.g., 10€) than the one selected in the previous trial (i.e., 20€). On the other hand, whenever the later reward was chosen (i.e., 40€), the subsequent trial increased the amount in the sooner condition (e.g. 30€). The size of the adjustment in the sooner reward decreased with successive choices; the first adjustment was half of the difference between the sooner and the later reward (in the example above, 10€) whereas, for subsequent choices, it was half of the previous adjustment (e.g., 5€; 2.50€; 1.25€; etc.). This procedure was repeated until the participant made five choices at one specific delay of delivery of the later option, after which, a new series of choices began at another delay of delivery of the later option (of the same or different temporal condition).
There was a third control condition, in which participants had to choose between €40 and a smaller amount of money, both available "now" (e.g. €40 now or €20 now) or both available in 365 days (€40 in 365 day or €20 in 365 days). The amount of the smaller option in the control condition was adjusted using the same titration procedure described above. All participants consistently chose the larger reward in this control condition, indicating that they adequately understood the task and had an appropriate sensitivity to reward. This condition was intended to verify participant’s attention and engagement during the task and was not included in the statistical analyses.
The order of delay blocks, as well as the different temporal conditions (Now, Not-now, and control condition), was randomized within subjects.
Self-reported questionnaires
Binge Eating Scale (BES): the Italian version of the 16‐item Binge Eating Scale (BES) [71, 72] was used to assess the severity of BE behavior. Scores range from 0 to 46, with a score ≥ 27 having conventionally served as a threshold value for identifying the presence of severe BE, ≥ 18 for moderate BE, and ≤ 17 for minimal or no BE [73]. This instrument has been widely used as a screening tool [73, 74]. It has good internal consistency reliability and high sensitivity and specificity for discriminating between binge eaters and non-binge eaters, presenting similar results to those obtained by reliable and supported semi-structured interviews [73–76]. Within this sample, Cronbach’s α was 0.87.
Barratt Impulsiveness Scale (BIS-11): the Italian version of the Barratt Impulsiveness Scale (BIS-11) [77, 78] was used to measure impulsivity. The scale consists of 30 items that evaluate motor, attention, and planning components. Participants were asked to rate how often impulsive traits were descriptive for them (e.g., “I act on impulse”, “I get easily bored when solving thought problems”, “I say things without thinking”). Each item is scored on a 4-point Likert scale ranging from 1 (= rarely or never) to 4 (= almost always or always). Scores range from 30 to 120, with higher scores indicating a higher level of impulsivity. The total score of the BIS-11 is an internally consistent measure and is widely used for the assessment of impulsiveness among the general population and selected patients [79]. Within this sample, Cronbach’s α was 0.83.
EEG recordings
Participants filled out the psychological scales previously described before having the EEG electrodes attached to their scalp. Participants sat on a chair at about 60 cm from the computer monitor measuring 34 × 27 cm (15.4 inches), and they were instructed to assume and maintain a relaxed position for the entire duration of the session. EEG data were continuously recorded (bandpass: 0.01–100 Hz, sampling rate: 1024 Hz; EB-Neuro Be-plus) from 56 scalp cup electrodes positioned according to a standard 10–10 system (electrical reference between AFz and FCz; ground electrode between Pz and Oz). Electrode impedance was kept smaller than 5 kΩ. Signals were stored on a computer for offline analysis. The EEG recording started 3 min before the TDt and ended 3 min after it. During the registration, the participant was seated comfortably and was asked to maintain open eyes, to reduce the number of blinks, to stay still, and to focus on the TDt.
Preliminary EEG data analysis
EEG data were pre-processed offline, by using the NPXLab 2016 software (available at http://www.brainterface.com; [80]). Raw EEG data were bandpass filtered between 0.5 and 100 Hz, notch filtered at 50 Hz and sampled at 512 Hz. Data were processed with Independent Component Analysis (ICA) to remove the eye-blinks artifacts. Remaining artifacts were removed by visual inspection. Cleaned data were segmented in single 1-s epochs (1000 ms post-stimulus) with respect to the stimulus onset (response options display) and analyzed in the frequency domain with respect to a baseline period of 1 s chosen in the rest period before the task. The frequency bands including delta (δ, 1–4 Hz), theta (θ, 4.5–8 Hz), alpha (α, 8.5–13 Hz), beta (β, 13.5–29.5 Hz), and gamma (γ, 30–40 Hz) were computed for each condition, using average Fourier cross-spectral matrices, with the LORETA (LOw-Resolution brain Electromagnetic TomogrAphy; [81]) KEY software package (v20181101; http://www.uzh.ch/keyinst/loreta#_Toc391372608).
Source localization of the EEG frequency bands was obtained using the sLORETA technique [82]. sLORETA employs the current density estimate given by the Minimum Norm solution and the localization inference based on standardized values of the current density estimates. In conditions of high signal-to-noise ratio, sLORETA has a zero-localization error. sLORETA solutions are computed using a realistic head model [83] within the source space (6239 voxels at 5 mm spatial resolution; [84]), and they are restricted to cortical grey matter and hippocampi, as determined by the probabilistic Talairach atlas [85].
In order to identify intracerebral electrical sources, we used the LORETA software package ‘ROI-maker 2’ to construct the region of interests (ROIs). We selected a list of 33 ROIs, including all voxels with coordinates corresponding to the respective Brodmann Areas (BA; Table 1). Current densities in the region of interest were then computed using the ‘sLORETA to ROI’ function.
Table 1.
Regions of interest (ROIs) for the EEG LORETA analysis
| ROI n) Name | Brodmann areas | Lobe | ROI n) Name | Brodmann areas | Lobe |
|---|---|---|---|---|---|
| 1) Angular G | 39 | Par, Temp L | 18) Paracentral Lobule | 3–7,31 | Fro, Par L |
| 2) Anterior Cing | 10,24–25,32–33 | Limbic L | 19)Parahippocampal G | 19–20,27–28,30,34–37 | Limbic, Occ L |
| 3) Cingulate G | 6,23–24,31–32 | Limbic L | 20) Postcentral G | 1–5,7,40,43 | Fro, Par L |
| 4) Cuneus | 7,17–19,23,30–31 | Occ L | 21) Posterior Cing | 18,23,29–31 | Limbic L |
| 5) Extra Nuclear | 13,47 | Fro L, Sub-lobar | 22) Precentral G | 4,6,9,43–44 | Fro, Par L |
| 6) Fusiform G | 18–20,36–37 | Temp, Occ L | 23) Precuneus | 7,18–19,23,31,39 | Par, Occ L |
| 7) Inf Fro G | 6,9–11,13,44–47 | Fro, Temp L | 24) Rectal G | 11 | Fro L |
| 8) Inf Occ G | 17,18,19 | Occ L | 25) Subcallosal G | 11,13,25,34,47 | Fro L |
| 9) Inf Par Lobule | 7,34,35,49,76,77 | Par L | 26) Sub-Gyral | 2,6–8,10,13,19–21, 31,37,39–40 | Fro, Limbic, Temp, Par L |
| 10) Inf Temp G | 15,16,17,32,57,58,59,74 | Limbic, Temp, Occ L | |||
| 11) Insula | 13,22,40,41,45,47 | Temp L, Sub-Lobar | 27) Sup Fro G | 6,8–11 | Fro L |
| 12) Lingual G | 17–19 | Occ L | 28) Sup Occ G | 19,39 | Temp L,, L |
| 13) Medial Fro G | 6,8–11,25,32 | Fro L | 29) Sup Par Lobule | 5,7,40 | Par L |
| 14) Middle Fro G | 6,8–11,46–47 | Fro L | 30) Sup Temp G | 13,21–22,38–39,41–42 | Temp L |
| 15) Middle Occ G | 18–19,37 | Occ L | 31) Supramarginal G | 39–40 | Temp, Par L |
| 16) Middle Temp G | 19–22,37–39 | Temp, Occ L | 32) Transverse Temp G | 41–42 | Temp L |
| 17) Orbital G | 11,47 | Fro L | 33) Uncus | 20,28,34,36,38 | Limbic L |
G: gyrus, L: lobe, Inf: inferior, Sup: superior, Fro: frontal, Par: parietal, Temp: temporal, Occ: occipital, Cing: cingulate
Data analysis
Preliminarily, we performed a power analysis to determine the sample size needed to detect a medium effect size (0.5). Power analysis was conducted using: (1) an alpha level of 0.05; (2) a power of 0.95; (3) number of groups = 4; (4) number of conditions = 2. According to statistical computing, a sample size of n = 24 was required for the ANOVA with repeated measures (within-between interaction). Power calculation was performed using the program G*Power 3.1 [86].
Statistical data analysis was performed using SPSS 26.0 for Windows. Descriptive statistics were reported in terms of mean and standard deviation [Mean (SD)] or absolute frequencies. The level of significance was set at 95%. Independent and paired-sample Student’s t tests or chi-square tests (χ2) were used to compare between- and within-group differences in socio-demographic and clinical variables between BE and NBE groups. Cohen’s d was used as a measure of effect size. A standardized effect size of 0.20–0.50 is considered small, 0.50–0.80 moderate, and > 0.80 large [87]. The Cramer’s φ was also used as a measure of the strength of association for the χ2 test [88]. The three magnitudes of effects of 0.10–0.30, 0.30–0.50, and > 0.50 are considered as small, medium, and large, respectively. Pearson’s correlation coefficient was used for the associations between BIS and BES scores and TD rate. The four magnitudes of Pearson’s coefficient of 0.20–0.40, 0.40–0.60, 0.60–0.80, and > 0.80 are considered as low, moderate, marked, and high, respectively.
TDt was assessed through the temporal discounting parameter (k) [89–91]. This is the rate at which the subjective value of a future reward decays with delay (TD rate), for each temporal condition (Now, Not-now). The hyperbolic function SV = 1/(1 + kD), where SV = subjective value (expressed as a fraction of the delayed amount), and D = delay between the two options at stake (in days), was fit to the data to determine the k constant of the best fitting TD function using a nonlinear, least squares algorithm. The larger the value of k (the steeper the discounting function), the more participants were inclined to choose smaller-sooner (SS) rewards over the larger-later (LL) rewards. The hyperbolic k constants were normally distributed after log-transformation, and the comparisons were performed using parametric statistical tests. Log-transformed k near to 0 describes a prevalent SS pattern of choice (i.e., higher delay discount) while very negative log-transformed k describes a prevalent LL pattern of choice (i.e., lower delay discount).
For this, we first carried out a correlation analysis between BIS scores, BES scores, and the log-transformed k of the Now and Not-now conditions. Due to the multiple comparisons, we applied the Bonferroni correction at a significance threshold of p = 0.006.
Subsequently, we divided our sample according to presence of BE (according to SCID-5-RV) and impulsivity trait (BIS median score) and carried-out a mixed analysis of variance (ANOVA) on log-transformed k values with BE group (NBE, BE) and BIS Group (Low BIS, High BIS) as between-subjects factors, and Temporal condition (Now, Not-now) as a within-subjects factor.
Regarding the EEG data, our focus was on assessing the EEG power spectra after the options display (before the response) in the two different conditions of the TDt (Now and Not-now, see the Methods section). We carried out 5 separate mixed ANOVAs, one for each of the 5 frequency bands, on the current density values with BIS Group (Low BIS, High BIS) and BE group (NBE, BE) as between-subjects factors, Temporal condition (Now, Not-now), Response (SS, LL), and the 33 ROIs (Table 1) as within-subjects factors.
Due to the multiple comparisons, we applied the Bonferroni correction at a threshold of p < 0.01. In all analyses, the post-hoc analyses were performed with the Newman-Keuls test.
Results
Sample characteristics and behavioral results
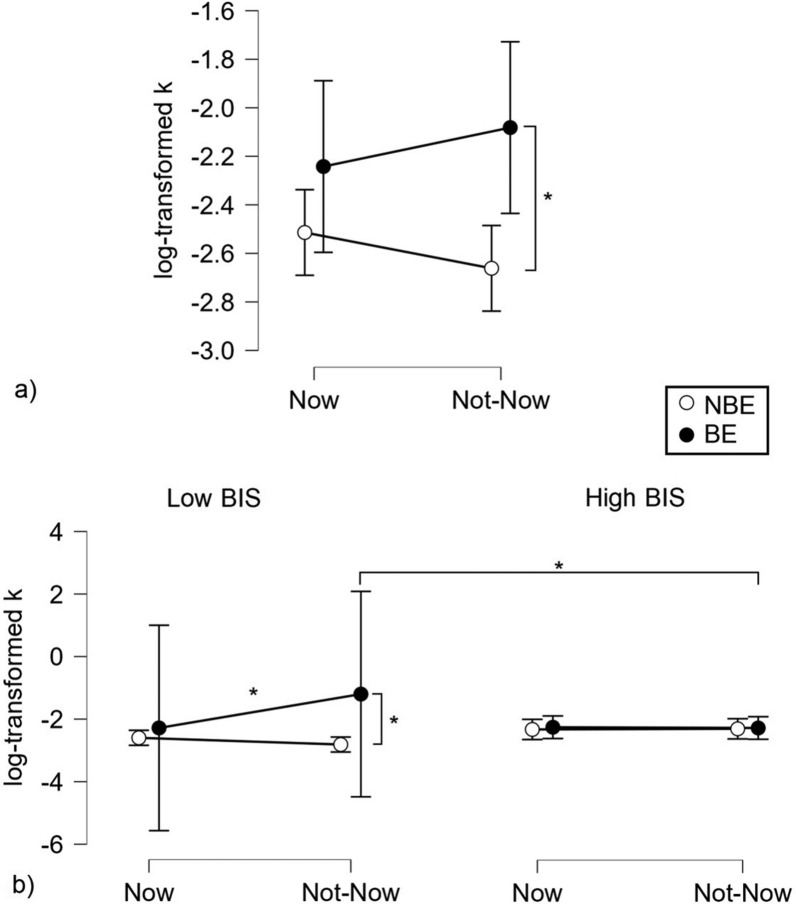
Included patients (Table 2) were mostly females (n = 17, 65.4%), overweight (n = 16, 61.5%), with a mean age of 32.30 years (SD = 10.39), and a high educational level (n = 23, 88.5%). Compared to the NBE patients, the BE group had a higher incidence of obesity (φ = 0.47), had higher BMI (d = 1.20), level of impulsivity (BIS-11, d = 1.52), BE (BES, d = 2.66), and increased TD in the Not-now condition (K-log NN, d = 0.92). No differences were found in age, gender, educational level, and delay discounting in the Now condition (K-log N) between the two groups (Fig. 1).
Table 2.
Socio-demographic and clinical characteristics of the study sample (N = 26)
| Variables | Total sample | NBE | BE | t/χ2 | p | d/φ |
|---|---|---|---|---|---|---|
| N = 26 | n = 13 | n = 13 | ||||
| Age, M(SD) | 32.30 (10.39) | 28.69 (5.96) | 35.92 (12.69) | 1.85 | 0.07 | 0.55 |
| Gender, n (%) | 0.17 | 0.68 | 0.08 | |||
| Male | 9 (43.6) | 4 (30.8) | 5 (38.5) | |||
| Female | 17 (65.4) | 9 (69.2) | 8 (61.5) | |||
| BMI | 31.11 (8.04) | 27.05(3.59) | 35.16 (9.28) | 2.93 | 0.007 | 1.20 |
| Weight class, n (%) | 5.85 | 0.016 | 0.47 | |||
| Obese | 10 (38.5) | 2 (15) | 8 (61) | |||
| Overweight | 16 (61.5) | 11 (84) | 5 (38) | |||
| Educational level, n (%) | 3.39 | .06 | 0.36 | |||
| Less than high school | 3 (11.5) | – | 3 (23.1) | |||
| With high school or more | 23 (88.5) | 13 (100) | 10 (76.9) | |||
| BIS-11 | 53.19 (7.73) | 48.61 (5.10) | 57.76 (7.28) | 3.71 | 0.001 | 1.52 |
| BES | 10.26 (9.18) | 3.07 (2.13) | 17.46 (7.67) | 6.50 | < 0.001 | 2.66 |
| K-log N | − 2.38 (0.63) | − 2.47 (0.72) | − 2.30 (0.53) | 0.65 | 0.52 | 0.28 |
| K-log NN | − 2.39 (0.56) | − 2.62 (0.42) | − 2.16 (0.60) | 2.27 | 0.03 | 0.92 |
BMI: Body Mass Index, BES: Binge Eating Scale, BIS-11: Barratt Impulsiveness Scale Version 11, K-log N: Now log transformed k, K-log NN: Not-now log-transformed k
Fig. 1.
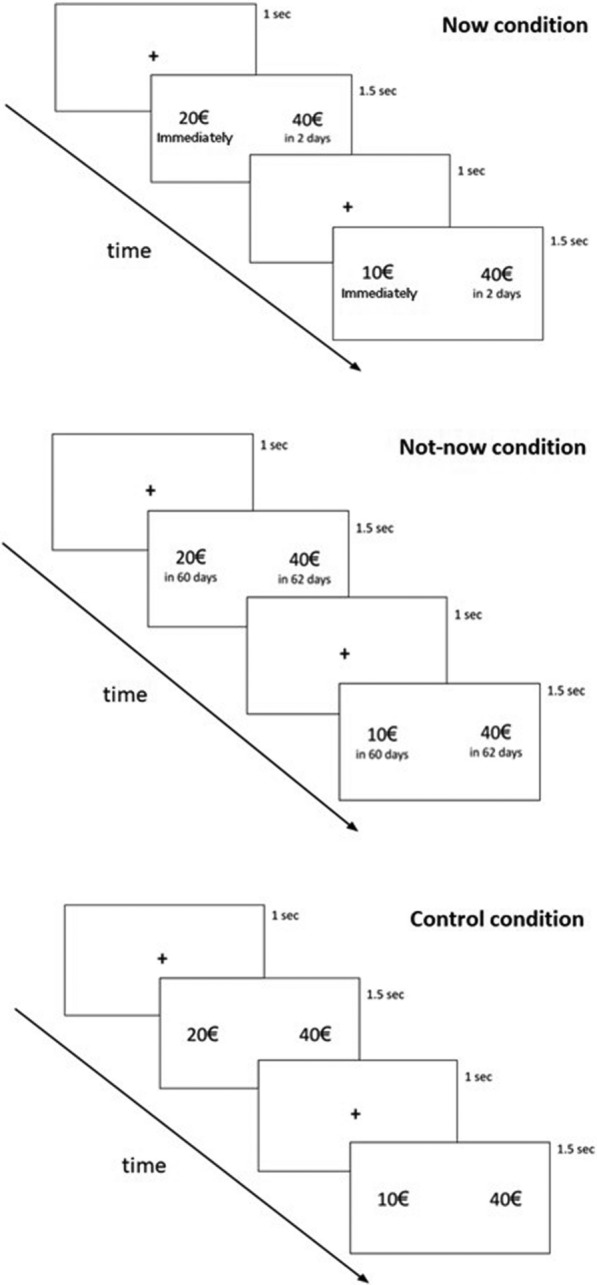
The three experimental conditions of the study
Behavioral results
The ANOVA performed on log-transformed k using BE and BIS groups as between factors, and the Temporal condition as a within-subject factor showed a significant effect of the BE group (F1, 22 = 5.315, p = 0.031, partial eta-squared = 0.195; where BE subjects showed higher k-log scores compared to low-BES subjects), a significant interaction BE group x BIS group (F1, 22 = 4.348, p = 0.049, partial eta-squared = 0.165), a significant interaction Temporal condition x BE group (F1, 22 = 5.164, p = 0.033, partial eta-squared = 0.190) and a significant triple interaction Temporal condition x BE group x BIS group (F1, 22 = 5.900, p = 0.024, partial eta-squared = 0.211) and no further significant result. According to the Newman-Keuls post-hoc analyses, the previously reported significant interaction BE group x BIS group was based on a difference, within the low BIS group, between NBE and BE subjects, the latter showing higher k-log scores (p = 0.025). The interaction Temporal condition x BE group was based on a difference between the two BE subgroups in the Not-now condition (p = 0.041; BE > NBE). The triple interaction was instead based on a higher k-log score of BE / low BIS patients in the Not-now compared to the Now condition (p = 0.004), on a higher score of the same subjects in the Not-now condition compared to NBE/low BIS subjects (p = 0.002), and on a higher score in the Not-now condition of BE/low BIS vs. BE/high BIS patients (p = 0.016). Overall, these findings show that patients with BE have more impulsive tendencies (higher TD) and that they show these tendencies for delayed rewards (Not-now condition).
Correlation analysis
The analysis showed a significant positive correlation effect size between BIS and BES scores in the large range (r = 0.59, p = 0.002), showing that higher impulsive subjects were also more prone to more severe BE behaviors. Neither the BIS nor the BES scores correlated with the log-transformed k. Effect sizes in the moderate positive range were found between BES scores and Not-now log-transformed k (r = 0.40, p = 0.047), therefore suggesting that participants who scored higher on BES also showed a tendency to choose SS rewards (i.e., higher TD) instead of LL rewards in the delayed condition (i.e., Not-now; 60 days or more). Additionally, a moderate positive correlation was also found between the Now and Not-now log-transformed k (r = 0.45, p = 0.019), meaning that a prevalent SS pattern of choices in the Now condition showed a tendency to a significant association with prevalent SS choice patterns in the Not-now condition and vice versa (Fig. 2).
Fig. 2.
Significant interactions: a Temporal condition x BE group; b Temporal condition x BE group x BIS group. Asterisks indicate significant post-hoc results
EEG results
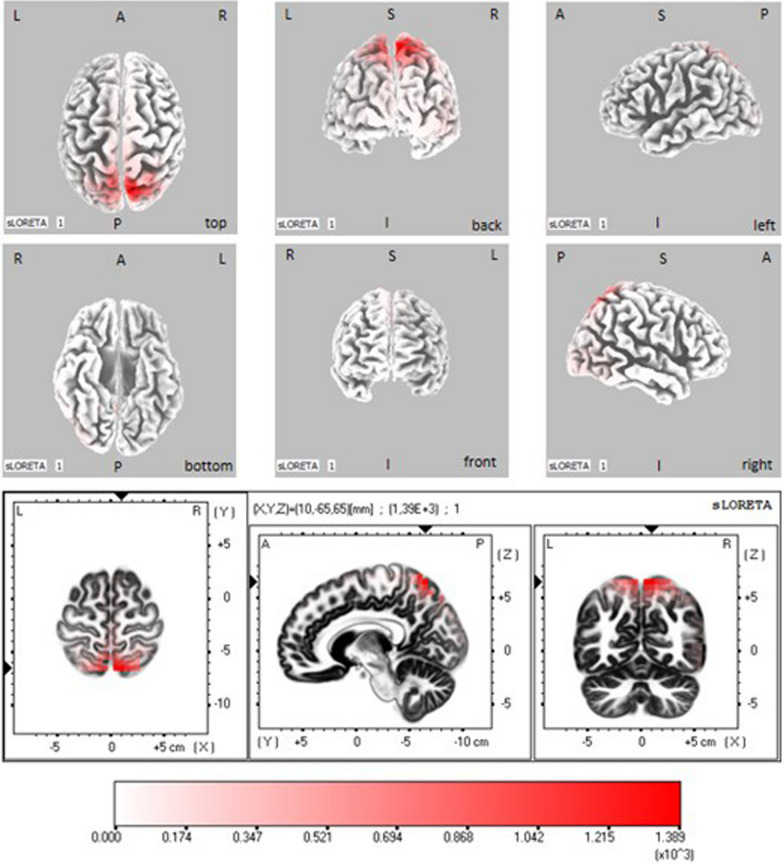
The 5 ANOVAs carried out on the LORETA current density for each frequency band showed a significant effect for the α band (Fig. 3) and no effects for the other bands.
Fig. 3.
EEG sLORETA statistical maps depicting grand-mean (all subjects) activations in alpha range during the post-stimulus period. The colored areas represent the spatial extent of voxels in the current source density. Top panel: significant results projected onto a fiducial cortical surface. Bottom panel: same results projected in an average brain MRI template (the slices are located at the indicated MNI coordinates). L: left, R: right, A: anterior, P: posterior, S: superior, I: inferior
Particularly, in the α band, a significant main effect of the ROI was found (F = 1.900, p = 0.003, partial eta-squared = 0.120). Visual inspection of mean results indicated that the ROI 29 (superior parietal lobule) presented lower LORETA activation levels with respect to all other ROIs. Subsequent Newman-Keuls post-hoc results showed that the ROI 29 differed significantly from all other ROIs except than from ROI 18 (paracentral lobule) and ROI 20 (postcentral gyrus), both located in the parietal lobe. The significance levels of the differences regarding ROI 29 were of p < 0.001 for the ROIs 1, 5, 7, 8, 12, 14, 15, 17, 24 and 28; of 0.001 < p < 0.010 for the ROIs 2, 4, 6, 10, 11, 13, 16, 19, 21, 23, 25, 27 and 33; of 0.01 < p < 0.05 for the ROIs 3, 9, 22, 26, 30, 31 and 32. Further significant post-hoc results were found between ROI 18 and ROIs 8, 12, 17, 24 and 28 (0.03 < p < 0.05), where ROI 18 showed always the lower activation levels.
These results indicate that EEG rhythms show a desynchronization in the alpha range (reduction of power) after the option presentation in the parietal lobe, but that they do not differ between the groups nor across experimental conditions.
Discussion
The main goal of this study was to investigate whether impulsivity and binge eating may interact with the TDt and underlying brain activity in outpatients with overweight or obesity who are starting treatment to achieve weight loss. The main result of this study is that patients with BE that self-reported to be less impulsive, showed higher temporal discount preferring sooner and smaller than larger and later rewards when both options are being delayed (Not-now condition). Furthermore, a preponderant role of α oscillations in the parietal lobe has been observed during the processes of reward choice. Our findings are consistent with a growing number of studies that have established that those who tend to choose immediate gratification at the expense of a greater long-term reward may be more likely to give in to food urges at the expense of long-term health outcomes, such as obesity, cardiovascular issues, or other related conditions [22, 31–33, 39]. This supports the recommendation to include psychological outcomes such as impulsivity and dysfunctional eating behaviors when designing a weight loss program to prevent a negative outcome.
In our first hypothesis, we expected that both groups of participants with higher levels of impulsivity and with BE behavior showed a preference for immediate gratification (i.e., increased delay discounting) in different conditions of rewards available sooner (Now condition) or respectively later (Not-now condition) as compared with those with lower impulsivity or without BE behavior. This hypothesis was supported only partially by our data. In fact, although patients with BE scored higher on impulsivity levels, a decline in perceiving reward when both options were being delayed (Not-now condition) was observed only in patients with BE that self-reported to be less impulsive. In contrast, no significant difference was found between subjects with high and low impulsivity on intertemporal choices. However, there were positive correlations between BES and the BIS scores and rates of temporal discounting in the Not-now condition. This suggests that stronger tendencies towards BE are associated with higher self-reported impulsivity. However, the increased discounting of delayed rewards when both options were delayed (Not-now condition) was observed in patients with BE who self-reported lower impulsivity. Overall, these results show that patients with BE tend to have more impulsive tendencies and perceive sooner rewards as more gratifying when both options are being delayed, when compared to patients without BE. This result favors the notion that the reduction in the subjective value of a delayed reward may be associated to the delay itself, rather than its amount and its delay separately influencing the reward’s choice, thus suggesting that the choice of immediate (or sooner) rewards is due to impulsive tendencies rather than a lack of behavioral inhibition [92].
Unexpectedly, impulsivity was associated to binge eating only when declared by the participants through a self-reported measure whereas inverse association was found by measuring impulsivity on a behavioral basis, namely by performing the discounting task. Further research is needed to ascertain whether this may be due to the unshared variance between psychological measures using different sources of information [93] or to a misguided self-perception of patients with BE towards less controlled behaviors. Another unexpected result concerned the main effect, which has been found only in the Not-now condition while impulsivity should manifest itself also in the immediate. However, self-reported impulsivity does not have a consistent relationship with discounting rate, as supported by the literature (e.g., [94]). Therefore, it is challenging to predict participants response dynamics in a complex task performed by our particular patients sample.
Although several previous studies identified a relation between temporal discounting and obesity, none of them have examined temporal discounting in relation to BE in patients under weight-loss treatment. Therefore, this study focused on subtyping outpatients with obesity by BE and then examining group differences. The current study is the first to provide empirical support for a presumptive BE pathway by which impulsivity and temporal discounting contribute to weight gain. This result is in line with evidence suggesting that patients in a weight-loss treatment are at a higher risk of adverse treatment outcomes if BE occurs in addition to overweight or obesity [15], and suggests that low self-reported impulsivity and increased discounting of delayed rewards as risk factors for negative weight loss program outcomes.
In our second hypothesis, we expected that impulsivity and binge eating behavior would be accompanied by a modulation of prefrontal cerebral rhythms during TDt. The hypothesis was not supported by our data. We found that only the α rhythm was modulated during the task (reduced power), and through LORETA source analysis we could observe that such effect occurred in the parietal lobe (more precisely in the superior parietal lobule and, to a lesser extent, in the paracentral lobule). This is not surprising, given the evidence suggesting that parietal areas and α-frequency brain oscillations may be involved in cognitive processes, including attention, problem solving, and decision making [95]. Due to the event-related nature of the present EEG analysis [96], the present reduction of power (desynchronization) of the α-rhythm means that during the task period (i.e. immediately after the options presentation) the α-rhythm was smaller in amplitude with respect to the baseline or rest period (i.e., event-related desynchronization), regardless from the experimental condition. According to a well-established acquisition of the literature in the field, α desynchronization follows and external influence which attracts attention of the subject and is associated to an opening of the thalamus gate which facilitates information flow to the cerebral cortex [97]. This result appears thus to be not specific for the present task. However, we can speculate that a judgment on reward can involve specific neural activations in the parietal lobe in people with overweight and obesity. This outcome, besides to structural alterations involving the parietal cortex in patients with obesity [98–100], would be in accordance with different recent studies which found that the parietal cortex plays also functionally a central role in eating disorders and impulsive behavior. [101] found altered occipito-parietal resting-state connectivity in patients with obesity, which they propose to be related to dysfunctions in perception, attention and value encoding of visual food cues (see also [102] for an earlier review). An fMRI study [103] reported altered activation in the posterior parietal cortex (as well as in the dorsolateral prefrontal cortex) in patients with obesity performing a task very similar to the TDt. In an oddball paradigm event-related EEG study, [104] found that the N200 and P300 EEG components, which are generated in the parietal cortex, are reduced in women with obesity. A further EEG study [105] found that the parietal cortex (precuneus) plays a central role in both food addicted and non-addicted people with obesity. Concerning healthy subjects, both prefrontal reward and frontoparietal control networks were proposed to be implicated in temporal discounting [106], with stronger activation of parietal regions being associated more to choices involving delayed rewards [107, 107–109]. Thus, activation of these regions may indicate also specific cognitive control when engaging in decision-making regarding rewards and delays. This being considered, our data corroborate previous evidence suggesting a widespread parietal and frontal network implicated in decision processes regarding future rewards. Furthermore, our result is in line with evidence suggesting that people with obesity have a reduced activation in brain areas associated with cognitive control, which can correspond with increased rates of TD [110–112] and predicts future weight gain [49, 113].
Limitations and future directions
The study has some limitations to consider. First, given its explorative nature, a consecutive non‐probabilistic small sample was used in this study. Future studies with probabilistic sampling procedures and larger sample size will be useful to investigate the involvement of impulsivity and BE in the decision process as well as the effects of age, education and BMI which were not balanced among our groups. Second, the cross‐sectional nature of our study does not allow us to determine the relation direction between impulsivity, BE, and temporal discounting over time, given that only a longitudinal design could help to clarify this point. Third, the task utilized hypothetical rewards, thus potentially underpowering the effects of the amount and delay sensitivity of participants on their temporal discounting choices [107]. Experiential rewards may have led to stronger frontal and parietal activations, due to a potentially enhanced experience and consequent evaluation of both rewards and delays. Future studies should aim to implement “real” rewards in their protocols in order to accentuate the effects associated with choices on temporal discounting tasks. Fourth, the study included individuals who volunteered for dietary intervention in tertiary care centers, thus limiting the generalizability to primary care patients or patients who do not seek treatment. Individuals with obesity or overweight actively seeking medical, dietary, and psychological help for their weight problem and eating behavior are likely to be more motivated to make behavioral changes and more aware of their psychological problems. Follow-up studies should investigate whether the role of impulsivity, BE, and TD is different in patients seeking treatment who completed the intervention program and those who are not seeking for any treatment or dropped out. Finally, in this study, several potentially relevant factors were not controlled for, such as an objective measure or document of past psychopathology, lifetime and current psychiatric conditions, quality of life, sleep quality, social support, medical comorbidity, physical activity, dietary habits, and inflammation activity.
Conclusion
In conclusion, the results of this study suggest that patients with overweight or obesity and BE tend to have more impulsive tendencies and perceive sooner rewards as more gratifying when both options are being delayed, compared to patients without BE. Furthermore, patients who scored higher in impulsivity have decreased α frequency oscillations in parietal areas when making choices regarding immediate or delayed rewards. We may speculate that people with overweight or obesity may prefer immediate rewards such as unhealthy food over long-term rewards. The tendency to favor immediate rewards may constitute an obstacle for obtaining adherence to treatment plans and to achieve weight loss goals for outpatients with overweight or obesity. Indeed, there has been interest in developing psychological treatments that strengthen inhibitory control and moderate impulsive behaviors [114]. "Top-down" approaches, exemplified by implementation intentions, concentrate on bolstering cognitive control to restrain impulsive behaviors [115]. Conversely, "bottom-up" approaches, like food-specific inhibitory control training, aim to modify automatic reactions to food cues by linking them with motor inhibition [116]. These strategies have demonstrated effectiveness in promoting healthy food choices and reducing consumption of unhealthy foods, even among individuals who are overweight or obese [117]. Clinicians are therefore encouraged to include psychological outcomes such as impulsivity and dysfunctional eating behaviors when designing a weight loss program to prevent a negative outcome [25, 118, 119]. Furthermore, as specific cortical brain areas seem to be associated with impulsivity and delay sensitivity, interventions aiming to increase delay tolerance through neurofeedback training or non-invasive brain stimulation [120, 121] programs may be particularly effective in more impulsive people [122, 123].
Acknowledgements
Thanks to Manuela Sellitto for her suggestions on temporal discounting task data processing.
List of abbreviations
- WHO
World Health Organization
- BMI
Body mass index
- BE
Bingeeating
- BED
Bingeeating disorder
- TD
Temporaldiscounting
- TDt
Temporaldiscounting task
- BIS
Barratt Impulsiveness Scale
- BES
Binge Eating Scale
- ICA
Independent Component Analysis
- SS
Smaller-sooner
- LL
Larger-later
- BA
Brodmann Areas
Author contributions
SF, methodology, data curation, formal analysis, writing original draft. VM, methodology, data curation, formal analysis, writing original draft. AD, methodology, data curation, formal analysis. LB writing review and editing. MG, writing review and editing, supervision. AB, conceptualization, methodology, writing original draft, supervision. PP, conceptualization, methodology, writing review and editing, supervision. CC, conceptualization, methodology, data curation, formal analysis, project administration, writing original draft. All authors have approved the final article.
Funding
Not applicable.
Data availability
For ethics or privacy issues of clinical data, data is not able to be made openly available but only to be made available via a request to the Authors.
Declarations
Ethics approval and consent to participate
The study was designed and carried out in accordance with the World Medical Association Declaration of Helsinki and its subsequent revisions (General Assembly of the World Medical Association, 2013) and was approved by the Ethics Committee of the Department of Psychological, Health, and Territorial Sciences of the University G. d’Annunzio of Chieti-Pescara (Prot. n. 254 of 03/14/2017).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original version of this article has been revised: the typo in the name of author, Luca Bovolon, has been corrected.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sara Ferracci and Valerio Manippa are co-first authors.
Change history
11/21/2024
A Correction to this paper has been published: 10.1186/s40337-024-01153-2
References
- 1.Obesity and overweight. World Health Organization; 2021. Available from:https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2.Kaiser J. Cholesterol forges link between obesity and breast cancer. Science. 2013;342(6162):1028–1028. 10.1126/science.342.6162.1028. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Hajifathalian K, Danaei G, Rimm EB, Woodward M, Ezzati M. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383(9921):970–83. 10.1016/s0140-6736(13)61836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 5.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-analysis. Epidemiol Rev. 2007;29:6e. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg AF, Burton DC, Jackson RJ. Economic and environmental costs of obesity: the impact on airlines. Am J Prev Med. 2004;27:264. [DOI] [PubMed] [Google Scholar]
- 9.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378:815–25. [DOI] [PubMed] [Google Scholar]
- 10.Byrne SM, Cooper Z, Fairburn CG. Psychological predictors of weight regain in obesity. Behav Res Ther. 2004;42(11):1341–56. 10.1016/j.brat.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Lowe MR. Dieting: Proxy or cause of future weight gain? Obes Rev. 2015;16(Suppl 1):19–24. 10.1111/obr.12252. [DOI] [PubMed] [Google Scholar]
- 12.Palascha A, van Kleef E, van Trijp HC. How does thinking in Black and White terms relate to eating behavior and weight regain? J Health Psychol. 2015;20(5):638–48. 10.1177/1359105315573440. [DOI] [PubMed] [Google Scholar]
- 13.Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle GR. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;26(9):37–46. 10.2147/DMSO.S89836.PMID:27013897;PMCID:PMC4777230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer RL, Devlin MJ, Walsh BT, Hasin D, Wing R, Marcus M, Stunkard A, Wadden T, Yanovski S, Agras S, Mitchell J &Nonas C. Binge eating disorder: a multisite field trial of the diagnostic criteria. Int J, 1992;11(3), 191–203. 10.1002/1098-108X(199204)11:3<191::AID-EAT2260110302>3.0.CO;2-S
- 15.Linde JA, Simon GE, Ludman EJ, Ichikawa LE, Operskalski BH, Arterburn D, Rohde P, Finch EA, Jeffery RWA. randomized controlled trial of behavioral weight loss treatment versus combined weight loss/depression treatment among women with comorbid obesity and depression. Ann Behav Med. 2011;41(1):119–30. 10.1007/s12160-010-9232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the national weight control registry. Am J Prev Med. 2014;46(1):17–23. 10.1016/j.amepre.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Decaluwé V, Braet C. Prevalence of binge-eating disorder in obese children and adolescents seeking weight-loss treatment. Int J Obes. 2003;27:404–9. [DOI] [PubMed] [Google Scholar]
- 18.Fandiño J, Moreira RO, Preissler C, Gaya CW, Papelbaum M, Coutinho WF, Appolinario JC. Impact of binge eating disorder in the psychopathological profile of obese women. Compr Psychiatry. 2010;51(2):110–4. 10.1016/j.comppsych.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J of Eat Disorders. 2007;40:S123–9. 10.1002/eat.20436. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed., text rev.). 2022. 10.1176/appi.books.9780890425787
- 21.Danner UN, Ouwehand C, van Haastert NL, Hornsveld H, de Ridder DTD. Decision-making impairments in women with binge eating disorder in comparison with obese and normal-weight women. Eur Eat Disord Rev. 2012;20:e56-62. 10.1002/erv.1098. [DOI] [PubMed] [Google Scholar]
- 22.Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences: A neuropsychological study of binge eating and obesity. Appetite. 2010;54:208–13. 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Svaldi J, Brand M, Tuschen-Caffier B. Decision-making impairments in women with binge eating disorder. Appetite. 2010;54:84–92. [DOI] [PubMed] [Google Scholar]
- 24.Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, Schreiber LRN, Gillan C, Fineberg NA, Sahakian BJ, Robbins TW, Harrison NA, Wood J, Daw ND, Dayan P, Grant JE, Bullmore ET. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatr. 2015;20:345–52. 10.1038/mp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence NS, O’Sullivan J, Parslow D, Javaid M, Adams RC, Chambers CD, Kos K, Verbruggen F. Training response inhibition to food is associated with weight loss and reduced energy intake. Appetite. 2015;95:17–28. 10.1016/j.appet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KE, Mason TB, Johnson JS, Lavender JM, Wonderlich SA. A systematic review of reviews of neurocognitive functioning in eating disorders: the state-of-the-literature and future directions. Int J Eat Disorders. 2018;51(8):798–821. 10.1002/eat.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann W, Friese M, Roefs A. Three ways to resist temptation: The independent contributions of executive attention, inhibitory control, and affect regulation to the impulse control of eating behavior. J Exp Soc Psychol. 2009;45:431e435. [Google Scholar]
- 28.Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415e422. [DOI] [PubMed] [Google Scholar]
- 29.Steward T, Menchón JM, Jiménez-Murcia S, Soriano-Mas C, & Fernández-Aranda F. Neural network alterations across eating disorders: A narrative review of fMRI studies. Curr Neuropharmacol. 2017 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 30.Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010;29:389e393. [DOI] [PubMed] [Google Scholar]
- 31.Bartholdy S, Rennalls S, Danby H, Jacques C, Campbell IC, Schmidt U, O’Daly OG. Temporal discounting and the tendency to delay gratification across the eating disorder spectrum. Eur Eat Disord Rev. 2017;25:344–50. 10.1002/erv.2513. [DOI] [PubMed] [Google Scholar]
- 32.Mole TB, Irvine MA, Worbe Y, Collins P, Mitchell SP, Bolton S, Harrison NA, Robbins TW, Voon V. Impulsivity in disorders of food and drug misuse. Psychol Med. 2015;45:771–82. 10.1017/S0033291714001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan W-S, Zhang R-R, Lan Y, Li Z-M, Li Y-H. Questionnaire-based maladaptive decision-coping patterns involved in binge eating among 1013 college students. Front Psychol. 2018;9:609. 10.3389/fpsyg.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odum AL. Delay discounting: I’m a k, you’re a k. J Exp Anal Behav. 2011;96:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellitti JS, Fazzino TL. Discounting of hyper-palatable food and money: associations with food addiction symptoms. Nutrients. 2023;15(18):4008. 10.3390/nu15184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrickson KL, Rasmussen EB, Lawyer SR. Measurement and validation of measures for impulsive food choice across obese and healthy-weight individuals. Appetite. 2015;90:254–63. 10.1016/j.appet.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Turel O, Xiao Z, He J, He Q. Impulsivity and neural mechanisms that mediate preference for immediate food rewards in people with vs without excess weight. Appetite. 2022;169:105798. 10.1016/j.appet.2021.105798. [DOI] [PubMed] [Google Scholar]
- 38.Verdejo-Román J, Vilar-López R, Navas JF, Soriano-Mas C, Verdejo-García A. Brain reward system’s alterations in response to food and monetary stimuli in overweight and obese individuals. Hum Brain Mapp. 2017;38(2):666–77. 10.1002/hbm.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bickel WK, Freitas-Lemos R, Tomlinson DC, Craft WH, Keith DR, Athamneh LN, Basso JC, Epstein LH. Temporal discounting as a candidate behavioral marker of obesity. Neurosci Biobehav R. 2021;129:307–29. 10.1016/j.neubiorev.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Buono FD, Whiting SW, Sprong ME. Comparison of temporal discounting among obese college students and obese adults. Behav Anal: Res Pract. 2015;15:139–47. 10.1037/bar0000015. [Google Scholar]
- 41.Daniel TO, Stanton CM, Epstein LH. The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychol Sci. 2013;24:2339–42. 10.1177/0956797613488780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarmolowicz DP, Cherry JBC, Reed DD, Bruce JM, Crespi JM, Lusk JL, Bruce AS. Robust relation between temporal discounting rates and body mass. Appetite. 2014;78:63–7. 10.1016/j.appet.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price M, Higgs S, Maw J, Lee M. A dual-process approach to exploring the role of delay discounting in obesity. PhysiolBehav. 2016;162:46–51. 10.1016/j.physbeh.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Villarejo C, Fernandez-Aranda F, Jimenez-Murcia S, Penas-Lledo E, Granero R, Penelo E, Tinahones FJ, Sancho C, Vilarrasa N, Montserrat-Gil de Bernabe M, Casanueva FF, Fernandez-Real JM, Fruhbeck G, De la Torre R, Treasure J, Botella C, Menchon JM. Lifetime obesity in patients with eating disorders: increasing prevalence, clinical and personality correlates. Eur Eat Disorders Rev. 2012;20:250–4. 10.1002/erv.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friederich HC, Wu M, Simon JJ, Herzog W. Neurocircuit function in eating disorders. Int J Eat Disorders. 2013;46:425–32. [DOI] [PubMed] [Google Scholar]
- 46.Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge-eating disorder. NeurosciBiobehav R. 2016;63:223–38. [DOI] [PubMed] [Google Scholar]
- 47.McClelland J, Kekic M, Bozhilova N, Nestler S, Dew T, Van den Eynde F, David AS, Rubia K, Campbell IC, Schmidt U. A randomised controlled trial of neuronavigated repetitive transcranial magnetic stimulation (rTMS) in anorexia nervosa. PLoS ONE. 2016. 10.1371/journal.pone.0148606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gluck ME, Viswanath P, Stinson EJ. Obesity, appetite, and the prefrontal cortex. Current Obes Rep. 2017;6(4):380–8. 10.1007/s13679-017-0289-0. [DOI] [PubMed] [Google Scholar]
- 49.Weygandt M, Mai K, Dommes E, Leupelt V, Hackmack K, Kahnt T, Rothemund Y, Spranger J, Haynes JD. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage. 2013;83:669–78. 10.1016/j.neuroimage.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Scarpazza C, Sellitto M, di Pellegrino G. Now or not-now? The influence of alexithymia on intertemporal decision-making. Brain Cognition. 2017;114:20–8. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y, Guan C, Wang Z, Yang W, Gao B. Electrocortical correlates of hypersensitivity to large immediate rewards in sensation seeking. Neuroimage. 2023;15(284): 120456. 10.1016/j.neuroimage.2023.120456. [DOI] [PubMed] [Google Scholar]
- 52.Torres A, Catena A, Megías A, Maldonado A, Cándido A, Verdejo-García A, Perales JC. Emotional and non-emotional pathways to impulsive behavior and addiction. Front Hum Neurosci. 2013;21(7):43. 10.3389/fnhum.2013.00043.Erratum.In:FrontHumNeurosci.2014;8:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherniawsky AS, Holroyd CB. High temporal discounters overvalue immediate rewards rather than undervalue future rewards: an event-related brain potential study. Cogn Affect Behav Neurosci. 2013;13(1):36–45. 10.3758/s13415-012-0122-x. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt B, Holroyd CB, Debener S, Hewig J. I can’t wait! Neural reward signals in impulsive individuals exaggerate the difference between immediate and future rewards. Psychophysiology. 2017;54(3):409–15. 10.1111/psyp.12796. [DOI] [PubMed] [Google Scholar]
- 55.Patalano AL, Lolli SL, Sanislow CA. Gratitude intervention modulates P3 amplitude in a temporal discounting task. Int J Psychophysiol. 2018;133:202–10. 10.1016/j.ijpsycho.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Patalano AL, Lolli SL, Sanislow CA. Brief report on the relationship between temporal discount rate and error related negativity for immediate versus future choice options. Int J Psychophysiol. 2020;151:1–6. 10.1016/j.ijpsycho.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Giustiniani J, Nicolier M, Teti Mayer J, Chabin T, Masse C, Galmès N, Pazart L, Trojak B, Bennabi D, Vandel P, Haffen E, Gabriel D. Event-related potentials (ERP) indices of motivation during the effort expenditure for reward task. Brain Sci. 2020;10(5):283. 10.3390/brainsci10050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dreo J, Attia D, Pirtošek Z, Repovš G. The P3 cognitive ERP has at least some sensory modality-specific generators: evidence from high-resolution EEG. Psychophysiology. 2017;54(3):416–28. 10.1111/psyp.12800. [DOI] [PubMed] [Google Scholar]
- 59.Guleken Z, Sutcubasi B, Metin B. The cognitive dynamics of small-sooner over large-later preferences during temporal discounting task through event-related oscillations (EROs). Neuropsychologia. 2021;162:108046. 10.1016/j.neuropsychologia.2021.108046. [DOI] [PubMed] [Google Scholar]
- 60.Pornpattananangkul N, Nusslock R. Willing to wait: elevated reward-processing EEG activity associated with a greater preference for larger-but-delayed rewards. Neuropsychologia. 2016;91:141–62. 10.1016/j.neuropsychologia.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rong Y, Chen N, Dong J, Li Q, Yue X, Hu L, Wei P. Expectations of immediate and delayed reward differentially affect cognitive task performance. Neuroimage. 2022;15(262): 119582. 10.1016/j.neuroimage.2022.119582. [DOI] [PubMed] [Google Scholar]
- 62.Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43(1):41–58. 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 63.Zacharia AA, Kaur S, Sharma R. Altered functional connectivity: a possible reason for reduced performance during visual cognition involving scene incongruence and negative affect. IBRO Neurosci Rep. 2022;21(13):533–42. 10.1016/j.ibneur.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gurja JP, Muthukrishnan SP, Tripathi M, Sharma R. Reduced resting-state cortical alpha connectivity reflects distinct functional brain dysconnectivity in Alzheimer’s disease and mild cognitive impairment. Brain Connect. 2022;12(2):134–45. 10.1089/brain.2020.0926. [DOI] [PubMed] [Google Scholar]
- 65.First MB, Williams JB, Karg RS, & Spitzer RL. Structured clinical interview for DSM-5-research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington: American Psychiatric Association. 2015.
- 66.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. 10.1016/0028-3932(71)90067-4. (PMID: 5146491). [DOI] [PubMed] [Google Scholar]
- 67.World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. 10.1001/jama.2013.281053. (PMID: 24141714). [DOI] [PubMed] [Google Scholar]
- 68.Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77(2):129–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellitto M, di Pellegrino G. Errors affect hypothetical intertemporal food choice in women. PLoS ONE. 2014;9(9): e108422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sellitto M, di Pellegrino G. Errors as a means of reducing impulsive food choice. JoVE. 2016;112: e53283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47–55. 10.1016/0306-4603(82)90024. [DOI] [PubMed] [Google Scholar]
- 72.Ricca V, Mannucci E, Moretti S, Di Bernardo M, Zucchi T, Cabras PL, & Rotella CM. Screening for binge eating disorder in obese individuals. 2000. [DOI] [PubMed]
- 73.Greeno C, Marcus MD, Wing R. Diagnosis of binge eating disorder: discrepancies between a questionnaire and clinical interview. Int J Eat Disorders. 1995;17(2):153–60. [DOI] [PubMed] [Google Scholar]
- 74.Freitas SR, Lopes CS, Appolinario JC, Coutinho W. The assessment of binge eating disorder in obese women: a comparison of the Binge Eating Scale with the structured clinical interview for the DSM-IV. Eat Behav. 2006;7(3):282–9. [DOI] [PubMed] [Google Scholar]
- 75.Celio AA, Wilfley DE, Crow SJ, Mitchell J, Walsh BT. A comparison of the binge eating scale, questionnaire for eating and weight patterns-revised, and eating disorder examination questionnaire with instructions with the eating disorder examination in the assessment of binge eating disorder and its symptoms. Int J Eat Disorders. 2004;36(4):434–44. 10.1002/eat.20057. [DOI] [PubMed] [Google Scholar]
- 76.Grupski AE, Hood MM, Hall BJ, Azarbad L, Fitzpatrick SL, & Corsica JA. Examining the Binge Eating Scale in screening for binge eating. 2013. [DOI] [PMC free article] [PubMed]
- 77.Fossati A, Di Ceglie A, Acquarini E, Barratt ES. Psychometric properties of an Italian version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J Clin Psychol. 2001;57(6):815–28. 10.1002/jclp.1051. (PMID: 11344467). [DOI] [PubMed] [Google Scholar]
- 78.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–74. [DOI] [PubMed] [Google Scholar]
- 79.Vasconcelos AG, Malloy-Diniz L, Correa H. Systematic review of psychometric proprieties of barratt impulsiveness scale version 11 (BIS-11). Clin Neuropsychiatry: J Treat Eval. 2012;9(2):61–74. [Google Scholar]
- 80.Bianchi L, Quitadamo LR, Abbafati M, Marciani MG, & Saggio G. Introducing NPXLab 2010: a tool for the analysis and optimization of P300 based brain-computer interfaces. 2nd International Symposium on Applied Sciences in Biomedical and Communication Technologies. IEEE. 2009; 1-4.
- 81.Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, Koukkou M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiat Res. 1999;90(3):169–79. 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 82.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24(1):5–12. [PubMed] [Google Scholar]
- 83.Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS. A standardized boundary element method volume conductor model. Clin Neurophysiol. 2002;113(5):702–12. [DOI] [PubMed] [Google Scholar]
- 84.Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34(4):1600–11. 10.1016/j.neuroimage.2006.09.024. (Epub 2007 Jan 4 PMID: 17207640). [DOI] [PubMed] [Google Scholar]
- 85.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–31. 10.1002/1097-0193(200007)10:3%3c120::aid-hbm30%3e3.0.co;2-8.PMID:10912591;PMCID:PMC6871915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faul F, Erdfelder E, Lang AG, Buchner AG. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 87.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Routledge. 1988
- 88.Cramer H. Mathematical Methods in Statistics. Princeton University Press. 1946
- 89.Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130(5):769–92. 10.1037/0033-2909.130.5.769.PMID:15367080;PMCID:PMC1382186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazur JE, An adjusting procedure for studying delayed reinforcement. In Commons ML, Mazur JE, Nevin JA, & Rachlin H (Eds.), The effect of delay and of intervening events on reinforcement value (pp. 55–73). Lawrence Erlbaum Associates, Inc. 1987.
- 91.Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55(2):233–44. 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frost R, McNaughton N. The neural basis of delay discounting: a review and preliminary model. NeurosciBiobehav R. 2017. 10.1016/j.neubiorev.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 93.Meyer GJ, Finn SE, Eyde LD, Kay GG, Moreland KL, Dies RR, Eisman EJ, Kubiszyn TW, Reed GM. Psychological testing and psychological assessment: a review of evidence and issues. Am Psychol. 2001;56:128–65. 10.1037/0003-066X.56.2.128. [PubMed] [Google Scholar]
- 94.Ikegami M, Sorama M. Differential neural correlates in the prefrontal cortex during a delay discounting task in healthy adults: an fNIRS study. Brain Sci. 2023;13(5):758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27(21):5796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfurtscheller G, da Lopessilva FH. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neuropsychol. 1999;110(11):1842–57. 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 97.Pfurtscheller G. Induced oscillations in the alpha band: functional meaning. Epilepsia. 2003;44(Suppl 12):2–8. 10.1111/j.0013-9580.2003.12001.x. [DOI] [PubMed] [Google Scholar]
- 98.Chen L, Zhao S, Wang Y, Niu X, Zhang B, Li X, Peng D. Genetic insights into obesity and brain: combine mendelian randomization study and gene expression analysis. Brain Sci. 2023;13(6):892. 10.3390/brainsci13060892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Contreras-Rodriguez O, Arnoriaga-Rodríguez M, Miranda-Olivos R, Blasco G, Biarnés C, Puig J, Fernandez-Real JM. Obesity status and obesity-associated gut dysbiosis effects on hypothalamic structural covariance. Int J Obes. 2022;46(1):30–8. 10.1038/s41366-021-00953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Syan SK, Owens MM, Goodman B, Epstein LH, Meyre D, Sweet LH, MacKillop J. Deficits in executive function and suppression of default mode network in obesity. Neuroimage Clin. 2019;24:102015. 10.1016/j.nicl.2019.102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang J, Dong D, Liu Y, Yang Y, Chen X, He Q, Lei X, Feng T, Qiu J, Chen H. Multivariate resting-state functional connectomes predict and characterize obesity phenotypes. CerebCortex. 2023;33(13):8368–81. 10.1093/cercor/bhad122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saruco E, Pleger B. A systematic review of obesity and binge eating-associated impairment of the cognitive inhibition system. Front Nutr. 2021;8:609012. 10.3389/fnut.2021.609012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W, Li G, Manza P, Hu Y, Wang J, Lv G, He Y, von Deneen KM, Yu J, Han Y, Cui G, Volkow ND, Nie Y, Ji G, Wang GJ, Zhang Y. Functional abnormality of the executive control network in individuals with obesity during delay discounting. Cereb Cortex. 2022;32(9):2013–21. 10.1093/cercor/bhab333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iceta S, Benoit J, Cristini P, Lambert-Porcheron S, Segrestin B, Laville M, Poulet E, Disse E. Attentional bias and response inhibition in severe obesity with food disinhibition: a study of P300 and N200 event-related potential. Int J Obesity. 2020;44(1):204–12. 10.1038/s41366-019-0360-x. [DOI] [PubMed] [Google Scholar]
- 105.De Ridder D, Manning P, Leong SL, Ross S, Sutherland W, Horwath C, Vanneste S. The brain, obesity, and addiction: an EEG neuroimaging study. Sci Rep. 2016;6:34122. 10.1038/srep34122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695): 503507. 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 107.Scheres A, De Water E, Mies GW. The neural correlates of temporal reward discounting. Wiley Interdiscip Rev: Cognit Sci. 2013;4(5):523–45. [DOI] [PubMed] [Google Scholar]
- 108.Sripada CS, Gonzalez R, Luan Phan K, Liberzon I. The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type, and trait impulsivity. Hum Brain Mapp. 2011;32(10):1637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Water E, Mies GW, Figner B, Yoncheva Y, van den Bos W, Castellanos FX, Scheres A. Neural mechanisms of individual differences in temporal discounting of monetary and primary rewards in adolescents. Neuroimage. 2017;153:198–210. [DOI] [PubMed] [Google Scholar]
- 110.McClelland J, Dalton B, Kekic M, Bartholdy S, Campbell IC, Schmidt U. A systematic review of temporal discounting in eating disorders and obesity: behavioral and neuroimaging findings. NeurosciBiobehav R. 2016;71:506–28. 10.1016/j.neubiorev.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 111.Stoeckel LE, Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE. Greater impulsivity is associated with decreased brain activation in obese women during a delay discounting task. Brain Imaging Behav. 2013;7(2):116–28. 10.1007/s11682-012-9201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, Haynes J-D. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 2015;109:318–27. [DOI] [PubMed] [Google Scholar]
- 113.Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58(2):582–92. 10.1016/j.appet.2011.11.029. (Epub 2011 Dec 4 PMID: 22166676). [DOI] [PubMed] [Google Scholar]
- 114.van Koningsbruggen GM, Veling H, Stroebe W, Aarts H. Comparing two psychological interventions in reducing impulsive processes of eating behaviour: Effects on self-selected portion size. Brit J Health Psych. 2017;19:767–82. [DOI] [PubMed] [Google Scholar]
- 115.Adriaanse MA, Vinkers CD, De Ridder DT, Hox JJ, De Wit JB. Do implementation intentions help to eat a healthy diet? A systematic review and meta-analysis of the empirical evidence. Appetite. 2011;56(1):183–93. 10.1016/j.appet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 116.Jones A, Di Lemma LC, Robinson E, Christiansen P, Nolan S, Tudur-Smith C, Field M. Inhibitory control training for appetitive behaviour change: A meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite. 2016;97:16–28. [DOI] [PubMed] [Google Scholar]
- 117.Vilà I, Carrero I, Redondo R. Reducing fat intake using implementation intentions: a meta-analytic review. Brit J Health Psych. 2017;22(2):281–94. [DOI] [PubMed] [Google Scholar]
- 118.Striegel-Moore RH, Wilson GT, DeBar L, Perrin N, Lynch F, Rosselli F, Kraemer HC. Cognitive behavioral guided self-help for the treatment of recurrent binge eating. J Consult Clin Psych. 2010;78(3):312–21. 10.1037/a0018915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loeb KL, Wilson GT, Gilbert JS, Labouvie E. Guided and unguided self-help for binge eating. Behav Res Ther. 2010;38(3):259–72. 10.1016/s0005-7967(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 120.Alhindi YA, Khalifa N, Al-Khyatt W, Idris I. The use of non-invasive brain stimulation techniques to reduce body weight and food cravings: a systematic review and meta-analysis. Clin Obes. 2023. 10.1111/cob.12611. [DOI] [PubMed] [Google Scholar]
- 121.Cavicchioli M, Sarzetto A, Erzegovesi S, Ogliari A. Is repetitive transcranial magnetic stimulation (RTMS) a promising therapeutic intervention for eating disorders and obesity? Clinical considerations based on a meta-analytic review. Clin Neuropsychatr. 2022;19(5):314–27. 10.3613/cnfioritieditore20220507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barth B, Mayer-Carius K, Strehl U, Wyckoff SN, Haeussinger FB, Fallgatter AJ, Ehlis AC. A randomized-controlled neurofeedback trial in adult attention-deficit/hyperactivity disorder. Sci Rep. 2021;11(1):16873. 10.1038/s41598-021-95928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cho BH, Kim S, Shin DI, Lee JH, Lee SM, Kim IY, Kim SI. Neurofeedback training with virtual reality for inattention and impulsiveness. Cyberpsychol Behav. 2021;7(5):519–26. 10.1089/cpb.2004.7.519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For ethics or privacy issues of clinical data, data is not able to be made openly available but only to be made available via a request to the Authors.