Abstract
STUDY QUESTION
What is the impact of co-designed, evidence-based information regarding the anti-Mullerian hormone (AMH) test on women’s interest in having the test?
SUMMARY ANSWER
Women who viewed the evidence-based information about the AMH test had lower interest in having an AMH test than women who viewed information produced by an online company selling the test direct-to-consumers.
WHAT IS KNOWN ALREADY
Online information about AMH testing often has unfounded claims about its ability to predict fertility and conception, and evidence suggests that women seek out and are recommended the AMH test as a measure of their fertility potential.
STUDY DESIGN, SIZE, DURATION
An online randomized trial was conducted from November to December 2022. Women were randomized (double-blind, equal allocation) to view one of two types of information: co-designed, evidence-based information about the AMH test (intervention), or existing information about the AMH test from a website which markets the test direct-to-consumers (control). A total of 967 women were included in the final analysis.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants were women recruited through an online panel, who were aged 25–40 years, living in Australia or The Netherlands, had never given birth, were not currently pregnant but would like to have a child now or in the future, and had never had an AMH test. The primary outcome was interest in having an AMH test (seven-point scale; 1 = definitely NOT interested to 7 = definitely interested). Secondary outcomes included attitudes, knowledge, and psychosocial and behavioural outcomes relating to AMH testing.
MAIN RESULTS AND THE ROLE OF CHANCE
Women who viewed the evidence-based information about the AMH test had lower interest in having an AMH test (MD = 1.05, 95% CI = 0.83–1.30), less positive attitudes towards (MD = 1.29, 95% CI = 4.57–5.70), and higher knowledge about the test than women who viewed the control information (MD = 0.75, 95% CI = 0.71–0.82).
LIMITATIONS, REASONS FOR CAUTION
The sample was more highly educated than the broader Australian and Dutch populations and some measures (e.g. influence on family planning) were hypothetical in nature.
WIDER IMPLICATIONS OF THE FINDINGS
Women have higher knowledge of and lower interest in having the AMH test when given evidence-based information about the test and its limitations. Despite previous studies suggesting women are enthusiastic about AMH testing to learn about their fertility potential, we demonstrate that this enthusiasm does not hold when they are informed about the test’s limitations.
STUDY FUNDING/COMPETING INTEREST(S)
This project was supported by an NHMRC Emerging Leader Research Fellowship (2009419) and the Australian Health Research Alliance’s Women’s Health Research, Translation and Impact Network EMCR award. B.W.M. reports consultancy for ObsEva and Merck and travel support from Merck. D.L. is the Medical Director of, and holds stock in, City Fertility NSW and reports consultancy for Organon and honoraria from Ferring, Besins, and Merck. K.H. reports consultancy and travel support from Merck and Organon. K.M. is a director of Health Literacy Solutions that owns a licence of the Sydney Health Literacy Lab Health Literacy Editor. No other relevant disclosures exist.
TRIAL REGISTRATION NUMBER
ACTRN12622001136796.
TRIAL REGISTRATION DATE
17 August 2022.
DATE OF FIRST PATIENT’S ENROLMENT
21 November 2022.
Keywords: informed decision-making, psychosocial outcomes, AMH test, intention, attitudes, knowledge, evidence-based, overuse, fertility
Introduction
The anti-Mullerian hormone (AMH) can be measured by a blood test and gives an indication of the number of oocytes in the ovaries, but not of oocyte quality (Dewailly and Laven, 2019; Hunt and Vollenhoven, 2020). Whilst the test is helpful in assisted reproduction as it roughly indicates the potential number of oocytes retrievable for in vitro fertilization or oocyte freezing (Broer et al., 2013), the test cannot reliably predict the chance of conceiving or the age of menopause for individual women (Depmann et al., 2018; Lin et al., 2021; Nelson et al., 2023). As such, AMH testing for women outside of fertility treatment settings is strongly discouraged (Practice Committee of the American Society for Reproductive Medicine, 2015; ACOG Committee Opinion, 2019). Despite this, the test is increasingly promoted as a way for women to find out about their fertility or reproductive timeline. Online companies in the USA, Australia, The Netherlands, and elsewhere now sell the test direct-to-consumers, falsely promising women detailed insights into their fertility potential (Johnson et al., 2023). Inaccurate information about the AMH test has also been identified on other websites including accredited fertility clinic websites (Copp et al., 2021).
The prevalence of misleading and false information has raised concerns that the test is being used outside of recommended settings (Copp et al., 2024). Indeed, our recent cross-sectional population-level survey examining AMH test usage found that, of the Australian women aged 18–55 years who reported having had an AMH test, one-third reported reasons not supported by evidence (Copp et al., 2023). For example, 19% had the test because they were considering getting pregnant soon and wanted to understand their chances of conceiving, and 9% had the test because they were curious about their fertility (Copp et al., 2023). This is concerning because the test is invalid for these purposes. In addition to undermining informed choice, misleading women into believing this test can reliably predict their fertility may have serious consequences. Previous research on polycystic ovary syndrome has illustrated how anticipated infertility can influence life choices and behaviour (Copp et al., 2019). For example, using the AMH test to plan pregnancy could give women a false sense of security about delaying pregnancy or unwarranted anxiety about their ability to conceive, pressuring them to try to conceive earlier than preferred or to undergo fertility treatment (O’Brien et al., 2020; Copp et al., 2021).
Studies in the USA, Australia, and The Netherlands have demonstrated that women are interested in ovarian reserve testing when uninformed about the test's limitations (Grootenhuis et al., 2021), and may change their reproductive timeline or consider fertility preservation (e.g. oocyte freezing) if their AMH result is low (Azhar et al., 2015; Evans et al., 2018; Hurley et al., 2018). These are high stake decisions and access to high-quality, accurate information to enable informed decision-making is therefore essential. Evidence is lacking on how information about the unreliability of the test affects women’s attitudes towards the test and their decision about whether to have the test. Given the plethora of misleading, commercially driven statements about the AMH test online, this study aimed to co-design evidence-based information about the AMH test and examine its impact on women’s interest in having the test, knowledge about, and attitudes towards the test.
Materials and methods
Study design
This was an online randomized controlled trial and is reported according to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials (Schulz et al., 2010). Participants were randomly allocated to one of two information pamphlets about the AMH test, either: (i) co-designed, evidence-based information about the AMH test, or (ii) content from an existing website promoting the test direct-to-consumers, using a balanced allocation ratio.
Ethics approval
This study was approved by the University of Sydney Human Research Ethics Committee (2022/177) and the University of Maastricht (FHML-REC/2022/060). The trial was prospectively registered in the Australian New Zealand Clinical Trials Registry (ACTRN12622001136796).
Intervention co-design and development
The intervention consisted of evidence-based information about the AMH test, including what the test does and does not measure and the potential benefits and harms of testing, including the limitations of the test (e.g. that it cannot predict chance of conceiving or timing of menopause for individual women; Supplementary Data File S1). The information was co-designed with all relevant stakeholders including seven consumers (four from Australia and three from The Netherlands) and four clinicians with different clinical backgrounds (two general practitioners (GPs) and two gynaecologists) in addition to the multidisciplinary study team with expertise in co-design, randomized controlled trials, obstetrics and gynaecology, assisted reproduction, and behavioural science. The co-design process was guided by the Agency for Clinical Innovation’s principles and process (Agency for Clinical Innovation, 2023).
Explore and understand
First, we reviewed and synthesized the current evidence on the utility of the AMH test (see previous work; Copp et al., 2021, 2023). Subsequently, we ran 1.5-hr long co-design workshops via Zoom with consumers in Australia and The Netherlands to gather experiences with the AMH test and impressions of the evidence, and to explore information needs.
Design and development
Based on the synthesized evidence and the consumer feedback, an information sheet was developed first in English which underwent several rounds of feedback and revision by the multidisciplinary team, consumers, and clinicians. To increase the reading ease and reduce the health literacy demands of the information, it was also revised using the Sydney Health Literacy Lab Health Literacy Editor (Ayre et al., 2023). This involved minimizing the use of jargon and the passive voice, as well as reducing sentence length and complexity. The information was then translated into Dutch and further reviewed by the Dutch consumer panel and a Dutch GP.
Pilot evaluation
After co-designing the intervention materials (Supplementary Data File S1), the study was piloted with 10 women (consumers) to test the data collection procedures and ensure the suitability of study materials and measures.
Control arm
The control information consisted of the content on an existing website selling the AMH test direct-to-consumers in Australia, formatted to visually match the intervention materials. Copies of the intervention and control materials are provided in Supplementary Data Files S1 and S2.
Participants and recruitment
Eligible participants were females aged 25–40 years living in Australia or The Netherlands who had never given birth, were not currently pregnant, would like to have a child now or in the future, had never had an AMH test, and were fluent in English or Dutch. These criteria reflect the demographic characteristics of the direct-to-consumer advertising target population for the AMH test in these two countries, which have both recently seen the emergence of online companies selling AMH tests direct-to-consumers (Johnson et al., 2023).
Participants were recruited through Dynata, an independent online social research company. Potential participants from the panel were directed by email to the study landing page where they could view the participant information statement in their own language (English or Dutch) and were required to indicate their informed consent before proceeding to the screening questions, the intervention or control information, and the questionnaire. Dynata panel participants receive modest compensation for their time in the form of points (equating to ∼$3–6 AUD). These points can later be redeemed for gift vouchers or donated to charities once several surveys have been completed and a certain number of points have been accrued.
Procedure
The questionnaire was administered using Qualtrics survey software, individually for each country. After completing the eligibility screening questions, participants were randomized to either the evidence-based information or the control information. Randomization was performed by the survey platform Qualtrics, which uses the Mersenne Twister pseudorandom number generator to create allocation sequences. Participants and the research team were blinded to the group allocation at the time of the randomization. After viewing the allocated information, participants completed several outcome measures. Finally, participants answered relevant health and sociodemographic questions. To minimize non-response on outcome measures, all questions were mandatory except the sociodemographic questions and free-text responses. After completing the questionnaire, participants randomized to the control group were shown a debrief statement which included the evidence-based information from the intervention materials.
Outcomes
Outcomes were assessed using several previously validated and adapted scales (see Table 1), as well as new measures (multiple choice, Likert scales, and open-ended responses) informed by the published literature and developed by the multidisciplinary study team. The primary outcome was interest in having an AMH test. Secondary outcomes included intention to discuss the AMH test with their doctor (Dolan et al., 2022), intention to get the test (Pickles et al., 2020), attitudes (Scherer et al., 2019), knowledge (Slater et al., 2022), emotional response to the information (Petrova et al., 2023), worry (Dolan et al., 2022), anticipated psychological reaction to having an AMH test (Vakkas et al., 2023), anticipated impact on family planning (Evans et al., 2018), and information satisfaction. Sociodemographic and health characteristics included age, relationship status, whether currently trying to conceive, perceived importance of having children (‘How important is it to you to have children one day?’ [1 = not important at all to 5 = very important]) (Prior et al., 2019), history of infertility, chronic conditions, family history of premature menopause, location, main language spoken at home, education, health literacy (the single-item health literacy screener; Chew et al., 2004), preference for more or less healthcare (the single item Medical Maximizer-Minimizer scale; Scherer and Zikmund-Fisher, 2020), and private health insurance status.
Table 1.
Outcome measures.
| Measure and reference | Items | Scale/responses and coding instructions |
|---|---|---|
| Interest in getting an AMH test | Now that you have read the information, are you interested in getting an AMH test? | Seven-point scale (1 = definitely NOT interested to 7 = definitely interested) |
| Intention to discuss the AMH test with doctor1 | After reading this information, would you talk to your doctor about getting an AMH test? |
|
| Intention2 | Which of the following best describes your intentions to get an AMH test? | Five-point scale (1 = I definitely will NOT get a AMH test to 5 = I definitely will get an AMH test) |
| Attitudes3 |
|
|
| Knowledge4 | Please indicate whether you think the statements below about the AMH test are true or false
|
|
| The Berlin Emotional Responses to Risk Instrument5 | How did you feel when you read the information about the AMH test?
|
|
| Worry about fertility1 | How worried are you about your chance of getting pregnant? |
|
| Anticipated psychological reaction of getting an AMH test6 | Getting an AMH test would make me feel:
|
|
| Anticipated impact on family planning7 |
|
Dichotomized into ‘yes’ vs ‘unsure’/‘no’. (If yes)
|
| Information satisfaction |
Please indicate how you felt about the AMH information on the five-point scale:
The information was…
|
Five-point scale (1 = strongly disagree to 5 = strongly agree) |
[R], reverse scored; (T), true; (F), false.
Adapted from Dolan et al., JAMA Network Open 2022; 5: e2216784.
Adapted from Pickles et al., PLoS One 2020; 15: e0227304.
Adapted from Scherer et al., Journal of Experimental Psychology: Applied 2019; 25: 149.
Adapted from Slater et al., Australian Journal of General Practice 2022; 51: 611–619.
Petrova et al., Risk Analysis 2023; 43: 724–746.
Adapted from Vakkas et al., ASPOG2023, Adelaide, Australia.
Adapted from Evans et al., Aust J Prim Health 2018; 24: 428–433.
Statistical analysis
Sample size
Based on the piloting of the survey materials, a total sample size of N = 800 (n = 400 participants per country, with equal allocation to the intervention or control group) was calculated to provide more than 90% power to detect the effect of the intervention on interest in getting an AMH test (0.24SDs = 0.4 units on a seven-point scale) at an alpha level of 0.01. This would also provide more than 80% power to detect a country by intervention interaction no smaller than 0.75 times the effect of the intervention alone. We intentionally over-sampled by 25% in each group (n = 500 per country, with n = 250 randomized to each arm within each country) to account for the possibility of smaller effect sizes, or removal due to data cleaning as described below.
Analyses
Statistical analysis was conducted in Stata/IC v17 (StataCorp). The data were first cleaned and checked for missing values, outliers, and invalid responders (failed attention check or repeat participants evidenced by identical responses, including free text). Baseline and demographic characteristics were then quantified, with the mean and SDs calculated for continuous variables, and frequencies and relative frequencies for categorical variables. Overall differences between randomized arms were analysed using linear, Poisson, logistic, and ordinal logistic regression models (except for impact on family planning which was examined using chi-square tests). Results are presented for main effects of information (effect of the study factor averaged across country). Country (Australia, The Netherlands) was also included as a categorical covariate for the primary outcome to examine for main effects as well as the interaction between country and information.
An inductive content analysis was conducted for the open-ended question ‘What do you think is the take-home message of the information you read about the AMH test?’ to extract patterns and/or themes, with the frequency of each identified theme calculated (Hsieh and Shannon, 2005). First, three researchers (T.C., L.A., F.V.M.) reviewed the free-text responses and each developed an initial list of recurring codes. These lists were then discussed and combined to create a coding framework. All free-text responses were then coded into the framework. To ensure consistency and rigour, 30% of free-text responses were double coded by two researchers independently (T.C. and L.A.). The level of agreement between the two researchers was tested using Cohen’s kappa and indicated a strong level of agreement (k = 0.757) (McHugh, 2012). Descriptive statistical analysis was then used to calculate the frequency of each code, with quotes selected to illustrate findings.
Results
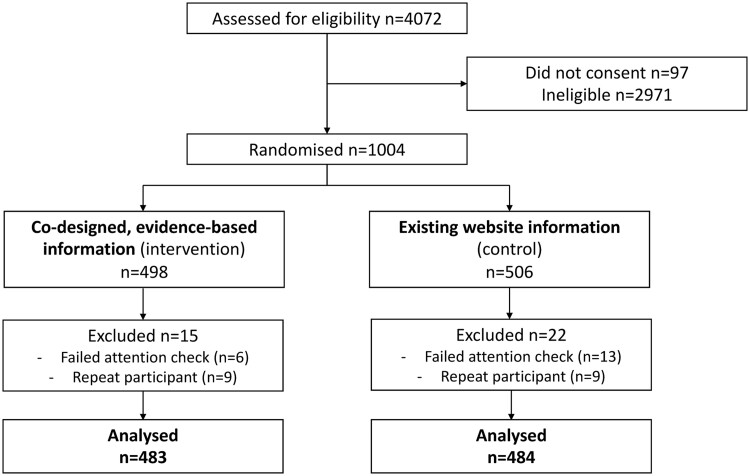
Of the 1004 eligible participants who were randomized, 37 were excluded due to failing the attention check question or repeat participation. This left a total of 967 participants for the final analysis (intervention n = 483, control n = 484, see Fig. 1).
Figure 1.
Participant flow diagram.
Sociodemographic and health characteristics
The sample characteristics by randomized group are reported in Table 2. Overall, 70% of participants were in a relationship, 7% reported a family history of premature menopause, 11% had previously heard about the AMH test, and 16% had experienced infertility. Almost all (95%) of participants spoke English or Dutch as their main language at home, 46% had a bachelor’s degree or higher and 80% had adequate health literacy according to the health literacy screener (Chew et al., 2004).
Table 2.
Characteristics of analysis sample (N = 967).
| Variable | Evidence-based information (intervention; n = 483) | Existing website information (control; n = 484) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 30.6 (4.18) | 30.3 (4.06) |
| n (%) | n (%) | |
|---|---|---|
| Country of residence | ||
| Australia | 230 (48) | 230 (48) |
| Netherlands | 253 (52) | 254 (52) |
| Relationship status | ||
| In a relationship (opposite sex) | 322 (67) | 309 (64) |
| In a relationship (same sex) | 16 (3) | 24 (5) |
| Not in a relationship | 142 (30) | 146 (30) |
| [missing] | 3 (1) | 5 (1) |
| Currently trying to conceive | ||
| No | 371 (77) | 369 (77) |
| Yes | 109 (23) | 111 (23) |
| [missing] | 3 (1) | 4 (1) |
| Importance of having children | ||
| Not important at all | 10 (2) | 6 (1) |
| Not very important | 34 (7) | 28 (6) |
| Neutral | 109 (23) | 119 (25) |
| Important | 183 (38) | 179 (37) |
| Very important | 144 (30) | 148 (31) |
| [missing] | 3 (1) | 4 (1) |
| Experience of infertility | ||
| Yes | 72 (15) | 77 (16) |
| No | 399 (83) | 395 (82) |
| Don’t know | 9 (2) | 8 (2) |
| [missing] | 3 (1) | 4 (1) |
| Chronic conditionsa | ||
| Polycystic ovary syndrome | 56 (12) | 40 (8) |
| Endometriosis | 24 (5) | 22 (5) |
| Thyroid disease | 32 (7) | 25 (5) |
| An autoimmune disease | 22 (5) | 24 (5) |
| Cancer | 5 (1) | 5 (1) |
| Primary ovarian insufficiency | 1 (0) | 1 (0) |
| Other | 9 (2) | 12 (2) |
| Family history of premature menopause | ||
| Yes | 39 (8) | 31 (6) |
| No | 369 (76) | 377 (78) |
| Don’t know | 71 (15) | 71 (15) |
| [missing] | 4 (1) | 5 (1) |
| Medical minimizer maximizer preference | ||
| Lean towards waiting and seeing | 244 (51) | 250 (52) |
| Lean towards taking action | 230 (48) | 225 (47) |
| [missing] | 9 (2) | 9 (2) |
| Residential location remoteness | ||
| Urban | 269 (56) | 272 (56) |
| Regional | 131 (27) | 122 (25) |
| Rural | 53 (11) | 59 (12) |
| Remote | 3 (1) | 1 (0) |
| [missing] | 27 (6) | 30 (6) |
| Main language spoken at home | ||
| English/Dutch | 450 (93) | 442 (91) |
| Other | 24 (5) | 27 (6) |
| [missing] | 9 (2) | 15 (3) |
| Education | ||
| High school or below | 96 (20) | 105 (22) |
| Diploma or certificate | 146 (30) | 163 (34) |
| Bachelor degree or above | 227 (47) | 201 (42) |
| [missing] | 14 (3) | 15 (3) |
| Health literacy screener | ||
| Low (‘inadequate’) health literacy | 105 (22) | 86 (18) |
| Adequate health literacy | 375 (78) | 392 (81) |
| [missing] | 3 (1) | 6 (1) |
| Private health insurance | ||
| Yes | 358 (74) | 358 (74) |
| No | 118 (24) | 120 (25) |
| [missing] | 7 (1) | 6 (1) |
| Prior knowledge of the AMH test | ||
| Yes | 52 (11) | 52 (11) |
| No | 414 (86) | 408 (84) |
| Don’t know | 14 (3) | 21 (4) |
| [missing] | 3 (1) | 3 (1) |
Chronic conditions were not mutually exclusive.
Main outcome
Interest in getting an AMH test (1 = definitely not interested to 7 = definitely interested)
Women who received the evidence-based information (intervention) had less interest in getting an AMH test (mean (M)=3.87, 95% CI = 3.71–4.03) than women who viewed the existing website information (control; M = 4.93, 95% CI = 4.77–5.09; mean difference (MD) = 1.05, P < 0.001, 95% CI = 0.83–1.30; Table 3).
Table 3.
Summary statistics of outcome measures, by randomized group.
| Outcome measure | Evidence-based information (intervention; n = 483) |
Existing website information
(control; n = 484) |
|
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | P-value | |
| Interest in getting an AMH test | 3.87 (3.71–4.03) | 4.93 (4.77–5.09) | <0.001 |
| Intention to get an AMH test | 2.84 (2.75–2.93) | 3.36 (3.27–3.45) | <0.001 |
| Attitudes towards the AMH test | 3.96 (3.86–4.06) | 5.25 (5.15–5.35) | <0.001 |
| Knowledge about the AMH test | 3.14 (2.98–3.30) | 2.39 (2.26–2.53) | <0.001 |
| Positive emotional reactions to information presented | 3.91(3.79–4.03) | 4.70 (4.58–4.83) | <0.001 |
| Negative emotional reactions to information presented | 3.47 (3.33–3.62) | 3.34 (3.20–3.48) | 0.193 |
| Worry about chances getting pregnant | 2.21 (2.13–2.28) | 2.14 (2.07–2.22) | 0.248 |
| Anticipated psychological reaction of having an AMH test | 2.98 (2.92–3.03) | 3.34 (3.29–3.40) | 0.001 |
| Information satisfaction | 3.94 (3.88–4.00) | 3.96 (3.90–4.02) | 0.605 |
|
| |||
| n (%) | n (%) | P-value | |
|
| |||
| Discuss with doctor—indicated ‘Yes’ | 174 (36%) | 254 (53%) | <0.001 |
| Impact on family planning intentions—indicated ‘Yes’ | 211 (44%) | 253 (52%) | 0.008 |
There was also a main effect of country, with women living in Australia having higher interest in having an AMH test than those living in The Netherlands, irrespective of information viewed (MD = 0.36, P = 0.027, 95% CI = 0.04–0.68).
Secondary outcomes
Discuss with doctor (yes/no/don’t know)
When asked if participants would talk to their doctor about getting an AMH test, 174 (36%) in the intervention and 254 (53%) in the control group indicated ‘yes’ (P < 0.001).
Intention to get an AMH test (1 = I definitely will NOT get an AMH test to 5 = I definitely will get an AMH test)
Intention to get an AMH test was statistically lower for those who viewed the evidence-based information (M = 2.84, 95% CI = 2.75–2.93) than those who viewed the control information (M = 3.36, 95% CI = 3.27–3.45; MD = 0.52, P < 0.001, 95% CI = 0.39–0.65).
Attitudes towards the test (1 = not at all to 7 = extremely)
Women who viewed the evidence-based information had less-positive attitudes towards the test (M = 3.96, 95% CI = 3.86–4.06) than women who viewed the control information (M = 5.25, 95% CI = 5.15–5.35; MD = 1.29, P < 0.001, 95% CI = 4.57–5.70). See Table 4 for mean scores on the individual attitude items.
Table 4.
Summary statistics for individual items for attitudes, knowledge, and anticipated actions regarding family planning, by randomized group.
| Attitude items (seven-point scale, higher scores=more positive attitudes) |
Intervention
Mean (SD) |
Control
Mean (SD) |
|---|---|---|
| How beneficial does an AMH test seem to you? | 3.96 (1.79) | 5.28 (1.31) |
| How harmful does an AMH test seem to you? [R] | 5.01 (1.51) | 5.32 (1.46) |
| Do you believe that getting an AMH test will give you reliable information about your fertility? | 3.32 (1.74) | 5.04 (1.23) |
| Do you believe that getting an AMH test will give you important information about your fertility? | 3.55 (1.75) | 5.26 (1.25) |
|
| ||
|
|
|
|
| ||
| 1. The AMH level is an indication of the number of eggs in the ovaries (T) | 345 (71) | 381 (79) |
| 2. The AMH level is an indication of the quality of the eggs in the ovaries (F) | 300 (62) | 225 (46) |
| 3. The AMH test can reliably predict fertility (likelihood of conceiving) (F) | 303 (63) | 95 (20) |
| 4. The AMH test can reliably predict age of menopause (F) | 330 (68) | 177 (37) |
| 5. Oral contraception use does not affect AMH results (F) | 240 (50) | 281 (58) |
|
| ||
| Anticipated actions for those who indicated ‘yes’ to an AMH result influencing their decision on when to start a family |
|
|
|
| ||
| Anticipated action if AMH result was low | ||
| I would bring forward plans to start a family/try and conceive | 124 (59%) | 163 (64%) |
| I would push back plans to start a family/try and conceive | 19 (9%) | 21 (8%) |
| I would reconsider my decision to start a family/try and conceive | 39 (18%) | 32 (13%) |
| I would consider elective egg freezing | 26 (12%) | 36 (14%) |
| Other (please specify) | 3 (1%) | 1 (0.5%) |
| Anticipated action if AMH result was normal or high | ||
| I would bring forward plans to start a family/try and conceive | 63 (41%) | 68 (43%) |
| I would push back plans to start a family/try and conceive | 55 (36%) | 45 (28%) |
| I would reconsider my decision to start a family/try and conceive | 25 (16%) | 28 (18%) |
| I would consider elective egg freezing | 8 (5%) | 14 (9%) |
| Other (please specify) | 2 (1%) | 5 (3%) |
[R], reverse scored; (T), true; (F), false.
Knowledge about the AMH test (true/false/don’t know)
For the knowledge items assessing information that was absent in the information viewed by the control group (items 2–4 regarding egg quality, fertility, and menopause), knowledge scores were consistently higher for those shown the evidence-based information (see Table 4). Overall total knowledge was also significantly higher in women who viewed the evidence-based information (M = 3.14, 95% CI = 2.98–3.30) compared to women who viewed the control information (M = 2.39, 95% CI = 2.26–2.53; MD = 0.75, P ≤ 0.001, 95% CI = 0.71–0.82).
Emotional reaction to the information presented (1 = not at all to 7 = extremely)
When participants were asked how they felt when they read the information about the AMH test, women in the control group reported more positive emotional reactions (i.e. assured, hopeful, relieved; M = 4.70, 95% CI = 4.58–4.83) compared to women who viewed the evidence-based information (M = 3.91, 95% CI = 3.79–4.03; MD = 0.80, P < 0.001, 95% CI = 0.62–0.97). However, there were no differences between groups in terms of negative reactions (i.e. anxious, afraid, worried; MD = 0.13, P = 0.193, 95% CI = −0.34 to 0.07).
Similarly, there were no differences between groups regarding participants’ worry about their chances of getting pregnant (MD = 0.06, P = 0.248, 95% CI = −0.17 to 0.04).
Anticipated psychological reaction of having an AMH test (1 = strongly disagree to 5 = strongly agree; higher scores = more positive anticipated reaction)
When asked how having an AMH test would make them feel, women randomized to the control information anticipated more positive emotions (e.g. more empowered, less anxious, etc) (M = 3.34, 95% CI = 3.29–3.40) than those who viewed the evidence-based information (M = 2.98, 95% CI = 2.92–3.03; MD = 0.37, P = 0.001, 95% CI = 0.29–0.45).
Impact on family planning intentions (yes/no/unsure)
When asked whether getting a low AMH result would influence their decision on when to start a family, those in the control group were more likely to indicate yes (52%, n = 253) than those in the intervention group (44% n = 211; P = 0.008).
For those who indicated ‘yes’, the top action selected for both groups was that they would bring forward their plans to conceive (see Table 4 for frequencies of all anticipated actions).
When asked if getting a normal or high AMH result would influence the decision on when to start a family, women who viewed the evidence-based information (n = 153 indicated ‘yes’; 32%) did not differ from those who viewed the control information (n = 160 indicated ‘yes’; 33%; P = 0.646).
Information satisfaction (1 = strongly disagree to 5 = strongly agree)
The mean total information satisfaction score was relatively high for both the evidence-based information (M = 3.94, SD = 0.72) and the control information (M = 3.96, SD = 0.65), with no statistical difference between groups (P = 0.605, 95% CI = −0.06 to 0.11) (Table 3).
Respondents’ interpretation of the take-home message of the information
When participants were asked what they thought was the take-home message of the information they read (free-text answer), the top three codes from the content analysis for women shown the evidence-based information were that (i) the AMH test was not reliable or helpful (34%, e.g. ‘That it’s not the crystal ball people make it out to be’), (ii) it provides information about quantity of eggs (8%), or (iii) it was only useful when having difficulty conceiving or undergoing fertility treatment (6%; see Table 5 for all codes and illustrative quotes).
Table 5.
Content analysis results for free-text question ‘What do you think is the take-home message of the information you read about the AMH test?’ by randomized group.
| Results for those randomized to the evidence-based information group (N = 483) | ||
|---|---|---|
| Code | Example quote | n (%) |
| Can’t tell you anything about fertility or quality of eggs, is unreliable, not helpful, or inaccurate | ‘AMH is not an accurate test to determine anything in terms of pregnancy chances and eggs quality’ | 165 (34%) |
| Not specific/unclear | ‘To use as a guideline’ | 44 (9%) |
| Can provide information about quantity of eggs | ‘The test is available for those who want to know that amount of eggs they have. It’s an option’ | 39 (8%) |
| Only useful when having difficulties conceiving or if the doctor thinks it’s necessary | ‘It’s a good test for doctors to interpret for IVF and egg freezing procedures. The average person shouldn’t feel the need to take one unless undergoing the following procedures.’ | 29 (6%) |
| Can provide information about fertility (i.e. chances to get pregnant) | ‘It is handy to consider doing the test if you want an idea about what your chances are with having a child’ | 26 (5%) |
| The test exists | ‘There’s a test if you want to find out’ | 23 (5%) |
| Other | ‘I haven’t heard of it, because I’ve never had it done’ | 22 (5%) |
| It’s important to take the test/worth getting | ‘AMH testing is important for women of childbearing age.’ | 19 (4%) |
| Don’t know/unsure | ‘I’m not sure.’ | 17 (4%) |
| Test could increase chances of pregnancy or can improve pregnancy | ‘It helps with pregnancy’ | 10 (2%) |
| People are being misinformed about the test/women need better information | ‘That people are misinformed and there needs to be more information shared about it.’ | 9 (2%) |
| Can provide information about egg quality | ‘AMH will help women understand the amount and quality of their eggs however does not measure fertility and may not work for those with PCOS or taking the pill’ | 8 (2%) |
| Helpful for decision-making re family planning/when to get pregnant | ‘Get tested if you are older, helps with planning a family’ | 8 (2%) |
| Empowerment language, e.g. Be in control, be better informed, be more aware of your fertility |
|
8 (2%) |
| Can give false sense of security, hope, stress, or pressure | ‘It could give women a false sense of security or cause stress or pressure to fall pregnant’ | 8 (2%) |
| I will get it when I’m older/when I’m trying to get pregnant | ‘When I’m a bit older I may get one’ | 4 (0.8%) |
| The only way to test your fertility is to try conceiving/there is no test of fertility | ‘Women shouldn’t rely on AMH tests when deciding on whether to start a family or not as they can’t predict quality of eggs. They can offer some insight for people doing IVF. The only way to get an accurate understanding of fertility is to try to conceive’ | 4 (0.8%) |
| I want more information | ‘I want to get more information on it’ | 4 (0.8%) |
| Informs reproductive timeline/menopause timing | ‘This test provides insight into my fertility and detects the onset of menopause.’ | 3 (0.6%) |
| Good for peace of mind | ‘That it is an option for women that need more reassurance about their fertility.’ | 3 (0.6%) |
| Is not useful when using the pill | ‘That it is for indicating number of eggs in ovaries and not at all a measure of fertility. Furthermore, the results can be affected by oral contraceptives and PCOS’ | 3 (0.6%) |
| Is expensive/costly | ‘don’t waste time or money’ | 2 (0.4%) |
| Results for those randomized to the control group (N = 484) | ||
|---|---|---|
| Code | Example quote | n (%) |
| Can provide information about fertility (i.e. chances to get pregnant) |
|
116 (24%) |
| Can provide information about quantity of eggs |
|
64 (13%) |
| Not specific/unclear | ‘that if you’re unsure there is a test to find out’ | 42 (9%) |
| It’s important to take the test/worth getting | ‘AMH is very important for women of childbearing age to test their own fertility.’ | 36 (7%) |
| The test exists | ‘To be aware that the test exists and it’s an option’ | 34 (7%) |
| Empowerment language, e.g. Be in control, be better informed, be more aware of your fertility | ‘Ability to check if you wish to do so, empowerment to take control of your health a bit more’ | 31 (6%) |
| Helpful for decision-making re family planning/when to get pregnant |
|
26 (5%) |
| Informs reproductive timeline/menopause timing | ‘This is a medical, reliable way to predict fertility. It can assist in decision making regarding timing of starting a family and may indicate beginnings of menopause.’ | 24 (5%) |
| Don’t know/unsure | ‘I’m not sure’ | 18 (4%) |
| Good for peace of mind | ‘To give women peace of mind’ | 15 (3%) |
| Other | ‘It should be advertised more’ | 14 (3%) |
| The test is easy and inexpensive | ‘There is an easy and relatively inexpensive way to check fertility’ | 10 (2%) |
| I will ask my doctor for this test/I should get this test | ‘That it’s probably time I look at organising to get a test done’ | 9 (2%) |
| Can provide information about quality of eggs | ‘It is a test to see how fertile and the quality of a woman’s eggs can be.’ | 7 (1%) |
| I will get it when I’m older/when I’m trying to get pregnant | ‘If your older like myself take the test so you can make informed decisions about starting a family’ | 7 (1%) |
| Can’t tell you anything about fertility or quality of eggs, is unreliable, not helpful, or inaccurate | ‘an indicator, not 100%’ | 7 (1%) |
| Test could increase chances of pregnancy or can improve pregnancy | ‘That is a worthwhile tool to assist women in their pregnancy journeys’ | 7 (1%) |
| The test is safe/not invasive | ‘It’s a really easy, non-invasive way to find out your chances of fertility’ | 6 (1%) |
| Can provide information about a fertility, e.g. PCOS or highlight a potential issue | ‘That having the AMH test can give you reliable information about your fertility, like how many eggs you have left, and also information about possible conditions like PCOS.’ | 5 (1%) |
| I want more information | ‘Would like to find out more’ | 5 (1%) |
| Only useful when having difficulties conceiving or if the doctor thinks it’s necessary | ‘The AMH test provides a clear analysis of a woman’s egg count and is also used in fertility treatments’ | 4 (0.8%) |
The top three codes for the control group were that (i) it provides information about chances of pregnancy (24%, e.g. ‘AMH testing can predict your chances of pregnancy’), (ii) it provides information about quantity of eggs (13%), or (iii) it was important to take the test (7%; see Table 5).
Discussion
Main findings
When supported with co-designed and evidence-based information about the AMH test, women have lower interest in having the test, lower intention to discuss the test with their doctor, less positive attitudes towards the test, and higher knowledge about the test than when commercial advertising of the AMH test is their source of information. Whilst women who viewed the evidence-based information reported less positive emotional reactions to the information (less assured, hopeful, relieved), an expected response to the evidence-base, there were no differences in terms of negative reactions (anxious, afraid, worried).
Comparison with existing literature
These results support findings from previous studies that show a high interest in AMH testing when women are uninformed about the test’s limitations (Azhar et al., 2015; Evans et al., 2018; Hurley et al., 2018; Grootenhuis et al., 2021). Although the mean score for those shown the evidence-based information was under the mid-point on the scale, indicating these women were not interested in AMH testing on average, a substantial proportion of this group was still interested despite being informed of the test’s limitations. This general enthusiasm for medical tests and information may in part be explained by the ingrained cultural and societal beliefs that ‘information is power’ and ‘more is better’, which are recognized as drivers of overdiagnosis and medical overuse (Pathirana et al., 2017). Interestingly, interest in testing was higher in Australian participants, which may reflect higher endorsement of these cultural beliefs than in the Netherlands. An interview study of experts on low-value care highlighted how, in the Netherlands, clinicians are less inclined to provide unnecessary care and patients are more accepting of the idea that testing is not always positive, perhaps due to the lower risk of litigation and their more Calvinistic attitudes compared to other countries (Verkerk et al., 2022). Overall, the findings from the current study indicate that when women are provided with the evidence and informed of the test’s limitations, they are less interested in having an AMH test. This is likely a reflection of the higher knowledge and less positive attitudes towards the test, suggesting that the women who viewed the evidence-based information had a more realistic view about what the test can and cannot do.
Interestingly, there were no differences between groups in terms of attitudes about whether an AMH test could be harmful, despite the intervention materials listing the potential downstream harms of inappropriate testing (i.e. false sense of security, needless worry, and pressure to conceive or undergo unnecessary procedures). This finding aligns with other research on low-value care which shows that people find potential downstream harms of medical overuse difficult to conceptualize (Brownlee and Korenstein, 2021), such as changing family planning behaviour based off an inaccurate test, psychological distress, and other downstream consequences (Korenstein et al., 2021). In the case of a simple blood test, it may be reasonable to presume that it does not cause harm, particularly as possible downstream harms can occur long after an inappropriate test, and may be difficult to trace back to the test itself (Brownlee and Korenstein, 2021). Further, the tendency of people to overestimate the benefits and underestimate the harms of medical interventions has been demonstrated in various medical contexts (Hoffmann and Del Mar, 2015). For example, female infertility patients were equally as willing to use a new hypothetical fertility treatment to improve a proxy or secondary IVF outcome (e.g. fertilization or implantation rate but not live birth rate) whether or not they were told that it may pose risk to themselves/their resulting pregnancy (Carrick et al., 2023).
Concerningly, information satisfaction was high in the control group, suggesting women considered the misleading information trustworthy and balanced. Given the prevalence of misleading information about the AMH test identified online (Copp et al., 2021; Johnson et al., 2023) and the potential harms of inappropriate AMH test use, women urgently need better access to evidence-based information about the AMH test. The information developed as part of this study may be useful for clinicians as well as online service providers in delivering unbiased, factual information to help women make an informed decision about whether to have the AMH test, as well as increase awareness of the test’s limitations. This will likely reduce inappropriate use of the AMH test, as well as any unfounded actions taken in response to the test result.
Strengths and limitations
To our knowledge, this is the first study to co-design and test the efficacy of evidence-based information about AMH test. Other strengths include the randomized design which enabled us to robustly assess the evidence-based information against a typical direct-to-consumer information source accessed by women, and that the trial was conducted among reproductive-aged women who have not yet had children, the target audience of oocyte freezing and AMH test advertising. A limitation of the study is that it was conducted in an online panel sample. It is not possible to know how much participants engaged with the information presented, although minimum time requirements were placed on the intervention and control information pages before participants were able to proceed to the questionnaire to increase the likelihood of them reading the information presented. The sample was also more highly educated than the broader Australian and Dutch populations, and some measures (e.g. influence on family planning) were hypothetical in nature.
Conclusion
When enabled to make an informed decision through the provision of evidence-based information, women have lower interest in having an AMH test, and less positive attitudes towards and higher knowledge of the AMH test. This study has produced crucial, evidence-based information for consumers, which, if adopted, will likely reduce inappropriate use of the AMH test.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the two consumer panels, the clinician advisory group and the participants who gave up their time for this study, as well as Anastasia Vakkas for leading the development of a measure of emotional response to AMH testing, which was adapted in the current study.
Contributor Information
T Copp, Faculty of Medicine and Health, School of Public Health, The University of Sydney, Sydney, NSW, Australia.
T van Nieuwenhoven, Faculty of Health, Medicine and Life Sciences, School of Public Health and Primary Care, Maastricht University, Maastricht, The Netherlands.
K J McCaffery, Faculty of Medicine and Health, School of Public Health, The University of Sydney, Sydney, NSW, Australia.
K Hammarberg, School of Public Health and Preventive Medicine, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia.
E Cvejic, Faculty of Medicine and Health, School of Public Health, The University of Sydney, Sydney, NSW, Australia.
J Doust, Australian Women and Girls’ Health Research Centre, School of Public Health, Faculty of Medicine, The University of Queensland, Brisbane, QLD, Australia.
S Lensen, Department of Obstetrics and Gynaecology, Royal Women’s Hospital, The University of Melbourne, Melbourne, VIC, Australia.
M Peate, Department of Obstetrics and Gynaecology, Royal Women’s Hospital, The University of Melbourne, Melbourne, VIC, Australia.
L Augustine, Faculty of Medicine and Health, School of Public Health, The University of Sydney, Sydney, NSW, Australia.
F van der Mee, Faculty of Health, Medicine and Life Sciences, School of Public Health and Primary Care, Maastricht University, Maastricht, The Netherlands.
B W Mol, Department of Obstetrics and Gynaecology, Monash University, Melbourne, VIC, Australia; Aberdeen Centre for Women’s Health Research, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK.
D Lieberman, City Fertility Centre Pty Ltd, Sydney, NSW, Australia.
J Jansen, Faculty of Health, Medicine and Life Sciences, School of Public Health and Primary Care, Maastricht University, Maastricht, The Netherlands.
Data availability
Data are available on reasonable request.
Authors’ roles
T.C., K.M., and J.J. conceived the study. T.C., T.v.N., E.C., K.J.M., K.H., J.D., S.L., M.P., B.W.M., D.L., and J.J. were involved in designing the study and developing the methods. T.C. and T.v.N. conducted the consumer workshops. T.C. conducted the statistical analyses with input from E.C. T.C., L.A., and F.v.d.M. conducted the content analyses. T.C. drafted the manuscript. All authors critically revised and approved the manuscript.
Funding
NHMRC Emerging Leader Research Fellowship (2009419 to T.C.) and The Australian Health Research Alliance’s Women’s Health Research, Translation and Impact Network EMCR award. The funder had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Conflict of interest
B.W.M. reports consultancy for ObsEva and Merck and travel support from Merck. D.L. is the Medical Director of, and holds stock in, City Fertility NSW and reports consultancy for Organon and honoraria from Ferring, Besins, and Merck. K.H. reports consultancy and travel support from Merck and Organon. K.M. is a director of Health Literacy Solutions that owns a licence of the Sydney Health Literacy Lab Health Literacy Editor. No other relevant disclosures exist.
References
- ACOG Committee Opinion. The use of Antimullerian hormone in women not seeking fertility care. Obstet Gynecol 2019;133:e274–e278. [DOI] [PubMed] [Google Scholar]
- Agency for Clinical Innovation. Co-Design Toolkit: Working as Equals in Leadership, Design and Decision Making. New South Wales (Australia: ): ACI, 2023. [Google Scholar]
- Ayre J, Bonner C, Muscat DM, Dunn AG, Harrison E, Dalmazzo J, Mouwad D, Aslani P, Shepherd HL, McCaffery KJ. Multiple automated health literacy assessments of written health information: development of the SHeLL (Sydney Health Literacy Lab) health literacy editor v1. JMIR Form Res 2023;7:e40645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar E, Seifer DB, Melzer K, Ahmed A, Weedon J, Minkoff H. Knowledge of ovarian reserve and reproductive choices. J Assist Reprod Genet 2015;32:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, Eijkemans MJ, Mol BW, Broekmans FJ; IMPORT Study Group. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update 2013;19:26–36. [DOI] [PubMed] [Google Scholar]
- Brownlee SM, Korenstein D. Better understanding the downsides of low value healthcare could reduce harm. BMJ 2021;372:n117. [DOI] [PubMed] [Google Scholar]
- Carrick M, Wilkinson J, Polyakov A, Kirkham J, Lensen S. How do IVF patients interpret claims about fertility treatments? A randomised survey experiment. Hum Fertil 2023;26:347–354. [DOI] [PubMed] [Google Scholar]
- Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–594. [PubMed] [Google Scholar]
- Copp T, Hersch J, Muscat D, McCaffery K, Doust J, Dokras A, Mol B, Jansen J. The benefits and harms of receiving a polycystic ovary syndrome diagnosis: a qualitative study of women’s experiences. Hum Reprod Open 2019;2019:hoz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp T, Nickel B, Lensen S, Hammarberg K, Lieberman D, Doust J, Mol BW, McCaffery K. Anti-Mullerian hormone (AMH) test information on Australian and New Zealand fertility clinic websites: a content analysis. BMJ Open 2021;11:e046927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp T, Pickles K, Smith J, Hersch J, Johansson M, Doust J, McKinn S, Sharma S, Hardiman L, Nickel B. Marketing empowerment: how corporations co-opt feminist narratives to promote non-evidence based health interventions. BMJ 2024;384:e076710. [DOI] [PubMed] [Google Scholar]
- Copp T, Thompson R, Doust J, Hammarberg K, Peate M, Lensen S, Cvejic E, Lieberman D, Mol BW, McCaffery KJ. Community awareness and use of anti-Müllerian hormone testing in Australia: a population survey of women. Hum Reprod 2023;38:dead111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depmann M, Eijkemans MJC, Broer SL, Tehrani FR, Solaymani-Dodaran M, Azizi F, Lambalk CB, Randolph JF Jr, Harlow SD, Freeman EW et al. Does AMH relate to timing of menopause? Results of an individual patient data meta-analysis. J Clin Endocrinol Metab 2018;103:3593–3600. [DOI] [PubMed] [Google Scholar]
- Dewailly D, Laven J. AMH as the primary marker for fertility. Eur J Endocrinol 2019;181:D45–D51. [DOI] [PubMed] [Google Scholar]
- Dolan H, McCaffery K, Houssami N, Cvejic E, Brennan M, Hersch J, Dorrington M, Verde A, Vaccaro L, Nickel B. Australian Women’s intentions and psychological outcomes related to breast density notification and information: a randomized clinical trial. JAMA Netw Open 2022;5:e2216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A, de Lacey S, Tremellen K. Australians’ understanding of the decline in fertility with increasing age and attitudes towards ovarian reserve screening. Aust J Prim Health 2018;24:428–433. [DOI] [PubMed] [Google Scholar]
- Grootenhuis A, van den Hoogen A, Broekmans F, Torrance H, van Os-Medendorp H, Ockhuijsen H. Young women’s opinions on the use of a blood test to predict the possibility of premature ovarian failure: a qualitative study. Hum Fertil (Camb) 2021;24:304–314. [DOI] [PubMed] [Google Scholar]
- Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2015;177:407–419. [DOI] [PubMed] [Google Scholar]
- Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–1288. [DOI] [PubMed] [Google Scholar]
- Hunt S, Vollenhoven B. Assessment of female fertility in the general practice setting. Aust J Gen Pract 2020;49:304–308. [DOI] [PubMed] [Google Scholar]
- Hurley EG, Ressler IB, Young S, Batcheller A, Thomas MA, DiPaola KB, Rios J. Postponing childbearing and fertility preservation in young professional women. South Med J 2018;111:187–191. [DOI] [PubMed] [Google Scholar]
- Johnson A, Thompson R, Nickel B, Shih P, Hammarberg K, Copp T. Websites selling direct-to-consumer anti-mullerian hormone tests. JAMA Netw Open 2023;6:e2330192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenstein D, Harris R, Elshaug AG, Ross JS, Morgan DJ, Cooper RJ, Cho HJ, Segal JB. To expand the evidence base about harms from tests and treatments. J Gen Intern Med 2021;36:2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Jing M, Zhu W, Tu X, Chen Q, Wang X, Zheng Y, Zhang R. The value of anti-Müllerian hormone in the prediction of spontaneous pregnancy: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12:695157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Davis SR, Kalantaridou S, Lumsden MA, Panay N, Anderson RA. Anti-Müllerian hormone for the diagnosis and prediction of menopause: a systematic review. Hum Reprod Update 2023;29:327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien Y, Kelleher C, Wingfield M. “So what happens next?” exploring the psychological and emotional impact of anti-Mullerian hormone testing. J Psychosom Obstet Gynaecol 2020;41:30–37. [DOI] [PubMed] [Google Scholar]
- Pathirana T, Clark J, Moynihan R. Mapping the drivers of overdiagnosis to potential solutions. BMJ 2017;358:j3879. [DOI] [PubMed] [Google Scholar]
- Petrova D, Cokely ET, Sobkow A, Traczyk J, Garrido D, Garcia‐Retamero R. Measuring feelings about choices and risks: The Berlin Emotional Responses to Risk Instrument (BERRI). Risk Anal 2023;43:724–746. [DOI] [PubMed] [Google Scholar]
- Pickles K, Kazda L, Barratt A, McGeechan K, Hersch J, McCaffery K. Evaluating two decision aids for Australian men supporting informed decisions about prostate cancer screening: a randomised controlled trial. PLoS One 2020;15:e0227304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 2015;103:e9–e17. [DOI] [PubMed] [Google Scholar]
- Prior E, Lew R, Hammarberg K, Johnson L. Fertility facts, figures and future plans: an online survey of university students. Hum Fertil (Camb) 2019;22:283–290. [DOI] [PubMed] [Google Scholar]
- Scherer LD, Valentine K, Patel N, Baker SG, Fagerlin A. A bias for action in cancer screening? J Exp Psychol Appl 2019;25:149–161. [DOI] [PubMed] [Google Scholar]
- Scherer LD, Zikmund-Fisher BJ. Eliciting medical maximizing-minimizing preferences with a single question: development and validation of the MM1. Med Decis Making 2020;40:545–550. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 2010;1:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A, Liew R, Peate M. Age-related fertility decline and elective oocyte cryopreservation: knowledge, attitudes and practices in a pilot study of general practitioners. Aust J Gen Pract 2022;51:611–619. [DOI] [PubMed] [Google Scholar]
- Vakkas A, Lensen S, Copp T, Hammarberg K, Simonis M, Lieberman D, Sandhu S, Peate M. ABSTRACT: exploring the psychosocial and behavioural impact of anti-Mullerian hormone (AMH) testing on women. In: ASPOG (ed). Exploring the Tapestry of Psychosocial Obstetrics and Gynaecology. Adelaide (Australia: ): ASPOG2023, 2023,15. [Google Scholar]
- Verkerk EW, Van Dulmen SA, Born K, Gupta R, Westert GP, Kool RB. Key factors that promote low-value care: views of experts from the United States, Canada, and the Netherlands. Int J Health Policy Manag 2022;11:1514–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.