Abstract
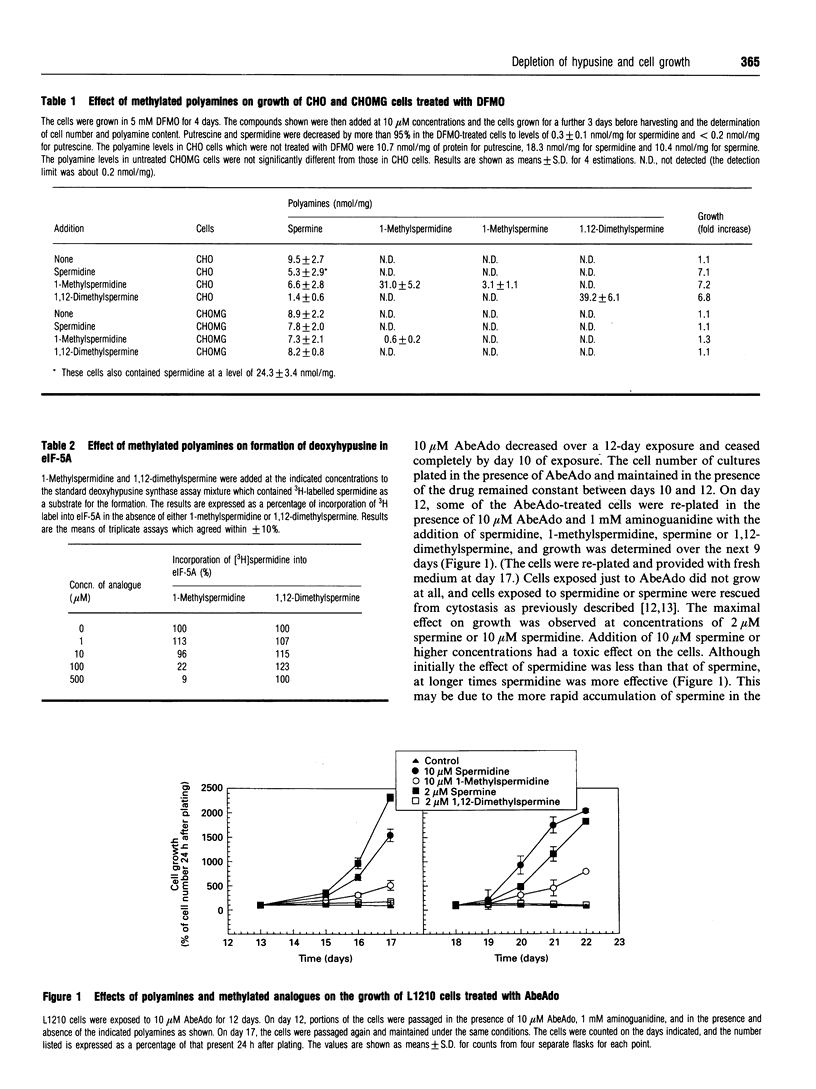
The abilities of the natural polyamines, spermidine and spermine, and of the synthetic analogues, 1-methylspermidine and 1,12-dimethylspermine, to reverse the effects of the S-adenosyl-L-methionine decarboxylase inhibitor 5'-([(Z)-4-aminobut-2-enyl]methylamino)-5'-deoxyadenosine (AbeAdo) on L1210-cell growth were studied. L1210 cells were exposed to AbeAdo for 12 days to induce cytostasis and then exposed to spermidine, spermine, 1-methylspermidine or 1,12-dimethylspermine in the continued presence of AbeAdo. AbeAdo-induced cytostasis was overcome by the natural polyamines, spermidine and spermine. The cytostasis was also reversed by 1-methylspermidine. 1,12-Dimethylspermine had no effect on the AbeAdo-induced cytostasis of chronically treated cells, although it was active in permitting growth of cells treated with the ornithine decarboxylase inhibitor, alpha-difluoromethylornithine. The initial 12-day exposure to AbeAdo elevated intracellular putrescine levels, depleted intracellular spermidine and spermine, and resulted in the accumulation of unmodified eukaryotic translation initiation factor 5A (eIF-5A). Exposure of these cells to exogenous spermidine, which is the natural substrate for deoxyhypusine synthase, resulted in a decrease in the unmodified eIF-5A content. 1-Methylspermidine, which was found to be a substrate of deoxyhypusine synthase in vitro, also decreased the levels of unmodified eIF-5A in the AbeAdo-treated cells. Although spermine is not a substrate of deoxyhypusine synthase, spermine was converted into spermidine in the L1210 cells, and spermine addition to AbeAdo-treated cells resulted in the appearance of both intracellular spermine and spermidine and in the decrease in unmodified eIF-5A. Exogenous 1,12-dimethylspermine, which was not metabolized to spermine or to 1-methylspermidine and was not a substrate of deoxyhypusine synthase in vitro, did not decrease levels of unmodified eIF-5A. The finding that AbeAdo-induced cytostasis was only reversed by polyamines and polyamine analogues that result in the formation of hypusine or an analogue in eIF-5A is consistent with the hypothesis [Byers, Wiest, Wechter and Pegg (1993) Biochem. J. 290, 115-121] that AbeAdo-induced cytostasis is due to the depletion of the hypusine-containing form of eIF-5A, which is secondary to the depletion of spermidine by inhibition of S-adenosyl-L-methionine decarboxylase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbruzzese A., Hanauske-Abel H. M., Park M. H., Henke S., Folk J. E. The active site of deoxyhypusyl hydroxylase: use of catecholpeptides and their component chelator and peptide moieties as molecular probes. Biochim Biophys Acta. 1991 Apr 8;1077(2):159–166. doi: 10.1016/0167-4838(91)90053-3. [DOI] [PubMed] [Google Scholar]
- Abbruzzese A., Park M. H., Folk J. E. Deoxyhypusine hydroxylase from rat testis. Partial purification and characterization. J Biol Chem. 1986 Mar 5;261(7):3085–3089. [PubMed] [Google Scholar]
- Bey P., Bolkenius F. N., Seiler N., Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985 Jan;28(1):1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Bey P., Seiler N. Specific inhibition of polyamine oxidase in vivo is a method for the elucidation of its physiological role. Biochim Biophys Acta. 1985 Jan 28;838(1):69–76. doi: 10.1016/0304-4165(85)90251-x. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. The role of polyamine reutilization in depletion of cellular stores of polyamines in non-proliferating tissues. Biochim Biophys Acta. 1987 Jan 20;923(1):125–135. doi: 10.1016/0304-4165(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Byers T. L., Bush T. L., McCann P. P., Bitonti A. J. Antitrypanosomal effects of polyamine biosynthesis inhibitors correlate with increases in Trypanosoma brucei brucei S-adenosyl-L-methionine. Biochem J. 1991 Mar 1;274(Pt 2):527–533. doi: 10.1042/bj2740527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. L., Ganem B., Pegg A. E. Cytostasis induced in L1210 murine leukaemia cells by the S-adenosyl-L-methionine decarboxylase inhibitor 5'-([(Z)-4-amino-2-butenyl]methylamino)-5'-deoxyadenosine may be due to hypusine depletion. Biochem J. 1992 Nov 1;287(Pt 3):717–724. doi: 10.1042/bj2870717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. L., Pegg A. E. Properties and physiological function of the polyamine transport system. Am J Physiol. 1989 Sep;257(3 Pt 1):C545–C553. doi: 10.1152/ajpcell.1989.257.3.C545. [DOI] [PubMed] [Google Scholar]
- Byers T. L., Wechter R., Nuttall M. E., Pegg A. E. Expression of a human gene for polyamine transport in Chinese-hamster ovary cells. Biochem J. 1989 Nov 1;263(3):745–752. doi: 10.1042/bj2630745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. L., Wiest L., Wechter R. S., Pegg A. E. Effects of chronic 5'-([(Z)-4-amino-2-butenyl]methylamino)-5'-deoxy- adenosine (AbeAdo) treatment on polyamine and eIF-5A metabolism in AbeAdo-sensitive and -resistant L1210 murine leukaemia cells. Biochem J. 1993 Feb 15;290(Pt 1):115–121. doi: 10.1042/bj2900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casero R. A., Jr, Pegg A. E. Spermidine/spermine N1-acetyltransferase--the turning point in polyamine metabolism. FASEB J. 1993 May;7(8):653–661. [PubMed] [Google Scholar]
- Danzin C., Marchal P., Casara P. Irreversible inhibition of rat S-adenosylmethionine decarboxylase by 5'-([(Z)-4-amino-2-butenyl]methylamino)-5'-deoxyadenosine. Biochem Pharmacol. 1990 Oct 1;40(7):1499–1503. doi: 10.1016/0006-2952(90)90446-r. [DOI] [PubMed] [Google Scholar]
- Hershey J. W., Smit-McBride Z., Schnier J. The role of mammalian initiation factor eIF-4D and its hypusine modification in translation. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):160–162. doi: 10.1016/0167-4781(90)90159-y. [DOI] [PubMed] [Google Scholar]
- Jakus J., Wolff E. C., Park M. H., Folk J. E. Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem. 1993 Jun 25;268(18):13151–13159. [PubMed] [Google Scholar]
- Klier H., Wöhl T., Eckerskorn C., Magdolen V., Lottspeich F. Determination and mutational analysis of the phosphorylation site in the hypusine-containing protein Hyp2p. FEBS Lett. 1993 Nov 22;334(3):360–364. doi: 10.1016/0014-5793(93)80712-4. [DOI] [PubMed] [Google Scholar]
- Lakanen J. R., Coward J. K., Pegg A. E. alpha-Methyl polyamines: metabolically stable spermidine and spermine mimics capable of supporting growth in cells depleted of polyamines. J Med Chem. 1992 Feb 21;35(4):724–734. doi: 10.1021/jm00082a013. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Flintoff W. F. Isolation of mutant mammalian cells altered in polyamine transport. J Cell Physiol. 1978 Dec;97(3 Pt 1):335–343. doi: 10.1002/jcp.1040970308. [DOI] [PubMed] [Google Scholar]
- Murphey R. J., Gerner E. W. Hypusine formation in protein by a two-step process in cell lysates. J Biol Chem. 1987 Nov 5;262(31):15033–15036. [PubMed] [Google Scholar]
- Park M. H., Wolff E. C., Folk J. E. Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors. 1993 May;4(2):95–104. [PubMed] [Google Scholar]
- Park M. H., Wolff E. C., Folk J. E. Is hypusine essential for eukaryotic cell proliferation? Trends Biochem Sci. 1993 Dec;18(12):475–479. doi: 10.1016/0968-0004(93)90010-k. [DOI] [PubMed] [Google Scholar]
- Park M. H., Wolff E. C., Smit-McBride Z., Hershey J. W., Folk J. E. Comparison of the activities of variant forms of eIF-4D. The requirement for hypusine or deoxyhypusine. J Biol Chem. 1991 May 5;266(13):7988–7994. [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan S., Quemener V., Moulinoux J. P., Knödgen B., Seiler N. On the degradation and elimination of spermine by the vertebrate organism. Int J Biochem. 1991;23(5-6):617–626. doi: 10.1016/0020-711x(87)90057-7. [DOI] [PubMed] [Google Scholar]
- Schnier J., Schwelberger H. G., Smit-McBride Z., Kang H. A., Hershey J. W. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jun;11(6):3105–3114. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwelberger H. G., Kang H. A., Hershey J. W. Translation initiation factor eIF-5A expressed from either of two yeast genes or from human cDNA. Functional identity under aerobic and anaerobic conditions. J Biol Chem. 1993 Jul 5;268(19):14018–14025. [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985 Jan 1;225(1):219–226. doi: 10.1042/bj2250219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Rennert O. M. Interconversion, catabolism and elimination of the polyamines. Med Biol. 1981 Dec;59(5-6):334–346. [PubMed] [Google Scholar]
- Seiler N. Functions of polyamine acetylation. Can J Physiol Pharmacol. 1987 Oct;65(10):2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- Smit-McBride Z., Dever T. E., Hershey J. W., Merrick W. C. Sequence determination and cDNA cloning of eukaryotic initiation factor 4D, the hypusine-containing protein. J Biol Chem. 1989 Jan 25;264(3):1578–1583. [PubMed] [Google Scholar]
- Smit-McBride Z., Schnier J., Kaufman R. J., Hershey J. W. Protein synthesis initiation factor eIF-4D. Functional comparison of native and unhypusinated forms of the protein. J Biol Chem. 1989 Nov 5;264(31):18527–18530. [PubMed] [Google Scholar]
- Wolff E. C., Park M. H., Folk J. E. Cleavage of spermidine as the first step in deoxyhypusine synthesis. The role of NAD. J Biol Chem. 1990 Mar 25;265(9):4793–4799. [PubMed] [Google Scholar]
- van den Berg G. A., Nagel G. T., Muskiet F. A., Halie M. R. Mass fragmentographic identification of polyamine metabolites in the urine of normal persons and cancer patients, and its relevance to the use of polyamines as tumour markers. J Chromatogr. 1985 May 3;339(2):223–231. doi: 10.1016/s0378-4347(00)84649-6. [DOI] [PubMed] [Google Scholar]


