Abstract
STUDY QUESTION
Does vitrification cryopreservation of embryos for more than 5 years affect the pregnancy outcomes after frozen embryo transfer (FET)?
SUMMARY ANSWER
Vitrification cryopreservation of good-quality blastocysts for more than 5 years is associated with a decrease in the implantation rate (IR) and live birth rate (LBR).
WHAT IS KNOWN ALREADY
Previous studies have predominantly focused on embryos cryopreserved for relatively short durations (less than 5 years), yet the impact of extended cryopreservation duration on pregnancy outcomes remains a controversial issue. There is a relative scarcity of data regarding the efficacy and safety of storing embryos for 5 years or longer.
STUDY DESIGN, SIZE, DURATION
This retrospective study involved 36 665 eligible vitrified-thawed embryo transfer cycles from 1 January 2016 to 31 December 2022, at a single fertility center in China.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Patients were divided into three groups according to embryo storage time: Group 1 consisted of 31 565 cycles, with storage time of 0–2 years; Group 2 consisted of 4458 cycles, with a storage time of 2–5 years; and Group 3 included 642 cycles, with storage time exceeding 5 years. The main outcome measures were IR and LBR. Secondary outcome variables included rates of biochemical pregnancy, multiple pregnancy, ectopic pregnancy, and miscarriage, as well as neonatal outcomes. Reproductive outcomes were analyzed as binary variables. Multivariate logistic regression analysis was used to explore the effect of preservation time on pregnancy outcomes after correcting for confounding factors. In addition, we also assessed neonatal outcomes, such as large for gestational age (LGA) and small for gestational age (SGA).
MAIN RESULTS AND THE ROLE OF CHANCE
IRs in the three groups (0–2, 2–5, and >5 years) were 37.37%, 39.03%, and 35.78%, respectively (P = 0.017), and LBRs in the three groups were 37.29%, 39.09%, and 34.91%, respectively (P = 0.028). After adjustment for potential confounding factors, compared with the 0–2 years storage group, prolonged embryo vitrification preservation time (2–5 years or >5 years) did not affect secondary outcomes such as rates of biochemical pregnancy, multiple pregnancy, ectopic pregnancy, and miscarriage (P > 0.05). But cryopreservation of embryos for more than 5 years reduced the IR (adjusted odds ratio (aOR) 0.82, 95% CI 0.69–0.97, P = 0.020) and LBR (aOR 0.76, 95% CI 0.64–0.91, P = 0.002). Multivariate stratified analysis also showed that prolonging the cryopreservation time of blastocysts (>5 years) reduced the IR (aOR 0.78, 95% CI 0.62–0.98, P = 0.033) and LBR (aOR 0.68, 95% CI 0.53–0.87, P = 0.002). However, no effect on cleavage embryos was observed (P > 0.05). We further conducted stratified analyses based on the number and quality of frozen blastocysts transferred, and the results showed that the FET results after transfers of good-quality blastocysts in the >5 years storage group were negatively affected. However, the storage time of non-good-quality blastocysts was not significantly associated with pregnancy outcomes. Regarding the neonatal outcomes (of singletons), embryo vitrification preservation time had no effect on preterm birth rates, fetal birth weight, or neonatal sex ratios. However, as the storage time increased, rates of SGA (5.60%, 4.10%, and 1.18%) decreased, while rates of LGA (5.22%, 6.75%, and 9.47%) increased (P < 0.05). After adjusting for confounding factors, the increase in LGA and the decrease in SGA were significantly correlated with the duration of storage time.
LIMITATIONS, REASONS FOR CAUTION
This was a retrospective study using data from a single fertility center, even though the data had been adjusted, our findings still need to be validated in further studies.
WIDER IMPLICATIONS OF THE FINDINGS
With the full implementation of the two-child policy in China, there may be more patients whose embryos have been frozen for a longer time in the future. Patients should be aware that the IR and LBR of blastocysts are negatively affected when the cryopreservation time is longer than 5 years. Couples may therefore consider shortening the time until FET treatment.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the National Nature Science Foundation of China (No. 82101672), Science and Technology Projects in Guangzhou (No. 2024A03J0180), General Guidance Program for Western Medicine of Guangzhou Municipal Health Commission (No. 20231A011096), and the Medical Key Discipline of Guangzhou (2021–2023). None of the authors have any conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: frozen embryo transfer, blastocysts, vitrification, storage time, live birth rate, implantation rate, neonatal outcomes
Introduction
IVF technology has a long developmental history, and embryo cryopreservation is an important step. Slow freezing (program freezing) technology was first used for embryo cryopreservation, while vitrification freezing was first applied in China in 2003 and fully implemented in 2012 (Zhu et al., 2005; Shi et al., 2012).
In 2016, China officially implemented a universal two-child policy, and so many patients wanted to transfer their embryos that had been preserved in the early years of vitrification technology. However, the high concentrations of cryoprotectants used in vitrification as well as the open system that directly exposes embryos to liquid nitrogen during storage may cause damage or osmotic toxicity in embryos that have been cryopreserved for a long time. Animal experiments on the long-term preservation of vitrified frozen embryos have shown variable results in terms of in vitro and in vivo embryo survival ability. The experimental results of mice showed that the rates of cryosurvival, fertilization, and embryonic development decreased with the extension of cryopreservation time (Yan et al., 2011), but the experimental results of bovine (Fang et al., 2014) or porcine (Sanchez-Osorio et al., 2010) embryos showed no significant effect. Given the significant interspecies physiological differences, although these animal models are referentially valuable for human, their predictive efficacy is somewhat limited for clinical applications. Although some clinical studies have evaluated the impact of the duration of embryo vitrification cryopreservation on results of frozen embryo transfer (FET), most have focused on embryos stored for less than 5 years. Some studies have shown that the duration of embryo cryopreservation does not affect the pregnancy outcome (Li et al., 2017; Ueno et al., 2018); however, other studies have shown that prolonging the vitrification preservation duration has a negative impact on pregnancy outcomes (Li et al., 2020; Zhang et al., 2021; Mao et al., 2022). A small-sample propensity score matching study suggested that if the duration of embryo cryopreservation is more than 5 years, the implantation rate (IR) and live birth rate (LBR) may be significantly reduced with the extension of freezing time (Cui et al., 2021). Nearly all studies with a storage duration exceeding 5 years have small sample sizes or are case reports, which limits the availability of data on the efficiency and safety of long-term embryo preservation. The lack of clinical evidence and exact guidelines makes the issue of embryo vitrification preservation extremely controversial. Therefore, this study aimed to explore the effect of a longer vitrification embryo preservation time (>5 years) on embryo pregnancy outcomes, such as IR and LBR, and to determine a safe vitrification embryo preservation time.
Materials and methods
Study population and design
A retrospective cohort study was conducted at a single fertility center from 1 January 2016 to 31 December 2022, including all women who had experienced at least one freeze-thaw cycle. The embryos of these patients were vitrified after 2012. The exclusion criteria were non-vitrification, pre-implantation genetic testing, uterine malformation, frozen oocytes, repeated vitrification-warming embryos, two-step transfers, incomplete information, and loss to follow-up. Finally, 36 665 cycles were included in the analysis. To investigate the effect of cryopreservation time on pregnancy outcomes, we divided the 36 665 cycles into three groups based on the embryo storage time: Group 1, stored for 0–2 years; Group 2, stored for 2–5 years; and Group 3, stored for more than 5 years. The study protocol was approved by the ethics committee of the Third Affiliated Hospital of Guangzhou Medical University, and written informed consent was obtained from each participant.
ART procedures
The women were monitored and managed according to the clinical protocols of the hospital. Ovarian stimulation protocols use recombinant follicle-stimulating hormone or human menopausal gonadotropin at 150–450 IU/day, and from a variety of protocols (short-term agonist regimens, long-term agonist regimens, and gonadotropin-releasing hormone antagonist regimens), and the characteristics of each patient (age, BMI, antral follicle count, and anti-Müllerian hormone) were used to determine which regimen was selected. Transvaginal oocyte retrieval was performed 35–36 h after hCG injection. ART was performed according to standard hospital operating procedures.
The choice of conventional IVF or ICSI was determined according to semen conditions and fertilization history. Embryos were incubated individually in 25 µl droplets of G-1 Plus™ medium (Vitrolife) under Ovoil (Vitrolife) and incubated at 37°C in an atmosphere of 6% CO2 and 5% O2. Day 3 embryos were transferred to G-2 Plus™ medium (Vitrolife) until Day 5/6. Fertilization assessment was performed 16–18 h after routine fertilization/injection, and embryo quality was assessed on Days 3, 5, and 6 after insemination.
A good-quality embryo on the third day was generally defined as an embryo derived from a two pronuclei zygote, with 7–9 blastomeres of equal size, <25% fragmentation, and no multinucleation. A good-quality blastocyst was generally defined as a blastocyst of stage 3 or above in the Gardner scoring criteria, with the inner cell mass and trophectoderm scores not including a C grade.
Embryo cryopreservation techniques and transfer protocols
The selection of embryos for freezing was based on the developmental stage of the embryo as well as the embryo count. Embryo freezing and thawing were performed according to the protocol of the Vitrification Kit (Kitazato Corp., Japan). For embryo freezing, cleavage-stage embryos were exposed to equilibration solution (ES) for 5 min at room temperature or blastocysts were exposed to ES for 2 min at 37°C. Embryos were then transferred to a vitrification solution and incubated for 45–60 s. Finally, small-volume embryos were transferred to Cryotop strips (Kitazato Corp., Tokyo, Japan) and immediately placed in liquid nitrogen. Embryos were stored in liquid nitrogen at −196°C with continuous manual monitoring. Each Cryotop strip in which embryos were stored contained 1–2 embryos.
During embryo thawing, embryos removed from the vehicle were first rapidly immersed in thawing solution (37°C) for 1 min and transferred to diluent for 3 min at room temperature and then transferred to washing solution for another 3 min (performed twice). All thawed embryos were cultured in a medium at 37°C in an incubator in the gas phase of 5% CO2 and 5% O2 until transfer. Embryos with less than 50% degraded cells were considered viable and could be transferred, whereas embryos with more than 50% degraded cells were considered inactive and were discarded.
The laboratory freezing and thawing procedures, embryo cryopreservation protocols, technical equipment, and storage tanks used were the same throughout the study.
Endometrial preparation for FET cycle and embryo transfer
According to the menstrual cycle and ovulation status of the patient, endometrial preparation for the FET cycle was determined based on whether the patient was going to use a natural or an artificial cycle. In patients with natural cycles, follicular development and ovulation were assessed using transvaginal ultrasonography and hormone levels. For patients with artificial cycles, estradiol valerate tablets (Progynova, Bayer, Germany) were administered orally daily, starting on Day 3 or 4 of menstruation. Once daily intramuscular progesterone (40 mg) was required when endometrial thickness was ≥7 mm. Ultrasound-guided embryo transfer was performed on Days 3 (cleavage-stage embryos) or 5 (blastocysts) after ovulation or on Days 4 (cleavage-stage embryos) or 6 (blastocysts) after progesterone exposure. All patients who underwent embryo transfer received progesterone as a post-transfer luteal support. Transvaginal ultrasound was performed 4–6 weeks after embryo transfer, and luteal support was continued until 10 weeks of gestation if the gestational sac and embryo heartbeat were detected.
Clinical outcomes
The primary outcomes were IR and LBR. The secondary outcome variables included rates of biochemical pregnancy, multiple pregnancies, ectopic pregnancy, and miscarriage, as well as neonatal outcomes. The embryo IR was calculated by dividing the number of gestational sacs detected using transvaginal ultrasonography (performed 28 days after embryo transfer) by the number of embryos transferred. A live birth was defined as a live baby delivered after 24 weeks of pregnancy. Biochemical pregnancy was defined as a diagnosis of pregnancy based on elevated serum hCG levels detected 14 days after embryo transfer but without a gestational sac. Multiple pregnancies were defined as the presence of multiple intrauterine fetuses simultaneously. Ectopic pregnancy was diagnosed using ultrasound or laparoscopic imaging of at least one ectopic pregnancy sac. Miscarriage was defined as the loss of fetal cardiac activity within 28 weeks of confirming clinical pregnancy. Only singletons were included to evaluate the relationship between storage time and neonatal outcomes. Neonatal outcomes included preterm birth (<37 weeks of gestation), small for gestational age (SGA; birth weight <10th percentile), large for gestational age (LGA; birth weight >90th percentile), appropriate for gestational age (AGA; birth weight between 10th and 90th percentile), newborn sex (male/female), and congenital defects.
Statistical analysis
All data were statistically analyzed using SPSS 22.0 for Windows (IBM, Armonk, NY, USA). Continuous variables were presented as the mean ± SD and analyzed by ANOVA test. Categorical variables are presented as counts and percentages and were compared using the chi-square or Fisher’s exact test, as appropriate. P-values <0.05 were considered statistically significant.
To investigate the effect of embryo storage time on pregnancy and neonatal outcomes, multivariable logistic regression analysis was conducted. Reproductive outcomes, including implantation, live birth, biochemical pregnancy, multiple pregnancies, ectopic pregnancy and miscarriage, and neonatal outcomes, including preterm birth, LGA, AGA, and SGA, were treated as binary variables. The potential confounding factors were adjusted in the models according to univariate analysis, and variables with P-value <0.10 were included in the multivariate regression model. Confounding factors included maternal age at oocyte retrieval, maternal BMI, type of infertility, main causes of infertility, number of oocytes retrieved, fertilization method, number of embryos transferred, development stage and quality of embryo transferred, number of previous embryos transferred, endometrial preparation method, and endometrial thickness. Considering that advanced maternal age significantly increases the risk of adverse reactions in newborns, we adjusted the age of FET. The storage duration group was included as a categorical variable, and Group 1 was used as the reference. The adjusted odds ratios (aOR) and 95% CI were calculated.
Results
A total of 36 665 cycles were included in our study, and the patients were divided into three groups according to embryo storage time: Group 1 consisted of 31 565 cycles, with storage time of 0–2 years; Group 2 consisted of 4458 cycles, with storage time of 2–5 years; and Group 3 included 642 cycles with storage time >5 years.
The demographic and clinical characteristics of the vitrified embryo transfer cycles are provided in Table 1. The mean age at oocyte retrieval among the patients included in the study was 32.31 ± 4.91 years, the mean age at FET was 33.16 ± 4.85 years, the mean BMI was 22.13 ± 3.18 kg/m2, and the average embryo cryopreservation time was 0.85 ± 1.24 years, with the longest preservation time being 9.5 years. With the longer storage times, the maternal age at oocyte retrieval and maternal BMI decreased, the mean number of oocytes retrieved increased, and the maternal age at FET increased (P < 0.001). The proportion with primary infertility and female infertility also increased with longer storage times (P < 0.001). IVF was the main fertilization method among the three groups. With longer storage time, there was an increase in the proportion of patients who transferred non-good-quality embryos, who had more than two transfers, and who had already given birth (P < 0.001). There was a significant difference in the number of patients receiving transfers of blastocysts rather than cleavage stage embryos among the three groups. In addition, there were differences in the distribution of endometrial preparation methods and endometrial thickness among the three groups (P < 0.001). There was no significant difference in the number of transferred embryos among the three groups (P > 0.05). Clinical parameters, including the IR (37.37%, 39.03%, and 35.78%, P = 0.017), LBR (37.29%, 39.09%, and 34.91%, P = 0.028), and multiple pregnancy rate (18.84%, 21.54%, and 22.11%, P = 0.005) showed statistically significant differences between the three groups. However, the survival rate (98.15%, 98.09%, and 97.86%), biochemical pregnancy rate (3.68%, 3.72%, and 3.79%), ectopic pregnancy rate (1.43%, 1.67%, and 1.40%), and miscarriage rate (18.83%, 17.50%, and 20.00%) were not significantly different among the three groups (P > 0.05).
Table 1.
Demographic and clinical characteristics of vitrified embryo transfer cycles.
| Variable | All cycles | Group 1 | Group 2 | Group 3 | P-value |
|---|---|---|---|---|---|
| 0–2 years | 2–5 years | >5 years | |||
| Number of thawing cycles, n | 36 665 | 31 565 | 4458 | 642 | |
| Number of frozen embryos transferred cycles, n (%) | 36 432 (99.36%) | 31 386 (99.43%) | 4413 (98.99%) | 633 (98.60%) | |
| Maternal age at oocyte retrieval (years) | 32.31 ± 4.91 | 32.67 ± 4.95 | 30.13 ± 3.97 | 29.38 ± 3.62 | <0.001 |
| <35 | 26 432 (72.55%) | 21 933 (69.88%) | 3912 (88.65%) | 587 (92.73%) | <0.001 |
| 35–37 | 5022 (13.78%) | 4627 (14.74%) | 359 (8.14%) | 36 (5.69%) | |
| ≥38 | 4978 (13.66%) | 4826 (15.38%) | 142 (3.22%) | 10 (1.58%) | |
| Maternal age at FET (years) | 33.16 ± 4.85 | 33.08 ± 4.97 | 33.36 ± 3.99 | 35.41 ± 3.69 | <0.001 |
| Storage time by vitrification (years) | 0.85 ± 1.24 | 0.41 ± 0.39 | 3.23 ± 0.78 | 6.03 ± 0.91 | <0.001 |
| Maternal BMI (kg/m2) | 22.13 ± 3.18 | 22.19 ± 3.20 | 21.78 ± 2.99 | 21.69 ± 3.02 | <0.001 |
| Type of infertility, n (%) | <0.001 | ||||
| Primary | 18 363 (50.40%) | 15 470 (49.29%) | 2538 (57.51%) | 355 (56.08%) | |
| Secondary | 18 069 (49.60%) | 15 916 (50.71%) | 1875 (42.49%) | 278 (43.92%) | |
| Main causes of infertility, n (%) | <0.001 | ||||
| Female factor | 19 057 (52.31%) | 16 046 (51.12%) | 2585 (58.58%) | 426 (67.30%) | |
| Male factor | 6204 (17.03%) | 5434 (17.31%) | 681 (15.43%) | 89 (14.06%) | |
| Male and female factors | 9258 (25.41%) | 8113 (25.85%) | 1034 (23.43%) | 111 (17.54%) | |
| Unexplained | 1913 (5.25%) | 1793 (5.71%) | 113 (2.56%) | 7 (1.11%) | |
| Number of oocytes retrieved | 15.15 ± 8.55 | 14.80 ± 8.60 | 17.14 ± 7.80 | 18.49 ± 8.04 | <0.001 |
| Fertilization method, n (%) | <0.001 | ||||
| IVF | 28 859 (79.21%) | 24 753 (78.87%) | 3575 (81.01%) | 531 (83.89%) | |
| ICSI | 6486 (17.80%) | 5668 (18.06%) | 719 (16.29%) | 99 (15.64%) | |
| IVF + ICSI | 1087 (2.98%) | 965 (3.07%) | 119 (2.70%) | 3 (0.47%) | |
| Number of embryos transferred, n (%) | 0.185 | ||||
| 1 | 18 620 (51.11%) | 16 101 (51.30%) | 2206 (49.99%) | 313 (49.45%) | |
| ≥2 | 17 812 (48.89%) | 15 285 (48.70%) | 2207 (50.01%) | 320 (50.55%) | |
| Development stage of embryo transferred, n (%) | <0.001 | ||||
| Cleavage embryo | 10 630 (29.18%) | 9543 (30.41%) | 948 (21.48%) | 139 (21.96%) | |
| Extended culture of cleavage embryos | 2388 (6.55%) | 1591 (5.07%) | 617 (13.98%) | 180 (28.44%) | |
| Blastocyst | 23 414 (64.27%) | 20 252 (64.53%) | 2848 (64.54%) | 314 (49.61%) | |
| Good-quality embryos transferred, n (%) | <0.001 | ||||
| 0 | 9408 (25.82%) | 7982 (25.43%) | 1226 (27.78%) | 200 (31.60%) | |
| 1 | 19 115 (52.47%) | 16 679 (53.14%) | 2159 (48.92%) | 277 (43.76%) | |
| 2 | 7909 (21.71%) | 6725 (21.43%) | 1028 (23.29%) | 156 (24.64%) | |
| Number of previous embryo transferred, n (%) | <0.001 | ||||
| 0 | 23 160 (63.57%) | 21 834 (69.57%) | 1171 (26.54%) | 155 (24.49%) | |
| 1 | 9030 (24.79%) | 7039 (22.43%) | 1756 (39.79%) | 235 (37.12%) | |
| ≥2 | 4242 (11.64%) | 2513 (8.01%) | 1486 (33.67%) | 243 (38.39%) | |
| Delivery achieved during this IVF/ICSI cycle | <0.001 | ||||
| Yes | 2366 (6.49%) | 273 (0.87%) | 1733 (39.27%) | 360 (56.87%) | |
| No | 34 066 (93.51%) | 31 113 (99.13%) | 2680 (60.73%) | 273 (43.13%) | |
| Endometrial preparation method, n (%) | <0.001 | ||||
| Natural | 11 038 (30.30%) | 9254 (29.48%) | 1568 (35.53%) | 216 (34.12%) | |
| Artificial | 22 475 (61.69%) | 19 538 (62.25%) | 2563 (58.08%) | 374 (59.08%) | |
| Others | 2919 (8.01%) | 2594 (8.26%) | 282 (6.39%) | 43 (6.79%) | |
| Thickness of the endometrium (mm) | 8.84 ± 1.60 | 8.81 ± 1.59 | 9.02 ± 1.68 | 8.80 ± 1.52 | <0.001 |
| Survival rate (%) | 58 300/59 406 (98.14%) | 49 112 (98.15%) | 7859/8012 (98.09%) | 1329/1358 (97.86) | 0.698 |
| Implantation rate (%) | 20 366/54 244 (37.55%) | 17 441/46 671 (37.37%) | 2584/6620 (39.03%) | 341/953 (35.78) | 0.017 |
| Biochemical pregnancy, n (%) | 1342 (3.68%) | 1154 (3.68%) | 164 (3.72%) | 24 (3.79%) | 0.981 |
| Live birth, n (%) | 13 651 (37.47%) | 11 705 (37.29%) | 1725 (39.09%) | 221 (34.91%) | 0.028 |
| Multiple pregnancy, n (%) | 3329 (19.23%) | 2802 (18.84%) | 464 (21.54%) | 63 (22.11%) | 0.005 |
| Ectopic pregnancy, n (%) | 253 (1.46%) | 213 (1.43%) | 36 (1.67%) | 4 (1.40%) | 0.695 |
| Miscarriage, n (%) | 3235 (18.68%) | 2801 (18.83%) | 377 (17.50%) | 57 (20.00%) | 0.285 |
FET, frozen embryo transfer.
Multivariate logistic regression analysis was performed after controlling for confounding factors. The impact of vitrification preservation time on pregnancy outcomes is shown in Table 2. In general, compared to the 0–2 years storage group, extending the vitrification preservation time (2–5 years or >5 years) of embryos did not influence the risk of secondary outcomes, including biochemical pregnancy, multiple pregnancy, ectopic pregnancy, and miscarriage rates (P > 0.05). However, compared to the 0–2 years storage group, a preservation time of more than 5 years reduced the IR (aOR 0.82, 95% CI 0.69–0.97, P = 0.020) and the LBR (aOR 0.76, 95% CI 0.64–0.91, P = 0.002).
Table 2.
Multivariate logistic regression analysis of the impact of storage time on pregnancy outcomes.
| P-value | aOR (95% CI) | |
|---|---|---|
| Implantation rate | ||
| 0–2 years | ref. | |
| 2–5 years | 0.225 | 0.96 (0.89–1.03) |
| >5 years | 0.020 | 0.82 (0.69–0.97) |
| Live birth rate | ||
| 0–2 years | ref. | |
| 2–5 years | 0.052 | 0.93 (0.87, 1.00) |
| >5 years | 0.002 | 0.76 (0.64, 0.91) |
| Biochemical pregnancy rate | ||
| 0–2 years | ref. | |
| 2–5 years | 0.353 | 1.09 (0.91, 1.29) |
| >5 years | 0.527 | 1.14 (0.75, 1.73) |
| Multiple pregnancy rate | ||
| 0–2 years | ref. | |
| 2–5 years | 0.786 | 1.02 (0.89, 1.17) |
| >5 years | 0.465 | 1.14 (0.81, 1.59) |
| Ectopic pregnancy rate | ||
| 0–2 years | ref. | |
| 2–5 years | 0.07 | 1.12 (0.99, 1.27) |
| >5 years | 0.314 | 1.17 (0.86, 1.61) |
| Miscarriage rate | ||
| 0–2 years | ref. | |
| 2–5 years | 0.324 | 1.07 (0.94, 1.21) |
| >5 years | 0.089 | 1.31 (0.96, 1.8) |
We further conducted a stratified logistic regression analysis based on the stages of embryo transfer (Table 3), and the results showed that frozen storage time did not affect the pregnancy outcomes of cleavage stage embryos, whether the transfer was straight after thawing or after extended culture after thawing. However, the frozen storage time of blastocysts for more than 5 years was negatively associated with the IR (aOR 0.78, 95% CI 0.62–0.98, P = 0.033) and LBR (aOR 0.68, 95% CI 0.53–0.87, P = 0.002). We further conducted a stratified analysis of blastocysts based on factors that clearly affect IR and LBR, including transfer number and blastocyst quality. Statistical differences in IR and LBR were found in some, but not all, subgroups (Table 4). In general, the stratified analysis results showed that the LBR was significantly decreased in the group with a cryopreservation time greater than 5 years, whether it was a single (aOR 0.71, 95% CI 0.50–0.99, P = 0.047) or double (aOR 0.60, 95% CI 0.42–0.85, P = 0.004) blastocyst transfer. Good-quality blastocysts stored for more than 5 years were negatively affected in terms of the IR (aOR 0.74, 95% CI 0.58–0.95, P = 0.020) and LBR (aOR 0.70, 95% CI 0.53–0.93, P = 0.013). However, for non-good-quality blastocysts, there was no significantly association between storage times and pregnancy outcomes.
Table 3.
Pregnancy outcomes and adjusted odds ratio after stratification analyses based on development stage of vitrified embryos transfer.
| Implantation rate | P-value | aOR (95% CI) | Live birth rate | P-value | aOR (95% CI) | |
|---|---|---|---|---|---|---|
| Cleavage embryo (n = 10 630) | 4899/17 567 (27.89%) | 3065/10 630 (28.83%) | ||||
| 0–2 years | 4262/15 663 (27.21%) | ref. | 2681/9543 (28.09%) | ref. | ||
| 2–5 years | 558/1654 (33.74%) | 0.433 | 1.06 (0.92–1.22) | 334/948 (35.23%) | 0.395 | 0.94 (0.81–1.09) |
| >5 years | 79/250 (31.60%) | 0.102 | 0.74 (0.52–1.06) | 50/139 (35.97%) | 0.414 | 0.86 (0.60–1.24) |
| Extended culture of cleavage embryos (n = 2388) | 1252/3871 (32.3%) | 802/2388 (33.58%) | ||||
| 0–2 years | 814/2757 (29.52%) | ref. | 493/1591 (30.99%) | ref. | ||
| 2–5 years | 349/877 (39.79%) | 0.538 | 0.93 (0.74–1.17) | 245/617 (39.71%) | 0.483 | 1.08 (0.87–1.35) |
| >5 years | 89/237 (37.55%) | 0.650 | 0.92 (0.65–1.31) | 64/180 (35.56%) | 0.843 | 1.04 (0.73–1.48) |
| Blastocyst (n = 23 414) | 14 215/32 977 (43.11%) | 9784/23 414 (41.79%) | ||||
| 0–2 years | 12 365/28 251 (43.77%) | ref. | 8531/20 252 (42.12%) | ref. | ||
| 2–5 years | 1677/4089 (41.01%) | 0.088 | 0.93 (0.85–1.01) | 1146/2848 (40.24%) | 0.156 | 0.94 (0.86–1.02) |
| >5 years | 173/464 (37.28%) | 0.033 | 0.78 (0.62–0.98) | 107/314 (34.08%) | 0.002 | 0.68 (0.53–0.87) |
Table 4.
Pregnancy outcomes and adjusted odds ratio based on the number and quality of vitrified blastocyst transplantation.
| Implantation rate | P-value | aOR (95% CI) | Live birth rate | P-value | aOR (95% CI) | |
|---|---|---|---|---|---|---|
| Number of transplanted blastocysts | ||||||
| Single blastocyst transferred (n = 14 024) | 6989/14 024 (49.84%) | 5590/14 024 (39.86%) | ||||
| 0–2 years | 6157/12 253 (50.25%) | ref. | 4929/12 253 (40.23%) | ref. | ||
| 2–5 years | 755/1607 (46.98%) | 0.620 | 1.03 (0.92–1.16) | 606/1607 (37.71%) | 0.108 | 0.91 (0.80–1.02) |
| >5 years | 77/164 (46.95%) | 0.997 | 0.99 (0.76–1.38) | 55/164 (33.54%) | 0.047 | 0.71 (0.50–0.99) |
| Double blastocyst transferred (n = 9390) | 7226/18 780 (38.48%) | 4194/9390 (44.66%) | ||||
| 0–2 years | 6208/15 998 (38.80%) | ref. | 3602/7999 (45.03%) | ref. | ||
| 2–5 years | 922/2482 (37.15%) | 0.098 | 0.90 (0.79–1.02) | 540/1241 (43.51%) | 0.223 | 0.92 (0.81–1.05) |
| >5 years | 96/300 (32.00%) | 0.008 | 0.64 (0.46–0.89) | 52/150 (34.67%) | 0.004 | 0.60 (0.42–0.85) |
| Quality of transplanted blastocysts | ||||||
| Non-good-quality (n = 4511) | 1751/6327 (27.68%) | 1130/4511 (25.05%) | ||||
| 0–2 years | 1516/5438 (27.88%) | ref. | 971/3858 (25.17%) | ref. | ||
| 2–5 years | 213/825 (25.82%) | 0.153 | 0.86 (0.70–1.06) | 144/604 (23.84%) | 0.135 | 0.85 (0.68–1.05) |
| >5 years | 22/64 (34.38%) | 0.238 | 1.44 (0.79–2.63) | 15/49 (30.61%) | 0.562 | 1.20 (0.64–2.25) |
| At least one good-quality (n = 18 903) | 12 464/26 477 (47.07%) | 8654/18 903 (45.78%) | ||||
| 0–2 years | 10 849/22 813 (47.56%) | ref. | 7560/16 394 (46.11%) | ref. | ||
| 2–5 years | 1464/3264 (44.85%) | 0.434 | 0.96 (0.88–1.06) | 1002/2244 (44.65%) | 0.704 | 1.02 (0.91–1.15) |
| >5 years | 151/400 (37.75%) | 0.020 | 0.74 (0.58–0.95) | 92/265 (34.72%) | 0.013 | 0.70 (0.53–0.93) |
The neonatal outcomes of singletons after vitrified embryo transfer are shown in Table 5. The results showed that the vitrification preservation times of the embryos did not affect the preterm birth rates, fetal birth weight, or newborn sex ratio. However, compared to the 0–2 years storage group, the gestational age of the 2–5 years storage group and >5 years storage group was significantly reduced (38.33 ± 1.82 weeks vs 38.07 ± 1.70 weeks and 37.53 ± 2.23 weeks, P < 0.001). As the storage time increased, the incidence of LGA was significantly increased (5.22%, 6.75%, and 9.47%, respectively; P < 0.01) and the incidence of SGA was significantly decreased (5.60%, 4.10%, and 1.18%, respectively; P < 0.01). After adjusting for a number of confounding factors, the increase of LGA and the decrease of SGA were significantly associated with the duration of storage.
Table 5.
Neonatal outcomes of singleton live births after vitrified embryo transfer cycles.
| Variable | All cycles | Group 1 | Group 2 | Group 3 | P-value | Group 2 vs Group 1 |
Group 3 vs Group 1 |
||
|---|---|---|---|---|---|---|---|---|---|
| 0–2 years | 2–5 years | >5 years | aOR(95% CI) | P-value | aOR(95% CI) | P-value | |||
| Single birth live babies, n (%) | 10 759 | 9272 | 1318 | 169 | |||||
| Gestational age (weeks) | 38.29 ± 1.82 | 38.33 ± 1.82 | 38.07 ± 1.7 | 37.53 ± 2.23 | <0.001 | ||||
| Preterm birth, n (%) | 920 (8.55%) | 788 (8.50%) | 114 (8.65%) | 18 (10.65%) | 0.606 | 0.93 (0.75–1.16) | 0.529 | 1.15 (0.69–1.92) | 0.601 |
| Birth weight (g) | 3214.13 ± 513.80 | 3218.06 ± 512.74 | 3219.51 ± 479.04 | 3199.02 ± 574.36 | 0.861 | ||||
| LGA, n (%) | 589 (5.47%) | 484 (5.22%) | 89 (6.75%) | 16 (9.47%) | 0.005 | 1.38 (1.09–1.75) | 0.009 | 2.00 (1.17–3.43) | 0.012 |
| AGA, n (%) | 9595 (89.18%) | 8269 (89.18%) | 1175 (89.15%) | 151 (89.35%) | 0.997 | 0.97 (0.80–1.19) | 0.800 | 0.98 (0.59–1.61) | 0.928 |
| SGA, n (%) | 575 (5.34%) | 519 (5.60%) | 54 (4.10%) | 2 (1.18%) | 0.004 | 0.69 (0.52–0.92) | 0.013 | 0.20 (0.05–0.80) | 0.023 |
| Newborn gender, n (%) | 0.908 | ||||||||
| Male | 5834 (54.22%) | 5033 (54.28%) | 708 (53.72%) | 93 (55.03%) | 1.03 (0.91–1.17) | 0.653 | 1.16 (0.84–1.60) | 0.354 | |
| Female | 4925 (45.78%) | 4239 (45.72%) | 610 (46.28%) | 76 (44.97%) | |||||
| Congenital defects, n (%) | 70 (0.65%) | 65 (0.60%) | 5 (0.38%) | 0 | |||||
LGA, large for gestational age; AGA, appropriate for gestational age; SGA, small for gestational age.
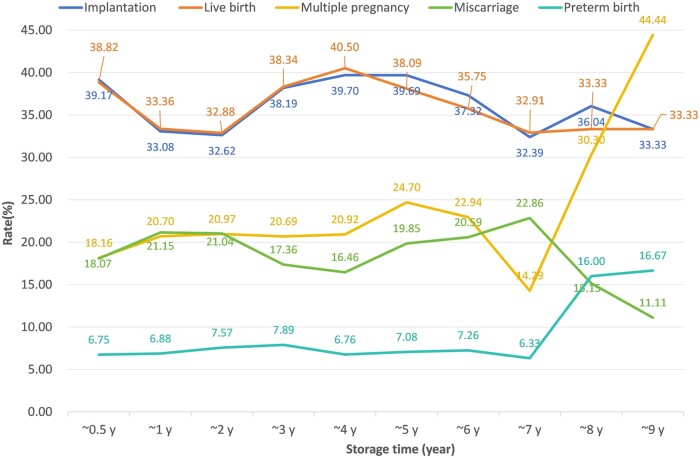
Figure 1 shows a line graph of storage time and clinical outcomes. When the storage time exceeded five years, IR and LBR gradually decreased with the duration of embryo preservation. The IR and LBR with 2–5 years storage time were similar to those stored for <0.5 years, while the IR and LBR with a 1–2 years of storage time were lower than those stored for <0.5 years.
Figure 1.
Line chart showing various clinical outcome indicators changing with embryo storage time. The horizontal axis represents the storage time of frozen-thawed embryos in FET cycles, while the vertical axis indicates the percentage of clinical indicators corresponding to each time interval, including the implantation and live birth rates, as well as the multiple pregnancy, miscarriage, and preterm birth rates.
Discussion
Principal findings
This is a comprehensive assessment of the relationship between vitrified embryo cryopreservation time and clinical outcomes, exploring whether long-term embryo preservation poses additional risks to pregnancy and neonatal outcomes after FET. Our study found that when the vitrification cryopreservation time of blastocysts was longer than 5 years, the IR and LBR of blastocyst transfer decrease, while long-term cryopreservation of cleavage-stage embryos had no effect on IR and LBR. In the neonatal outcomes of singleton live births, as the cryopreservation time increased, the rates of SGA decreased while LGA rates increased.
Results and research implications
To obtain the first fetus, most individuals undergo embryo transfer within 2 years of oocyte retrieval. With the full implementation of the two-child policy in China, many individuals with early frozen embryos flocked to hospitals, hoping to achieve their desire to give birth again through FET. The first child of these individuals may be 2 years old or older, indicating that the remaining embryos were frozen for at least 2 years, with some frozen for 5 years or longer. Previous studies have shown that prolonged cryopreservation times not only increase chromosomal aberrations in embryos (Mozdarani and Moradi, 2007; Yan et al., 2011), but also allow embryos to undergo irreversible damage caused by ionizing radiation and cosmic rays (Chen et al., 2000). At low temperatures, enzymes cannot repair this damage, which may lead to DNA breakage or other harmful forms of DNA damage in the embryos. In addition, racemization of amino acids accumulated during long-term cryopreservation and DNA detoxification can lead to cell damage (Kennedy et al., 1994). Therefore, to provide patients with safe and satisfactory FET results, it is necessary to understand the effect of embryo freezing times exceeding 2 years or even 5 years on pregnancy and newborn outcomes.
Almost all studies on human embryo vitrification have been conducted within a cryopreservation period of less than 2 years (Li et al., 2020; Zhang et al., 2021; Mao et al., 2022) or 5 years (Li et al., 2017; Ueno et al., 2018), and the impact on reproductive outcomes is not consistent. In addition, clinical pregnancy and LBRs are influenced by double embryo transfer, and therefore IRs can better reflect the potential of embryos. Therefore, it was necessary to conduct a comprehensive analysis of long-term embryo freezing, including implantation and LBRs, as well as known factors that affect clinical outcomes, such as embryo stage, number, and quality, in order to better solve problems in clinical practice. Our study included 36 665 freeze-thaw cycles, aiming to evaluate the effect of vitrification preservation time on cleavage-stage embryos and blastocysts, in order to provide clinical evidence.
We studied embryos that had been cryopreserved for more than 5 years, and found that when the embryo cryopreservation time exceeded 5 years, IR and LBR decreased significantly. However, IR and LBR did not decrease in the 2–5 years storage group, which is different from the results of previous studies showing that clinical outcomes decreased with the extension of embryo cryopreservation time (Li et al., 2020; Hu et al., 2022; Mao et al., 2022). This may be related to the short duration of embryo cryopreservation (mostly within 2 years) in previous studies, which included patients who underwent their first vitrified embryo transfer following a freeze-all strategy or fresh transfer. If these patients failed to undergo their first transfer or to conceive within 1 year, it indicates that other medical factors may be present that are more likely to reduce clinical outcomes than extended cryopreservation (1–2 years). This is consistent with our line chart, where the IR and LBR of embryos frozen for 1–2 years decreased compared to those of embryos frozen within 1 year. The IR and LBR of embryos frozen for 2–5 years increased to levels similar to those frozen for 0.5 years. Therefore, a survey questionnaire can be considered to confirm the reasons for delayed transfer in patients.
We found that with prolonged cryopreservation times, the proportion of patients undergoing their first transfer significantly decreased (69.57%, 26.54%, and 24.49%, respectively) (Table 1). This difference may be due to the different proportion of patients who had already given birth among the three groups. Only 0.87% of patients in the <2 years storage group had already given birth through a previous transfer, while 39.27% and 56.87% of patients in the 2–5 and >5 years storage groups had already given birth and wished to conceive again (Table 1). Therefore, successful transfer and the need for a second child led to long-term freezing and subsequent thawing of the embryos. We adjusted for multiple confounding factors, including the number of previous embryos transferred, and still found that long-term freezing for >5 years storage time would reduce IR and LBR.
Embryonic stage has been documented to affect the LBR (Zhang et al., 2021; Glujovsky et al., 2022). Considering the possibility of confounding factors affecting the results, hierarchical logistic regression analyses were performed. Previous studies had shown that there was no significant difference in FET results after long-term cryopreservation of cleavage stage embryos, but they were all small-scale studies (Li et al., 2017, 2023) or only analyzed the preservation time within 2 years (Zhang et al., 2021). Our research indicates that there is no significant difference in IR and LBR for cleavage stage embryo transfer after long-term cryopreservation. This may be related to changes in clinical practice. Prior to 2018, embryologists selected multiple high-quality day-3 embryos for cryopreservation in order to transfer two times, and the surplus embryos from the same batch were frozen after blastocyst culture. After 2018, embryologists only guaranteed a maximum of one opportunity for day-3 embryo transfer or freezing, and the surplus embryos or all embryos were cultured into blastocysts and then frozen. When thawing, blastocysts were preferred, so this clinical change may have led to the existence of potential residual confounding factors. In addition, some patients undergo extended culture of their cleavage embryos after thawing to further screen the embryos, resulting in changes in clinical outcomes.
After stratifying the embryonic stages, we found a significant negative association between cryopreservation of blastocyst-stage embryos for more than 5 years and pregnancy outcomes, especially for good-quality blastocysts. The conclusions of most studies on the cryopreservation time of blastocysts have been inconsistent. Some studies have shown that long-term cryopreservation does not affect clinical outcomes, but the sample size was very small (Li et al., 2023), closed carriers were used, and confounding factors were not adjusted for Wirleitner et al. (2013), or a large time span of 2–7 years was used as the long-term cryopreservation group (Ueno et al., 2018; Ma et al., 2023). On the contrary, other studies have suggested that long-term cryopreservation of vitrified blastocysts might have a negative impact on pregnancy outcomes, but there were still some biases, such as small sample size (Zheng et al., 2023) and preservation times within 2 years (Zhang et al., 2021). The theoretical risk of vitrification is the use of very high concentrations of cryoprotectants, which may lead to osmotic damage and biochemical cytotoxicity (Nagy et al., 2020). Secondly, during long-term cryopreservation, the viability of embryos may be affected by temperature fluctuations and radiation. Improper maintenance of the cryo-tanks, failure to maintain sufficient liquid nitrogen levels, repeated opening of the cryo-tanks, or movement of the samples, may cause the embryo may experience transient warming during these processes, which may affect the developmental potential of the embryos. Although the incidence rate is low, these risks may increase with the extension of frozen storage time. In addition, studies have shown that vitrification freezing can reduce mouse embryo viability and increase chromosomal aberrations, depending on the duration of cryopreservation (Mozdarani and Moradi, 2007; Yan et al., 2011). Another study suggests that repeated fluctuations in temperature to −80°C can lead to a significant decrease in the number, activity, and metabolic characteristics of placental pluripotent stromal cells after thawing, as well as an increase in apoptosis (Pogozhykh et al., 2017).
It is worth noting that we observed a significant increase in the rate of multiple pregnancies due to the transfer of more than one embryo in patients who were at least 5 years older than when their embryos were frozen. This may be related to the decrease in LBRs. Therefore, we performed a stratification on the number of transferred embryos to further analyze the impact of long-term cryopreservation on LBRs. We found that the LBR was still significantly decreased in patients who were transferred with a single blastocyst that had been cryopreserved for over 5 years. In addition, compared to embryos frozen for 5 years (line chart in Fig. 1), the LBR of embryos frozen for 6–7 years was also decreased, even without an increase in the rate of multiple pregnancies. This might exclude the potentially confounding probability of the multiple pregnancy per se on the LBR. Although patients with an embryo storage period of 8–9 years showed a high multiple pregnancy rate, premature birth rate, and low LBR, the number of patients in these categories was relatively small and therefore further research is needed. These results may indicate the potential risks of long-term cryopreservation of the blastocysts.
Consistent with previous research findings, our study showed that, compared to a preservation time of less than 2 years, a preservation time of 2–5 years had no effect on the pregnancy outcomes of cleavage embryos (Li et al., 2017), nor did it affect the pregnancy outcomes of blastocysts (Ma et al., 2023). This also indicates that vitrification cryopreservation of embryos or blastocysts for less than 5 years is relatively safe. The decline in fecundity is attributed to a lower oocyte yield and, most importantly, higher oocyte aneuploidy rates in older women (Franasiak et al., 2014). There is no doubt that long-term preservation can reduce the negative impact of increased age at oocyte retrieval on pregnancy outcomes and improve cumulative outcomes (Loreti et al., 2024). Therefore, when providing medical consultation to patients, it is necessary to inform them that cryopreservation of embryos for more than 5 years is more effective compared to an increase in oocyte retrieval age, but it is still recommended to thaw and transfer embryos as soon as possible within 5 years to achieve a live birth, rather than relying on medical technology to delay childbirth.
At present, there is limited data on the impact of cryopreservation time on perinatal outcomes, mainly focusing on average gestational age and weight, and it is believed that it will not affect neonatal outcomes (Li et al., 2017; Ma et al., 2023). Similar to these studies, our results showed no significant differences in the preterm birth rates, birth weight, or newborn sex ratio among the groups. However, it is noteworthy that the 2–5 years storage group and the >5 years storage group had an increased incidence of LGA and decreased incidence of SGA compared with the 0–2 years storage group, which was used as the control group. This is consistent with previous studies (Cui et al., 2021; He et al., 2023). The underlying mechanisms of these changes are still largely unclear and speculated to be related to modifications in the embryonic epigenome (Ishihara et al., 2014), which requires further research. Interestingly, we also found that the gestational age of newborns in the 2–5 years storage group and the >5 years storage group decreased with the duration of embryo cryopreservation compared with the 0–2 years storage group, but the average gestational age still exceeded 37 weeks (Ueno et al., 2018).
This study has several advantages. Firstly, due to the large sample size, it reports results with a storage time of >5 years, providing more evidence for the safety of long-term storage. Secondly, this study included as many known factors as possible related to pregnancy outcomes to ensure a more objective analysis. In addition, the stage, number, and quality of embryos transferred have been shown to be associated with FET outcomes (Zhu et al., 2020). This study conducted a comprehensive stratified analysis based on these factors to further explore the impact of cryopreservation time on reproductive outcomes in different subgroups.
However, there are also some limitations. Firstly, owing to the retrospective nature and acquisition of data from a single fertility center, inevitable selection bias persists. Secondly, although the sample size of the cryopreserved embryo group over a longer time span is larger compared to previous studies, it remains relatively small, which affects further stratified analysis, such as combining the number and quality of blastocysts. Thirdly, although the freezing and thawing procedures in the laboratory have not changed, personnel turnover may have an impact on the outcome. Lastly, we did not conduct a long-term follow-up of children born after FET treatment, limiting our ability to provide more convincing evidence regarding potential effects on offspring development.
Conclusion
In conclusion, the duration of vitrification preservation did not affect the IR and LBR of cleavage stage embryos, however, good-quality blastocyst vitrification preservation for >5 years was associated with a lower IR and LBR. It is important to note that this study still has some limitations that warrant cautious interpretation.
Acknowledgements
The authors thank all the doctors, embryologists, and nurses in the Department of Reproductive Medicine, Third Affiliated Hospital of Guangzhou Medical University, and particularly thank Dr Li Li for revising the format of the manuscript.
Contributor Information
Shaoquan Zhan, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Chenxing Lin, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Qiwang Lin, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Jiayu Gan, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Chunyan Wang, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Yang Luo, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Jianqiao Liu, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Hongzi Du, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Hanyan Liu, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, The People’s Republic of China.
Data availability
The data will be available on reasonable request to the corresponding author.
Authors’ roles
S.Z. and C.L. analyzed the data and drafted the manuscript; Q.L. selected the data and revised the manuscript; J.G., C.W., and Y.L. selected the data and provided critical feedback on the content; J.L. revised the manuscript and participated in the interpretation of results and discussion; H.D. and H.L. designed the study and revised the manuscript. All authors approved the final version of the manuscript for publication.
Funding
National Nature Science Foundation of China (82101672); Science and Technology Projects in Guangzhou (2024A03J0180); General Guidance Program for Western Medicine of Guangzhou Municipal Health Commission (20231A011096); Medical Key Discipline of Guangzhou (2021–2023).
Conflict of interest
None declared.
References
- Chen SU, Lien YR, Chen HF, Chao KH, Ho HN, Yang YS. Open pulled straws for vitrification of mature mouse oocytes preserve patterns of meiotic spindles and chromosomes better than conventional straws. Hum Reprod 2000;15:2598–2603. [DOI] [PubMed] [Google Scholar]
- Cui M, Dong X, Lyu S, Zheng Y, Ai J. The impact of embryo storage time on pregnancy and perinatal outcomes and the time limit of vitrification: a retrospective cohort study. Front Endocrinol (Lausanne) 2021;12:724853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Zeng S, Fu X, Jia B, Li S, An X, Chen Y, Zhu S. Developmental competence in vitro and in vivo of bovine IVF blastocyst after 15 years of vitrification. Cryo Letters 2014;35:232–238. [PubMed] [Google Scholar]
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 2014;101:656–663.e1. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Quinteiro Retamar AM, Alvarez Sedo CR, Ciapponi A, Cornelisse S, Blake D. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2022;5:CD002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Jiang R, Ren X, Jin L, Jiang Y. The safety of human embryos following long-term cryopreservation (>6 years) on vitrification. Cryo Letters 2023;44:178–184. [PubMed] [Google Scholar]
- Hu KL, Hunt S, Zhang D, Li R, Mol BW. The association between embryo storage time and treatment success in women undergoing freeze-all embryo transfer. Fertil Steril 2022;118:513–521. [DOI] [PubMed] [Google Scholar]
- Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril 2014;101:128–133. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Reader SL, Swierczynski LM. Preservation records of micro-organisms: evidence of the tenacity of life. Microbiology (Reading) 1994;140 (Pt 10): 2513–2529. [DOI] [PubMed] [Google Scholar]
- Li J, Yin M, Wang B, Lin J, Chen Q, Wang N, Lyu Q, Wang Y, Kuang Y, Zhu Q. The effect of storage time after vitrification on pregnancy and neonatal outcomes among 24 698 patients following the first embryo transfer cycles. Hum Reprod 2020;35:1675–1684. [DOI] [PubMed] [Google Scholar]
- Li W, Zhao W, Xue X, Zhang S, Zhang X, Shi J. Influence of storage time on vitrified human cleavage-stage embryos froze in open system. Gynecol Endocrinol 2017;33:96–99. [DOI] [PubMed] [Google Scholar]
- Li X, Guo P, Blockeel C, Li X, Deng L, Yang J, Li C, Lin M, Wu H, Cai G et al. Storage duration of vitrified embryos does not affect pregnancy and neonatal outcomes after frozen-thawed embryo transfer. Front Endocrinol (Lausanne) 2023;14:1148411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti S, Darici E, Nekkebroeck J, Drakopoulos P, Van Landuyt L, De Munck N, Tournaye H, De Vos M. A 10-year follow-up of reproductive outcomes in women attempting motherhood after elective oocyte cryopreservation. Hum Reprod 2024;39:355–363. [DOI] [PubMed] [Google Scholar]
- Ma Y, Sun M, Wen T, Ding C, Liu LW, Meng T, Song J, Hou X, Mai Q, Xu Y. Storage time does not influence pregnancy and neonatal outcomes for first single vitrified high-quality blastocyst transfer cycle. Reprod Biomed Online 2023;47:103254. [DOI] [PubMed] [Google Scholar]
- Mao Y, Tang N, Luo Y, Yin P, Li L. Effects of vitrified cryopreservation duration on IVF and neonatal outcomes. J Ovarian Res 2022;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdarani H, Moradi SZ. Effect of vitrification on viability and chromosome abnormalities in 8-cell mouse embryos at various storage durations. Biol Res 2007;40:299–306. [PubMed] [Google Scholar]
- Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril 2020;113:241–247. [DOI] [PubMed] [Google Scholar]
- Pogozhykh D, Pogozhykh O, Prokopyuk V, Kuleshova L, Goltsev A, Blasczyk R, Mueller T. Influence of temperature fluctuations during cryopreservation on vital parameters, differentiation potential, and transgene expression of placental multipotent stromal cells. Stem Cell Res Ther 2017;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Osorio J, Cuello C, Gil MA, Parrilla I, Almiñana C, Caballero I, Roca J, Vazquez JM, Rodriguez-Martinez H, Martinez EA. In vitro postwarming viability of vitrified porcine embryos: effect of cryostorage length. Theriogenology 2010;74:486–490. [DOI] [PubMed] [Google Scholar]
- Shi W, Xue X, Zhang S, Zhao W, Liu S, Zhou H, Wang M, Shi J. Perinatal and neonatal outcomes of 494 babies delivered from 972 vitrified embryo transfers. Fertil Steril 2012;97:1338–1342. [DOI] [PubMed] [Google Scholar]
- Ueno S, Uchiyama K, Kuroda T, Yabuuchi A, Ezoe K, Okimura T, Okuno T, Kobayashi T, Kato K. Cryostorage duration does not affect pregnancy and neonatal outcomes: a retrospective single-centre cohort study of vitrified-warmed blastocysts. Reprod Biomed Online 2018;36:614–619. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Vanderzwalmen P, Bach M, Baramsai B, Neyer A, Schwerda D, Schuff M, Spitzer D, Stecher A, Zintz M et al. The time aspect in storing vitrified blastocysts: its impact on survival rate, implantation potential and babies born. Hum Reprod 2013;28:2950–2957. [DOI] [PubMed] [Google Scholar]
- Yan J, Suzuki J, Yu XM, Qiao J, Kan FW, Chian RC. Effects of duration of cryo-storage of mouse oocytes on cryo-survival, fertilization and embryonic development following vitrification. J Assist Reprod Genet 2011;28:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu S, Hao G, Wu X, Ren H, Zhang Y, Yang A, Bi X, Bai L, Zhang Y et al. Prolonged cryopreservation negatively affects embryo transfer outcomes following the elective freeze-all strategy: a multicenter retrospective study. Front Endocrinol (Lausanne) 2021;12:709648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Mo M, Zhang H, Xu S, Xu F, Wang S, Zeng Y. Prolong cryopreservation duration negatively affects pregnancy outcomes of vitrified-warmed blastocyst transfers using an open-device system: A retrospective cohort study. Eur J Obstet Gynecol Reprod Biol 2023;281:68–75. [DOI] [PubMed] [Google Scholar]
- Zhu GJ, Jin L, Zhang HW, Li YF, Wei YL, Hu J. [Vitrification of human cleaved embryos in vitro fertilization-embryo transfer]. Zhonghua Fu Chan Ke Za Zhi 2005;40:682–684. [PubMed] [Google Scholar]
- Zhu Q, Lin J, Gao H, Wang N, Wang B, Wang Y. The association between embryo quality, number of transferred embryos and live birth rate after vitrified cleavage-stage embryos and blastocyst transfer. Front Physiol 2020;11:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available on reasonable request to the corresponding author.