Abstract
Deer management (e.g., reduction) has been proposed as a tool to reduce the acarological risk of Lyme disease. There have been few opportunities to investigate Ixodes scapularis (blacklegged tick) and Borrelia burgdorferi sensu stricto dynamics in the absence of white-tailed deer (Odocoileus virginianus) in midwestern North America. A pair of islands in Lake Michigan presented a unique opportunity to study the role of alternative hosts for the adult stage of the blacklegged tick for maintaining a tick population as a deer herd exists on North Manitou Island but not on South Manitou Island, where coyotes (Canis latrans) and hares (Sylvilagus lepus) are the dominant medium mammals. Additionally, we were able to investigate the maintenance of I. scapularis and B. burgdorferi in small mammal communities on both islands, which were dominated by eastern chipmunks (Tamias striatus). From 2011 to 2015, we surveyed both islands for blacklegged ticks by drag cloth sampling, bird mist netting, and small and medium-sized mammal trapping. We assayed questing ticks, on-host ticks, and mammal biopsies for the Lyme disease pathogen, B. burgdorferi. We detected all three life stages of the blacklegged tick on both islands. Of the medium mammals sampled, no snowshoe hares (Lepus americanus, 0/23) were parasitized by adult blacklegged ticks, but 2/2 coyotes (Canis latrans) sampled on South Manitou Island in 2014 were parasitized by adult blacklegged ticks, suggesting that coyotes played a role in maintaining the tick population in the absence of deer. We also detected I. scapularis ticks on passerine birds from both islands, providing support that birds contribute to maintaining as well as introducing blacklegged ticks and B. burgdorferi to the islands. We observed higher questing adult and nymphal tick densities, and higher B. burgdorferi infection prevalence in small mammals and in adult ticks on the island with deer as compared to the deer-free island. On the islands, we also found that 25% more chipmunks were tick-infested than mice, fed more larvae and nymphs relative to their proportional abundance compared to mice, and thus may play a larger role compared to mice in the maintenance of B. burgdorferi. Our investigation demonstrated that alternative hosts could maintain a local population of blacklegged ticks and an enzootic cycle of the Lyme disease bacterium in the absence of white-tailed deer. Thus, alternative adult blacklegged tick hosts should be considered when investigating deer-targeted management tools for reducing tick-borne disease risk, especially when the alternative host community may be abundant and diverse.
Keywords: Ixodes scapularis, Borrelia burgdorferi sensu stricto, island, Lyme disease, white-tailed deer, eastern chipmunk
Introduction
Lyme disease (LD) is the most commonly reported vector-borne disease of humans in Michigan and the United States (MDHHS, 2016; Schwartz et al., 2017). In North America, LD is caused primarily by the bacterium Borrelia burgdorferi sensu stricto (hereafter B. burgdorferi), which is transmitted in eastern North America by the blacklegged tick, Ixodes scapularis (Schwartz et al., 2017). Lyme disease is a growing public health problem as blacklegged tick populations continue to expand, and the number of human cases continues to increase nationally (Mead, 2015).
Deer management (e.g., population reduction) has been proposed as a method to reduce the acarological risk for LD (Telford III, 2002) as white-tailed deer (Odocoileus virginianus) serve as the main host for adult blacklegged ticks in heavily Lyme endemic northern temperate forests (Piesman et al., 1979). Deer population reduction is a controversial topic, and more data are needed to evaluate its effectiveness. Experiments manipulating deer populations can be logistically and socially difficult or infeasible (Garrott, 1995; Messmer et al., 1997). Because other vertebrates may serve as alternative hosts when deer populations are reduced (Kugeler et al., 2016), we assessed whether other hosts could sustain populations of I. scapularis on an island without deer and compared it with another island with similar ecology and a deer population. The two islands that were studied included, North Manitou Island (NMI), which had a deer herd, and South Manitou Island (SMI) which did not.
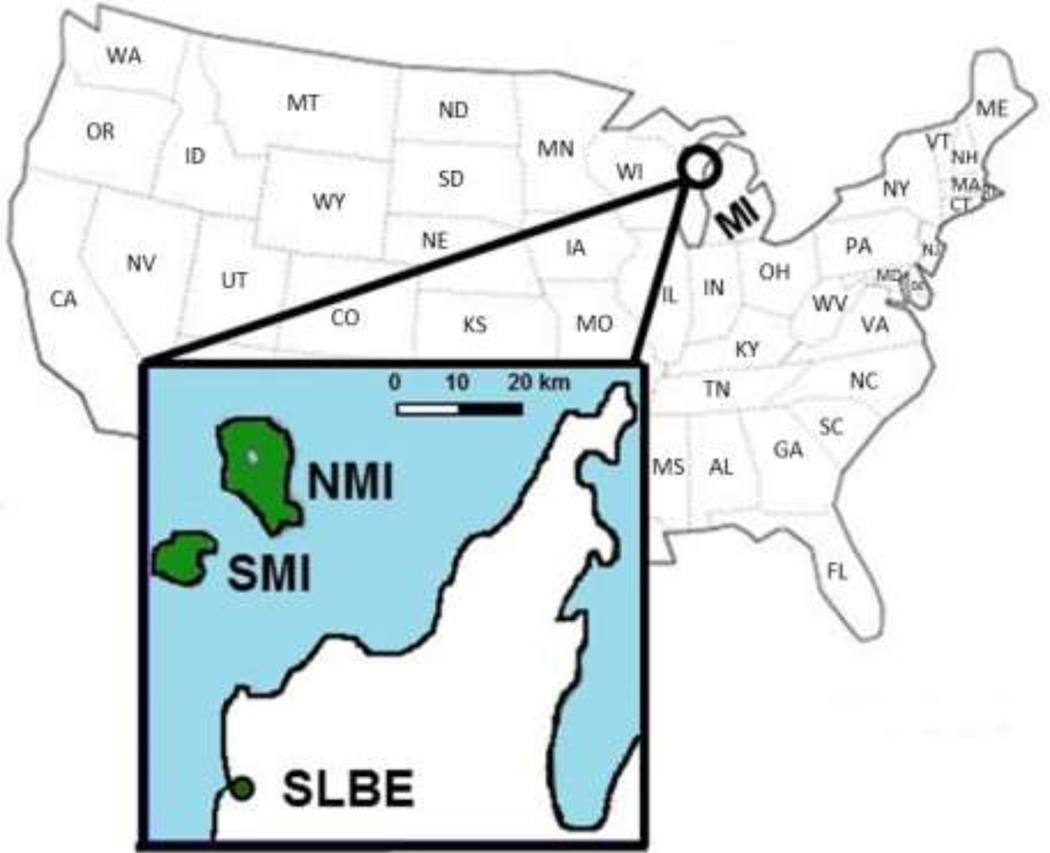
North and South Manitou Islands are a part of Sleeping Bear Dunes National Lakeshore located in Michigan’s northwest Lower Peninsula (Figure 1). The blacklegged tick and LD pathogen emerged in southwestern Michigan in the early 2000’s (Foster, 2004), and Sleeping Bear Dunes was at the leading edge of the expansion along the Lake Michigan shoreline in 2009 (Hamer et al., 2010; Figure 1). It was therefore surprising when a deer hunter reported abundant blacklegged ticks on NMI in the fall of 2010 to the Michigan Department of Health and Human Services. Historically, no ticks had been reported by park staff or visitors (personal communication with National Park Service staff), although surveillance for ticks had not been conducted on the Manitou Islands.
Figure 1. Study sites:
The inset shows two Lake Michigan islands, North Manitou Island (NMI) and South Manitou Island (SMI). The islands are part of the Sleeping Bear Dunes National Lakeshore, which is in the northwestern Lower Peninsula of Michigan.
In 1926, humans introduced deer to NMI, but deer were never introduced to SMI (National Park Service, 2016a). If deer were detected on SMI (e.g., as a consequence of swimming from NMI or walking from NMI during the winter), they were removed. Other known vertebrate wildlife on both islands were similar and comprised many species known to feed blacklegged immature ticks and maintain the LD pathogen, including white-footed mice (Peromyscus leucopus) and eastern chipmunks (Tamias striatus) (National Park Service, 2018) which we now refer to simply as ‘mice’ and ‘chipmunks.’ Mice are more abundant than chipmunks at sites in the Midwest and the Northeast where LD is endemic (Slajchert et al., 1997; Mather et al., 1989; Hamer et al., 2010; Sidge, 2016). However, anecdotal reports received prior to this investigation suggested the opposite may be true on the islands with more chipmunks present than mice. The only two medium-sized mammal species inhabiting the islands during the study were snowshoe hares (Lepus americanus) and coyotes (Canis latrans) (National Park Service, 2018; Sleeping Bear Dunes National Lakeshore, 2000).
Between 2011 and 2015, we investigated the ecology of blacklegged ticks and B. burgdorferi on the islands. Our objectives and predictions were:
To determine whether a population of blacklegged ticks and enzootic cycle of B. burgdorferi can be maintained on an island without deer. We predicted that adventitious nymphal and adult ticks could be present due to migrating birds, but no larval ticks would be detected due to the lack of deer (Elias et al., 2011). If alternative hosts could sustain a tick population, then larvae would be detected.
To determine and compare the presence and abundance of the blacklegged tick in vegetation (i.e., host-seeking) and on small mammal hosts between islands, and to determine and compare B. burgdorferi infection prevalence in blacklegged tick populations between islands. We predicted that there would be a smaller blacklegged tick population and lower B. burgdorferi infection prevalence in ticks and mammals on the island devoid of deer (SMI) compared to the island with deer (NMI).
To compare the relative roles of chipmunks and mice in feeding and infecting blacklegged ticks. We hypothesized that chipmunks would play a larger role in feeding and infecting blacklegged ticks on both islands due to the anecdotal reports received from park personnel indicating that chipmunk abundance was greater on the islands compared to the mainland, and possibly greater than mouse abundance. We also predicted that nymphal blacklegged tick infestation prevalence would be higher on chipmunks, and therefore, B. burgdorferi infection prevalence would be greater among chipmunks compared to mice on both islands (Mannelli et al., 1993; Slajchert et al., 1997; Schmidt et al., 1999).
Materials and Methods
Field sites and mammal communities-
Each island constituted a single field site. North Manitou Island (NMI) is approximately 19 km from the mainland and is 58 km2 with 32 km of shoreline. South Manitou Island (SMI) is approximately 5 km from NMI and is 21.4 km2 with 16 km of shoreline (National Park Service, 2016b). Both islands consist of mixed hardwood, oak-dominated, forests and sandy, open dunes. Currently, there are no year-round residents on either island, and no domestic pets are permitted. Aside from park personnel, visitors come to the islands either for day trips or weekend long hiking/camping trips. In addition to the white-tailed deer on NMI, wildlife on both islands includes small rodents such as mice and chipmunks, and only two medium-sized mammal species: snowshoe hares and coyotes (National Park Service, 2018; Sleeping Bear Dunes National Lakeshore, 2000). Historically, raccoons (Procyon lotor) were present on NMI, but no raccoons were present on either island during our study (based on personal communication with National Park Service staff and observations made by field personnel). Since 1984, a week-long deer hunt had taken place annually on NMI to control the deer herd and preserve the natural vegetation, with an average of 119 deer harvested per year from 1984–2014 (standard deviation: 176) (personal communication with National Park Service staff). Prior to a report from a hunter indicating that he had observed blacklegged ticks on NMI in Fall 2010, the state health department and university researchers had not received any reports of blacklegged ticks on the islands, and no tick surveys had been performed on either island.
Both islands were sampled from 2011–2015, with small animal and questing tick sampling taking place at minimum in June of each year (2011–2014), when all three life stages of I. scapularis were active. Coyote trapping took place on SMI (deer-free) in October 2014 during the adult I. scapularis activity peak. Additional sampling of small animals, vegetation, passerine birds, and hares took place as listed in Supplement 1. For reference, throughout the discussion section, the Manitou Islands were compared to a Lyme disease endemic site along the Lake Michigan coast of Michigan’s Lower Peninsula (Hamer et al. 2010). This site was chosen as a mainland comparison because it had been studied by our lab for numerous years, using the same methodologies and sampling design, and all three life stages of the tick could reliably be collected.
Questing tick sampling –
Each site was sampled for questing ticks by dragging a 1 m2 corduroy cloth along the forest floor as described by Hamer et al. (2010) along six 250 m transects situated in oak-dominated, closed-canopy forests. Drag sampling was performed (5–10 m on both sides of the transect), where the cloth was examined every 20 m, and any ticks that were found attached to the cloth or to the researcher were collected and stored in 70% ethanol. A minimum of 1000 m2 (i.e., along at least 4 of the 6 different transects) was dragged during each site visit.
Small mammal trapping –
Small mammal trapping, targeting mice and chipmunks, was conducted following Hamer et al. (2010). Briefly, we trapped small mammals along the same six transects we sampled for questing ticks. Along each transect, 25 live traps (7.62 × 8.89 × 22.86 cm, H. B. Sherman Traps, Tallahassee, FL) were spaced 10 m apart and baited with crimped oats for two consecutive nights. Traps were placed in locations where rodent activity would be likely (e.g., near trees, downed logs, not in open areas). Traps were set in the evening (18:00–20:30) and checked the following morning (05:00–07:00). We identified all captured mammals to species and sex, examined them for ticks, and biopsied both ears using a 2-mm biopsy punch (Miltex Instruments, York, PA). Ticks and ear biopsies were stored separately in 70% ethanol. To determine if an animal was recaptured from the previous day, the healing status of the ear from the biopsy punch was determined. For consistency, this determination was made by the same technicians throughout the study. Recaptured animals that had been caught the previous day were only examined for ticks; animals recaptured from a previous trapping period were biopsied again. To make sure tick infestation counts were not affected by tick removal the previous day, we estimated tick infestation prevalence using data from the first time an animal was captured whether it was first captured on the first day or the second day of the trapping period. Tick counts from individuals captured in different trapping periods were considered independent. All captured mammals were released at the point of capture. The number of trap nights per trapping period was adjusted for tripped traps as follows: number of traps set - (0.5 * no. of tripped traps). Animal handling procedures were approved by MSU’s Institutional Animal Care and Use Committee (IACUC) Animal Use Form #: 06/12–103-00.
Snowshoe hare trapping –
To determine whether snowshoe hares (hares) serve as an alternative host for adult blacklegged ticks on the deer-free island, hares were trapped on SMI from June-September 2012 and 2014. Trapping took place for two consecutive nights at least once per month. The traps were placed near the small mammal traps on the southeast side of the island where the hares appeared to be most abundant and most active, and where there were habitats suitable for blacklegged ticks. Four to six wooden box traps were baited with cut apples and set in the evening and then checked the following morning. Captured hares were anesthetized using ketamine hydrochloride (Ketaset; Fort Dodge, Overland Park, KS) and xylazine hydrochloride (Rompun; Bayer Health Care, Kansas City, KS). To minimize stress, a towel was used to cover the eyes and the tick checking was limited to ten minutes. Anesthesia was reversed by administering yohimbine hydrochloride (Antagonil; Wildlife Laboratories, Fort Collins, CO). Animals were released at the point of capture.
Coyote trapping –
To determine whether coyotes serve as an alternative host for adult blacklegged ticks on the deer-free island, coyotes were trapped in October 2014 using 3.5 EZ grip padded leg hold traps with off-set jaws and swivel (Olsen et al., 1986; Phillips, 1996; Frame and Meier, 2007). A total of twenty-six traps were set throughout SMI in locations where surveys for coyote signs (e.g., feces and/or footprints) conducted during the preceding summer months had indicated activity. Four traps were set for nine consecutive nights and the remaining twenty-two traps were set for eight consecutive nights. Traps were visually inspected and checked every 6–8 hours. Scent lures targeting coyotes (Weiser Western Lure, Melrose, MT) were placed near the traps.
Captured coyotes were anesthetized using ketamine hydrochloride (Ketaset; Fort Dodge, Overland Park, KS) and xylazine hydrochloride (Rompun; Bayer Health Care, Kansas City, KS) using a syringe pole (Kreeger et al., International Edition). Once the animal was fully anesthetized, each animal was sexed by inspection, examined for ticks, blood sampled via jugular vein, biopsied in both ears using a 4-mm biopsy punch (Miltex Instruments, York, PA), and ear-tagged (National Band and Tag, Newport, KY). Examination for ticks was limited to ten minutes, after which anesthesia was reversed by administering yohimbine hydrochloride (Antagonil; Wildlife Laboratories, Fort Collins, CO). Animals were released at the point of capture.
To further verify that the coyotes could serve as reproductive hosts for blacklegged ticks, live adult engorged female blacklegged ticks collected from coyotes were brought back to the laboratory at MSU and stored individually at 95% relative humidity. The females were kept until larval hatching was observed. Wildlife procedures were approved by MSU’s IACUC and the National Park Service’s IACUC (Protocol #MWR_SLBE_Sidge_Coyote.Foxes_2014.A2).
Bird mist-netting –
Birds were captured on both islands between June and August to assess their role in tick/pathogen maintenance and then only on SMI in September and October of 2014 to assess their potential role in introducing ticks to SMI. On each island, six 12-m mist-nets (Avinet, Dryden, NY) were used to capture songbirds. Nets were placed on the southeast side of SMI and on the eastern side of NMI where conditions allowed for potential bird flyways, and there was minimal risk of disturbance by island hikers/campers. Briefly, nets were run from 06:00–12:00, weather permitting, and were checked every 30 minutes. Captured birds were weighed, identified to species and sex, measured (wing and tail length), searched for ticks, and leg-banded with federally issued bands prior to release. Mist-netting was performed under the U.S. Geological Survey Bird Banding Laboratory Federal Bird Banding Permit #23629.
Tick identification and pathogen detection –
All ticks were identified morphologically to species and life stage using dichotomous keys (Keirans and Clifford, 1978; Sonenshine, 1979; Durden and Keirans, 1996). To optimize limited resources, improve coverage of the host population, and limit statistical concerns associated with assessing on-host tick infection prevalence where a large number of ticks came from only a few highly-infested hosts, we assayed up to three randomly selected blacklegged and/or Haemaphysalis leporispalustris ticks per life stage per host per capture (Hamer et al., 2010). One ear biopsy per animal and all questing blacklegged ticks were assayed. Except for questing blacklegged tick samples from 2014, we extracted total DNA from ticks and ear biopsies using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) per the manufacturer’s animal tissue protocol with modifications described previously (Hamer et al., 2010). Borrelia burgdorferi was detected using a quantitative polymerase chain reaction (qPCR) assay targeting a fragment of the 16S rDNA gene (Tsao et al., 2004) as previously described (Hamer et al., 2010). DNA from the 2014 tick samples was extracted using the QIAcube HT automated nucleic acid system and the cador Pathogen 96 QIAcube HT Kit (Qiagen) as described elsewhere (Graham et al., 2016), and we tested these extracts for B. burgdorferi, Anaplasma phagocytophilum, and Babesia microti using a pair of multiplex real-time PCR assays (Hojgaard et al., 2014) as described by Johnson et al. (2017a) (Supplement 2). Both real-time PCR assays can detect multiple B. burgdorferi sensu lato (sl) spp. (Barbour et al., 2009; Graham et al., 2018). Based on an analysis of primer-probe specificity, and previous reports that B. burgdorferi sl spp. other than B. burgdorferi ss are rare in I. scapularis from the Upper Midwest (Barbour et al., 2009; Pritt et al., 2016; Johnson et al., 2017b; Johnson et al., 2018), we treated all samples that tested positive for B. burgdorferi as B. burgdorferi sensu stricto in our analyses.
Statistical analyses –
Fisher’s exact test was used to assess differences in overall capture success between sites. This was performed using GraphPad Software, Inc. (La Jolla, CA), with α = 0.05. In order to assess differences in average infestation prevalence, infection prevalence, and annual capture success between sites and within the same site over time, linear regression was used, and/or we calculated the z-score and associated two-tailed probabilities using a web-based calculator, Social Science Statistics (Stangroom, 2016), with the assumption of a normal distribution and equal variance and α = 0.05. The 95% binomial confidence intervals for proportions (Agresti and Coull, 1998) were calculated using GraphPad Software, Inc. (La Jolla, CA).
Results
Validation of PCR assays –
Prior to testing the 2014 samples, we confirmed that both PCR assays yielded consistent results by assaying a subset of samples (n = 304 ticks and tissues) collected from NMI, SMI, and the Sleeping Bear Dunes mainland in 2012 and 2013. Both assays yielded the same B. burgdorferi results for 97.4% of the samples; there was no significant difference between the proportion of B. burgdorferi positives from each assay (Fisher’s exact test: p = 0.798).
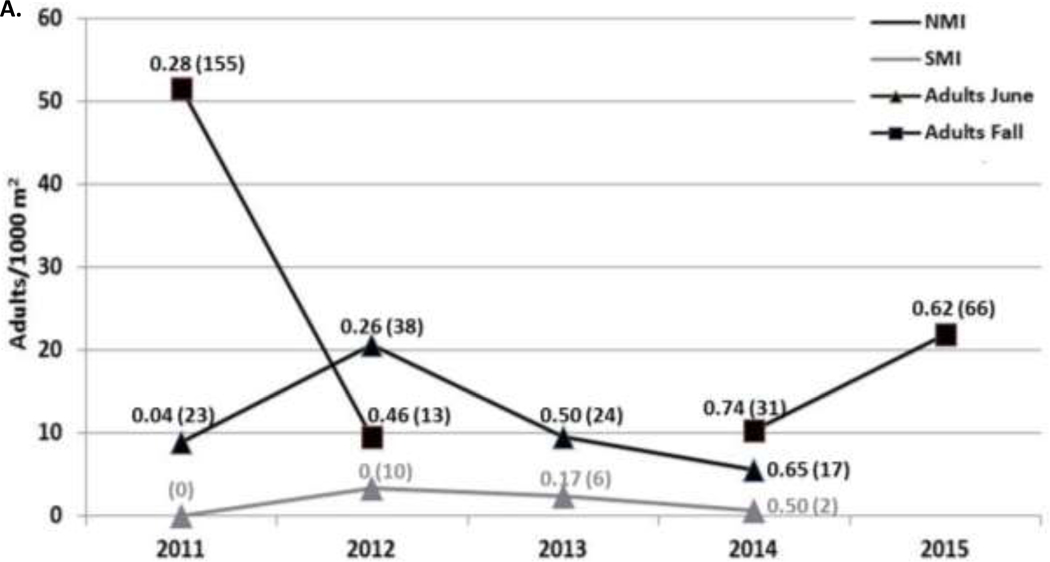
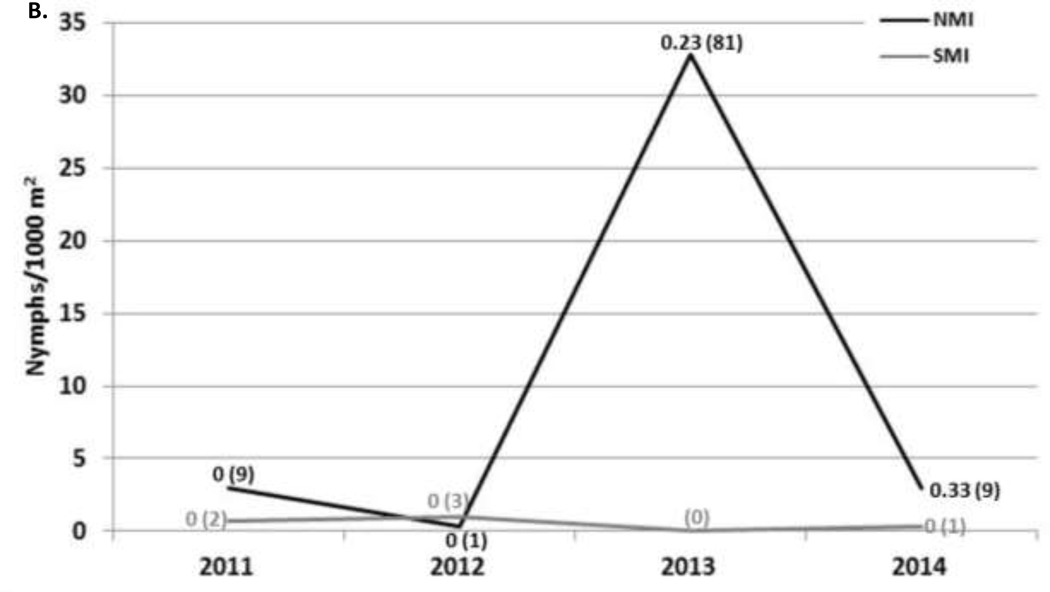
Questing ticks (Figure 2) –
Figure 2. Questing tick density/1000 m2 and B. burgdorferi infection prevalence on NMI (black) and SMI (gray) in June:
A) I. scapularis adults in June (triangle) each year (2011–2014), adults in the fall (square) each year (2011–2015) except 2013, and B) nymphs each June (2011–2014). Fall questing tick sampling only took place on NMI. The proportion of ticks infected on NMI (black) and SMI (gray) appears near each point with the total number of ticks tested indicated in parentheses.
A total of 74,246 m2 and 64,850 m2 were sampled by drag cloth on NMI and SMI, respectively throughout the study. On NMI, 1,084 adults, 130 nymphs, and 605 larvae I. scapularis were collected. On SMI, all three life stages of I. scapularis were also collected: 104 adults, 7 nymphs, and 7 larvae. The only other tick species collected by drag sampling was H. leporispalustris (5 nymphs and 39 larvae). In June, when all three life stages were active, the adult density over four years of collection on NMI and SMI was 11.3 (standard deviation: 6.5) and 1.6 (standard deviation: 1.5), respectively (Figure 2). In June, the nymphal density over four years of collection on NMI and SMI was 8.8 (standard deviation: 15.4) and 0.5 (standard deviation: 0.4), respectively (Figure 2).
Borrelia burgdorferi infection in questing ticks (Figure 2) –
Overall, 36.9% of 868 adults and 26.0% of 127 nymphal questing blacklegged ticks collected on NMI over all months sampled from 2011–2015 were infected with B. burgdorferi. On SMI, 19.2% of 104 adult and 0% of 7 nymphal questing I. scapularis were infected with B. burgdorferi. The adult infection prevalence was significantly greater on NMI compared to SMI (z-score: 3.6, p < 0.001).
The infection prevalence of questing adult ticks in June significantly increased from 2011–2014 (R2 = 0.99; p = 0.004) on NMI. The infection prevalence of questing adult ticks in the fall also increased from 2011–2014 (R2 = 0.77; p = 0.122) on NMI. Due to the small sample size of infected nymphs on NMI, and of infected adults and nymphs on SMI, statistical trends over time were not analyzed for these populations.
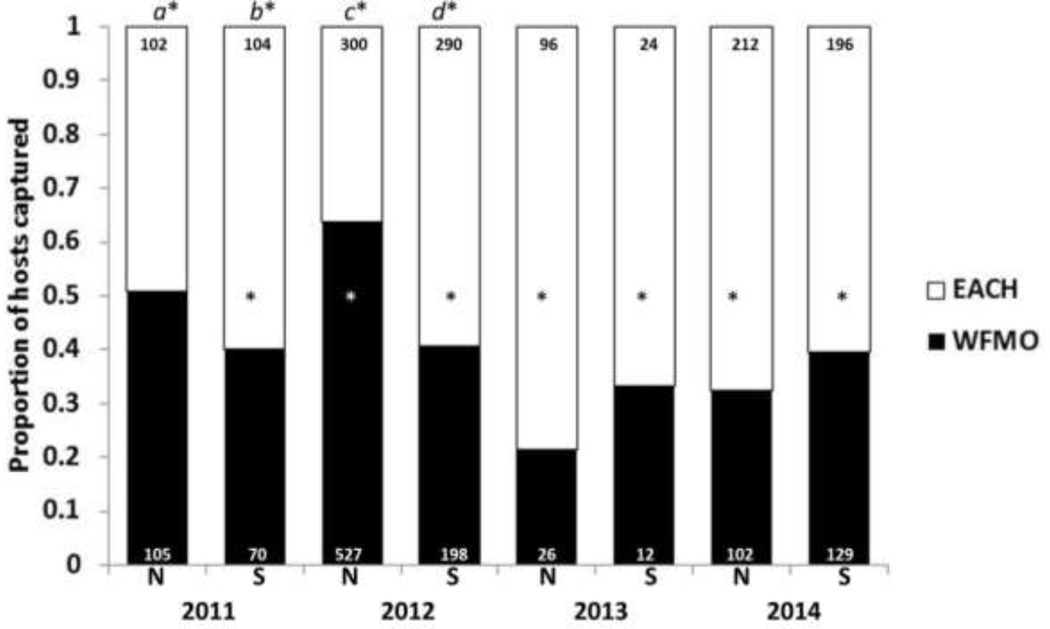
Small mammal captures (Figure 3) –
Figure 3. Proportion of white-footed mice (WFMO; black) and eastern chipmunks (EACH; white) captured each year (2011–2014) on NMI (N) and SMI (S):
The number within each bar indicates the total number of unique mice (black) and unique chipmunks (white) captured; this only includes first-time captures within a trapping session. The asterisk within the bar itself indicates that there was a statistically significant difference (z-score) between WFMO and EACH capture success for that year/site. The letter and asterisk above the bar indicate a statistically significant difference (z-score) between sites (the proportions of WFMO and EACH captured on NMI vs. the proportions of WFMO and EACH captured on SMI in a given year). (a*, b*: 2011; c*, d*: 2012).
On NMI, from 2011–2014, with 5,167 adjusted trap nights, we captured a total of 907 (17.9% trapping success) mice, of which 16% were recaptures from the previous day, and a total of 838 (16.2% trapping success) chipmunks, of which 15% were recaptures from the previous day. On SMI, from 2011–2014, with 5,121 adjusted trap nights, we captured a total of 486 (9.5% trapping success) mice, of which 16% were recaptures from the previous day, and a total of 769 (15.0% trapping success) chipmunks, of which 20% were recaptures from the previous day.
Additional small mammal species that were captured from 2011–2014 on NMI included meadow voles (Microtus pennsylvanicus) (n = 122), northern short-tailed shrews (Blarina brevicauda) (n = 3), and fox squirrels (Sciurus niger) (n = 3). On SMI from 2011–2014, the only additional species captured was the northern short-tailed shrew (n = 9). Because mice and chipmunks made up 93.2% and 99.3% of the captures on NMI and SMI respectively, we focused our analyses on these two species.
To compare relative abundances of mice and chipmunks between islands and also over time on each island, we evaluated the proportion of uniquely captured individuals within a trapping period (i.e., if an individual was captured twice within a trapping period, it was only counted once). Overall, with all trap years combined, there were no statistically significant differences between mouse capture success on NMI in comparison to SMI (Fisher’s Exact Test: p = 0.097; p = 0.183) nor chipmunk capture success between the islands (Fisher’s Exact Test: p = 1.000; p = 0.834 excluding previous day recaptures). When considering each year individually on NMI, there was a significant difference between mice and chipmunk captures in three of the four years with more mice than chipmunks captured in 2012 and more chipmunks than mice in 2013 and 2014 (Figure 3). On SMI, capture success of chipmunks was significantly greater compared with mice (z-score and associated two-tailed probability: p < 0.001) for each year of the study.
Ixodes scapularis infestation on small mammals (Figures 4–6) –
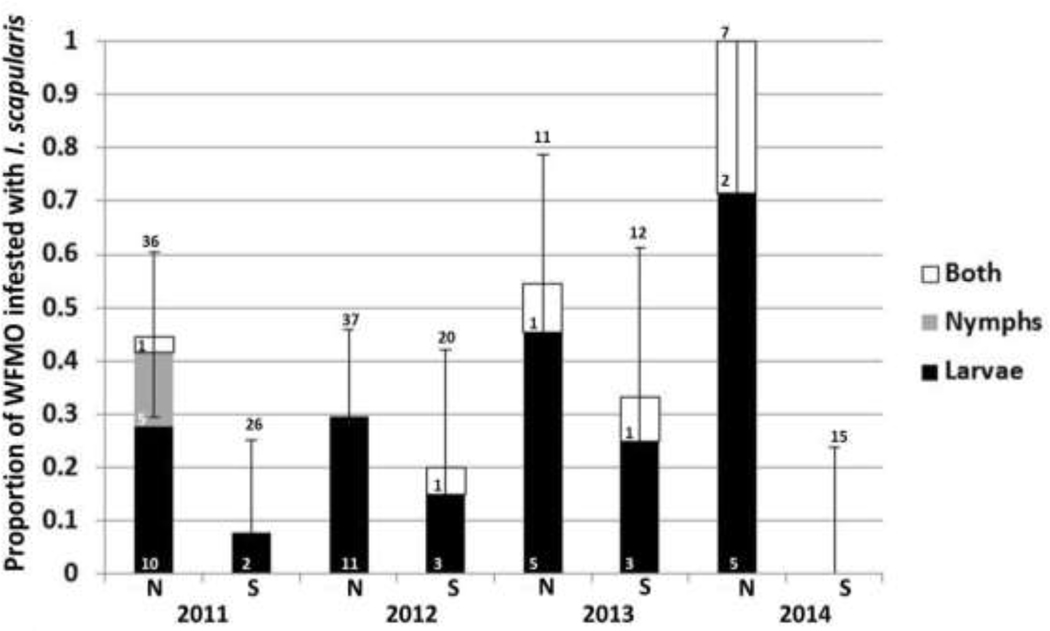
Figure 4. Proportion of white-footed mice (WFMO) infested with I. scapularis larvae (black), nymphs (gray), and both life stages (larva and nymph) simultaneously (white) on North Manitou Island (N) and South Manitou Island (S) in June 2011–2014:
The number above each bar represents the total number of unique WFMO captured and only includes first-time captures within a trapping session. This is a non-cumulative stacked graph. The number of individual WFMO infested with each life stage is indicated within the corresponding shaded bar. The 95% binomial confidence interval error bars correspond to the proportion of mice infested with at least one I. scapularis tick.
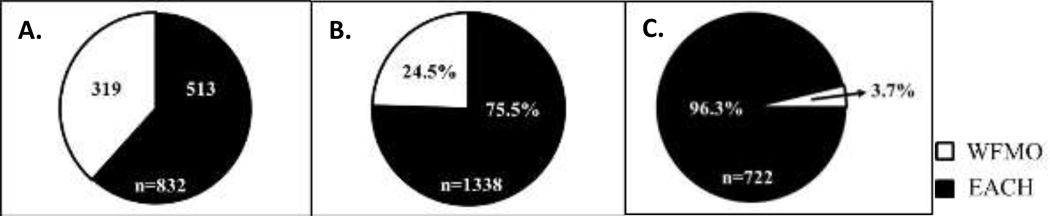
Figure 6. A comparison of the relative abundance of captured white-footed mice, eastern chipmunks and associated larval and nymphal blacklegged tick burdens on NMI and SMI (combined) in June (2011–2014) and August (2012, 2014) for only first-time captures within a trapping session:
Number of white-footed mice (WFMO; white) and eastern chipmunks (EACH; black) captured, with the total number of mice and chipmunk captures indicated at the bottom of the pie chart (A). The percent of larvae (B) and nymphs (C) that were removed from eastern chipmunks (EACH; black) and white-footed mice (WFMO; white) with the total number of ticks collected indicated at the bottom of the corresponding pie chart.
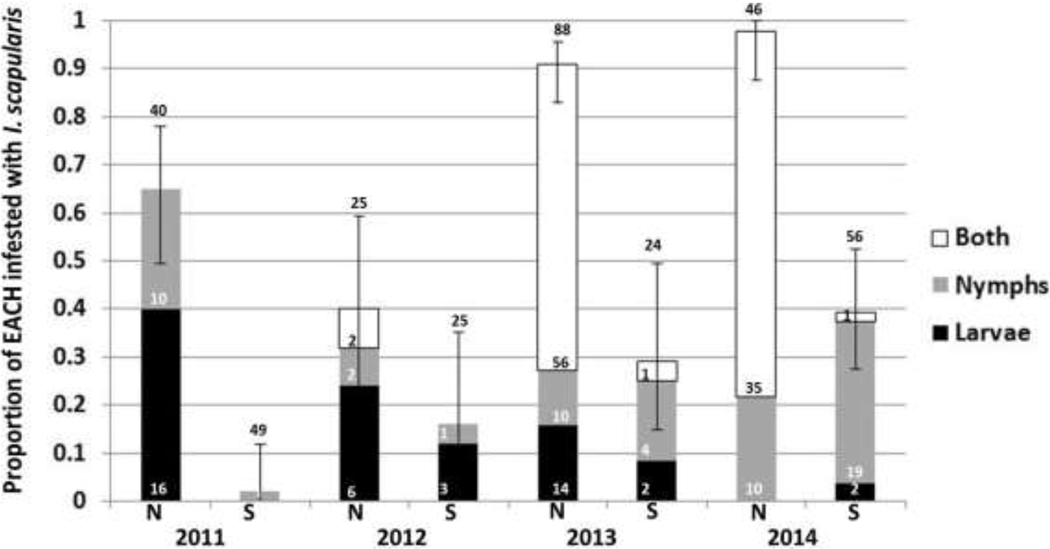
Both immature stages of the blacklegged tick were found attached to small mammals captured on NMI and SMI (Figures 4, 5). Immature stages were detected on small mammals every June throughout the study on both islands. When the proportions of mice and chipmunks infested with immature I. scapularis ticks were combined, the infestation prevalence on NMI compared to SMI was 0.24 (standard deviation: 0.30) versus 0.02 (standard deviation: 0.03). Both mice and chipmunks combined on NMI were significantly more infested than mice and chipmunks combined on SMI in June of 2011, 2013, and 2014 (p < 0.001 each year; Figure 4, 5).
Figure 5. Proportion of eastern chipmunks (EACH) infested with I. scapularis larvae (black), nymphs (gray), and both life stages (larva and nymph) simultaneously (white) on North Manitou Island (N) and South Manitou Island (S) in June 2011–2014:
The number above each bar represents the total number of unique EACH captured and only includes first-time captures within a trapping session. This is a non-cumulative stacked graph. The number of individual EACH infested with each life stage is indicated within the corresponding shaded bar. The 95% binomial confidence interval error bars correspond to the proportion of chipmunks infested with at least one I. scapularis tick.
Across the calendar year, on both islands, mice and chipmunks were consistently captured each month with on-host larval abundance peaking in June and August and a slight on-host nymphal peak in June. When only these peak tick abundance months (June and August) were considered for nymphs and larvae, chipmunks fed significantly more larvae (z-score: −6.8, p = 0; Figure 6) and nymphs (z-score: −16.4, p = 0; Figure 6) relative to their proportional abundance compared to mice.
Borrelia burgdorferi infection in I. scapularis removed from small mammals –
Borrelia burgdorferi was detected in both immature stages of the tick removed from small mammals on both islands (Supplement 3). On NMI, we collected infected larvae and nymphs from mice and chipmunks every June (except for June 2012, when no nymphs were detected on white-footed mice). There was a significant increase in infection prevalence between the first year of the study (June 2011) and the last year (June 2014) in larvae and nymphs removed from both mice (p = larvae: 0.002; nymphs: 0.001) and chipmunks (p = larvae: 0.001; nymphs: <0.001). Borrelia burgdorferi was not detected in the June SMI mammal ticks until the last two years of the study, when larvae attached to mice and chipmunks and nymphs attached to chipmunks were infected.
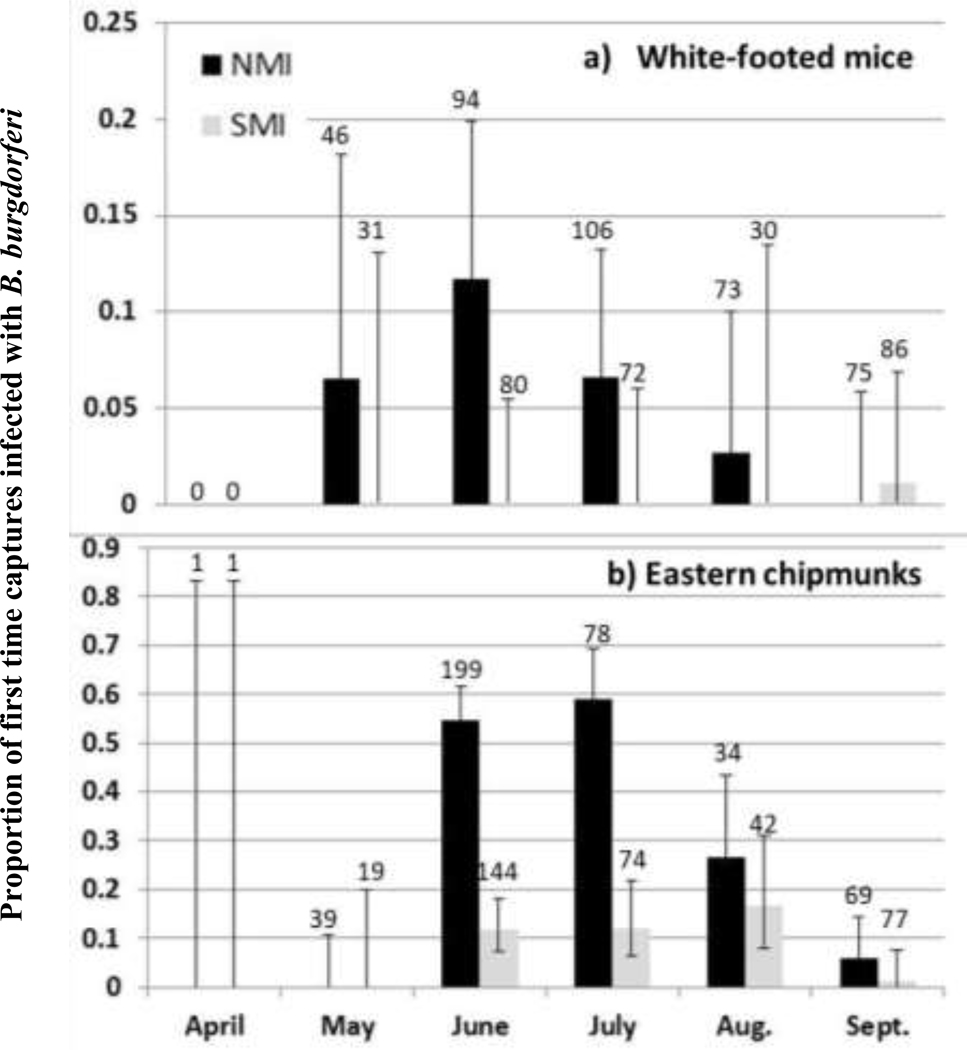
Borrelia burgdorferi infection in small mammal biopsies (Figure 7) –
Figure 7. Proportion of B. burgdorferi infected tissue biopsies from a) white-footed mice and b) eastern chipmunks across the calendar year:
This represents monthly samples from 2011–2014 from NMI (black, left) and SMI (gray, right). The number of samples tested is indicated above each bar. Error bars correspond to a 95% binomial confidence interval. Note the difference in scale for the y-axis for the two graphs.
A total of 394 and 299 mouse tissue biopsies were tested from NMI and SMI, respectively. A total of 420 and 357 chipmunk tissue biopsies were tested from NMI and SMI, respectively. Overall, small mammal infection prevalence was significantly greater on NMI (42.1%) than SMI (7.4%) (z-scores: 2011: 3.0, p = 0.003; 2012: 4.2, p < 0.001; 2013: 4.8, p < 0.001; 2014: 3.1, p = 0.002). Both mice and chipmunks were infected every June on NMI; overall infection prevalence was 55% and 12% in chipmunks and mice, respectively. We detected the pathogen in chipmunks from SMI every June from 2012–2014 (overall infection prevalence was 12%) but not in mice.
Across the calendar year, mouse infection prevalence peaked in June on NMI, while chipmunk infection prevalence did not decrease until after July. The only time that mice were found to be infected on SMI was in September 2014. On SMI, chipmunks were infected June-September, with the proportion infected being relatively consistent from June - August and then declining in September.
Snowshoe hares-
A total of 23 unique hares were captured (15 in 2012 and 8 in 2014) on SMI. None of the hares were infested with I. scapularis ticks. Seventeen hares were infested with H. leporispalustris and a total of 3,491 H. leporispalustris were removed (48% larvae, 37% nymphs, and 15% adults). A total of 311 larvae (27 pools), 33 nymphs, and 55 adults were tested for B. burgdorferi; none were positive. Tissue biopsies from seven hares were tested for the presence of B. burgdorferi; none were positive.
Coyotes –
Two adult female coyotes were captured on SMI. Both coyotes were infested with adult I. scapularis ticks; no other tick species were detected. A total of 37 and 30 adult ticks were removed from each of the two coyotes. Of the 67 total ticks, 46.3% were female and 53.7% were male. Seven engorged females oviposited, and all larvae hatched. Ear biopsies were only collected from one of the coyotes, and B. burgdorferi was not detected.
Birds (Supplement 4) –
During the breeding season in 2014, a total of 167.6 and 273.5 net hours were accumulated on NMI and SMI, respectively. An additional 77.3 net hours were accrued on SMI during the fall migration period. On NMI, 49 unique individuals comprising six different bird species were captured. These six species included chipping sparrow (Spizella passerine), American redstart (Setophaga ruticilla), red-eyed vireo (Vireo olivaceus), northern waterthrush (Parkesia noveboracensis), gray catbird (Dumetella carolinensis), and American robin (Turdus migratorius). Of these birds, six (12.2%) were infested with at least one I. scapularis tick. The infested birds included five American robins and one American redstart. A total of two larvae and six nymphs were collected and tested for B. burgdorferi. Two of the American robins (4.1% of captured birds) were found to host infected ticks (one larva and two nymphs).
On SMI, 127 unique individuals were captured representing 26 different bird species, including all 6 species captured on NMI (Supplement 4). Among the birds captured on SMI, only one American redstart (0.79% of captured birds) hosted at least one I. scapularis tick and this bird was captured during the migratory period. A total of one I. scapularis larva was removed and tested. This tick was positive for B. burgdorferi.
Discussion
How are adult blacklegged ticks fed on an island in the absence of deer?
Larger mammals, in particular white-tailed deer, are responsible for feeding the majority of adult blacklegged ticks and completing the tick’s life cycle (Telford et al., 1988). However, on deer-free SMI, larval ticks were detected both on small mammal hosts and questing within the vegetation, implying that alternative medium/large mammal hosts on SMI were able to feed enough adult ticks to maintain the tick population in the absence of white-tailed deer.
Coyotes served as hosts for adult I. scapularis on SMI. Two coyotes were captured on SMI, and we found both to be infested with adult I. scapularis ticks. The SMI coyote tissue that was tested for the presence of B. burgdorferi was negative, however since B. burgdorferi is not transmitted transovarially, absence of infection in coyotes is not relevant to maintenance of the spirochete on SMI (Telford et al. 1988). Oviposition by multiple coyote-fed, engorged female ticks confirmed that coyote-fed I. scapularis collected on SMI could complete their life cycle. Given the lack of other alternative hosts for adult blacklegged ticks on SMI, we conclude that coyotes are most likely the host for maintaining a reproductive population of blacklegged ticks on the deer-free island.
Although eastern cottontail rabbits (Sylvilagus floridanus) have previously been shown to feed Ixodes species of ticks and become infected with the Lyme disease pathogen (Telford and Spielman, 1989a; Telford and Spielman, 1989b), this was not observed for snowshoe hares on SMI. Throughout this study, twenty-three unique hares were captured, and none were infested with any stage of I. scapularis even though hares were captured when adult blacklegged ticks were active on the islands (questing adult blacklegged ticks were collected in June, July, and September on one or both islands). Furthermore, B. burgdorferi was not detected in any of the ear biopsies taken nor in the H. leporispalustris feeding on the hares.
Migratory passerine birds may play a crucial role in long distance dispersal of immature blacklegged ticks (Ogden et al., 2008; Brinkerhoff et al., 2011; Hamer et al., 2012b). Migratory birds have been estimated to disperse 50–175 million I. scapularis across Canada every spring (Ogden et al., 2008). Although our mist-netting efforts were limited on SMI, we detected I. scapularis ticks on passerine birds during fall migration, providing support for the possibility that birds may be introducing ticks to SMI. Following deer removal on Monhegan Island, ME, Elias et al. (2011) used uncertainty analysis to show that the tick density on the island was equivalent to expected imported tick densities, indicating that all I. scapularis ticks on Monhegan Island were bird-derived. However, unlike on Monhegan Island, where no alternative hosts for adult blacklegged exist, on SMI we found questing I. scapularis larvae and I. scapularis larvae attached to small mammals in the absence of deer (Rand et al., 2004; Elias et al., 2011). Thus, although birds may continue to introduce I. scapularis and B. burgdorferi to SMI, these data support the hypothesis that blacklegged ticks were established on SMI and could not just be adventitious ticks dispersed by migrating avian species.
The low tick and pathogen levels on SMI were likely a function of not only the recent emergence of the blacklegged tick at Sleeping Bear Dunes (Hamer et al. 2010), but also the coyote population size. Although population estimates could not be performed, based on our capture success and the footprints observed during the trapping effort, we estimate that there was a minimum of four coyotes present on SMI in 2014. Therefore, in the presence of sufficient small mammal hosts for immature I. scapularis, even if there are limited numbers of alternative (non-deer) adult blacklegged tick hosts, a population of blacklegged ticks can be maintained. We hypothesize that if there were more coyotes on SMI, then the island could have a larger tick population and pose a greater LD risk.
How does the blacklegged tick population and B. burgdorferi infection prevalence compare between the island with deer and the island without deer?
Contrary to our hypothesis, all three life stages of the blacklegged tick were detected questing on both islands indicating that blacklegged ticks were established on both islands, despite the lack of white-tailed deer on SMI. Furthermore, an infected tick population was confirmed on SMI. However, as we hypothesized, the tick population on SMI was smaller than NMI. Significant differences existed between the I. scapularis populations on the two islands; we collected approximately 10.4, 18.6, and 86.4 times as many host-seeking adults, nymphs, and larvae, respectively, on NMI as we collected on SMI.
In samples taken throughout the June study period, the density of questing adult I. scapularis was greater than that of nymphs on both islands. This is counterintuitive and opposite of what Hamer et al. (2010) observed at a Lyme disease endemic mainland site approximately 359 km south of the islands along the west coast of Michigan. Although mammal densities were not reported with this work, given that there are more alternative host species for the adult life stage in addition to deer in mainland areas, host-finding success might be lower on the Manitou Islands and therefore, island adult ticks may be relatively more frequently collected via drag cloth. Indeed, Rand et al. (2004) reported that adult questing tick density increased on Monhegan Island, ME immediately after deer removal. Along the same lines but in the opposite direction, Ginsberg and Zhioua (1999) found that if host densities were high enough to allow a large proportion of questing ticks to find hosts, the loss of free-living ticks could influence density estimates based on flagging. This was illustrated on Fire Island, NY where questing adult tick densities were much lower on the island as a result of the ticks being unavailable to flag since they were on the highly abundant deer hosts (Ginsberg and Zhioua, 1999). Similarly, the lower questing nymphal density on the Manitou Islands may be due to high host-finding success of nymphs given the high abundance of small mammal hosts.
Borrelia burgdorferi was detected in questing ticks, in small mammals, and in ticks removed from small mammals on both islands. The detection of B. burgdorferi in larvae removed from small mammals on SMI illustrated that despite the lack of deer on the island, an enzootic cycle existed. As predicted, however, higher infection prevalences of B. burgdorferi were observed on NMI. The overall questing adult infection prevalence on NMI (~37%) is similar to other areas in the north central U.S. (Hamer et al., 2014) and was almost double that on SMI (~19%). Additionally, infected host-seeking nymphs were collected on NMI, but not on SMI, which may have been due in part to the low number of nymphs collected (which was a function of the smaller population size owing to scarcity of hosts for adult ticks).
What is the comparative role of chipmunks to mice in feeding and infecting blacklegged ticks?
The two primary small mammal hosts captured on the islands were eastern chipmunks and white-footed mice, both important hosts for immature blacklegged ticks and B. burgdorferi (Mather et al., 1989). The islands provided a unique opportunity to investigate enzootic maintenance of B. burgdorferi in an environment where chipmunk abundance was similar to or greater than mouse abundance. On NMI, chipmunk and mice capture rates were similar most years. On SMI, the capture rate for chipmunks was consistently higher than for mice. This is unlike what has been reported at most mainland sites in the Midwest and the Northeast. At an Illinois field site, the capture rate of mice was almost twice that of chipmunks (Slajchert et al., 1997), and at a field site in Massachusetts, the ratio of mice to chipmunks was 15:1 (Mather et al., 1989). A higher ratio of mice to chipmunks was also observed at multiple sites within Michigan, including two sites on Sleeping Bear Dunes’ mainland (Hamer et al., 2010; Sidge, 2016).
Chipmunks played an important role in feeding both immature stages of the blacklegged tick on the two Manitou Islands. Previous research has shown that mice are typically infested with more larvae than chipmunks and chipmunks are infested with more nymphs than mice (Mannelli et al., 1993; Slajchert et al., 1997; Schmidt et al., 1999). Hamer et al. (2010) also observed this pattern at a Lyme disease endemic site in Michigan’s Lower Peninsula. During the peak larval activity months on the islands (June and August), however, chipmunks fed more larvae and more nymphs than mice. Chipmunks were also active throughout the entire summer and into the fall; a “summer lull” was not observed on the Manitou Islands, unlike what has been reported in the literature (Dunford, 1972; Hamer et al., 2012a). Therefore, island chipmunks were able to continue to contact ticks as opposed to chipmunks in other locations.
Chipmunks significantly contributed to the enzootic maintenance of B. burgdorferi on the islands. The larval I. scapularis population on NMI fed on a large population of infected mice (12% infection prevalence in June) and chipmunks (55% infection prevalence in June). The abundance of infected chipmunks and the larger role that chipmunks played relative to mice in feeding larvae may have further contributed to the B. burgdorferi infection prevalence observed in questing nymphs (26.0%) on NMI, which was comparable to that in areas in the Midwest and Northeast where the tick has been established longer and Lyme disease is endemic (Gatewood et al., 2009; Barbour et al., 2009; Johnson et al., 2017a). In addition to having a higher infection prevalence and feeding relatively more larvae, chipmunks may also have served as a more persistent reservoir over time. As chipmunks typically live longer than white-footed mice (Tryon and Snyder, 1973; Wolff et al., 1988), not only do they have more opportunities to become infected, but if they maintain their infectivity, they may also serve as a more reliable source of B. burgdorferi over time, infecting multiple cohorts of larval I. scapularis (McLean et al., 1993; Slajchert et al., 1997). Unfortunately, we did not mark the animals with permanent identifiers, so we could not evaluate the longevity of infection within individuals in this study. However, on SMI, B. burgdorferi was first detected in chipmunks in 2012 but it was not until 2014 that B. burgdorferi was detected in mice. This further supports the important role of chipmunks as reservoirs for B. burgdorferi and in establishing enzootic cycles.
Implications for Lyme Disease Risk Management and Future Research
Our data from an offshore island confirm the findings of others that blacklegged tick abundance and B. burgdorferi prevalence can be reduced greatly if white-tailed deer are completely removed. However, even in the absence of deer, alternative medium/large mammalian hosts, such as coyotes, can maintain blacklegged ticks, and consequently enzootic cycles. Therefore, when contemplating deer population reduction or elimination for tick-borne disease management (or other approaches such as host-targeted acaricides or anti-tick vaccines), the potential role of alternative hosts for “rescuing” the tick population should be evaluated.
The importance of chipmunks for contributing to the maintenance of B. burgdorferi is strongly supported by our data. While chipmunk abundance on the Manitou Islands was most likely greater than those usually found on mainland sites, these data support the inclusion of chipmunks when designing reservoir-targeted strategies to disrupt the B. burgdorferi enzootic cycle to reduce LD risk (e.g., bait tubes, bait boxes, and OspA-targeted vaccination). In addition, researchers and managers should consider host community composition when implementing reservoir-targeted strategies in order to maximize efficiency.
Future research efforts should address host association, feeding success, oviposition success of adult ticks fed on alternative hosts; contribution of alternative hosts to feeding and infecting immature ticks; and the relationship between alternative host densities and tick population sizes.
Supplementary Material
Acknowledgements
We would like to thank the following Tick Team members for their assistance with field and/or lab work: T. Anderson, D. Arsnoe, I. Arsnoe, J. Assenmbacher, M. Bammer, C. Bateman, L. Bragg, J. Calogero, J. Chung, M. Clayson, K. Ferguson, G. Grzesiak, S. Han, G. Hickling, A. Janssen, E. Johnston Flies, A. Klingler, L. Kramer, L. Makielski, S. Manning, K. Miazgowicz, M. Nowicki, J. O’Malley, G. Pang, L. Paxton, N. Rochte, A. Schroeder, J. Sidge, K. Sidge, M. Sidge, N. Sidge, P. Sidge Senior, P. Sidge Junior, K. Signs, N. Spala, J. Stych, S. Szabo, C. Vivian, E. Walker, J. Webeck, and M. Wiseman. We would also like to thank the staff at Sleeping Bear Dunes National Lakeshore for allowing us to perform fieldwork within the park, for providing boat rides to/from the islands, housing on the islands, and for continual support and encouragement. In particular, we would like to thank: D. Chew, D. Hendrick, S. Jennings, R. Newhouse, D. Schroeder, K. Skerl, and D. Steele. We thank the USDA APHIS Wildlife Services for lending coyote traps. Specifically, we thank A. Bowden for sharing his coyote trapping expertise. We thank the Michigan Department of Natural Resources for permission to trap coyotes, hares, and other small mammals. We thank J. Owen for permitting J. Sidge to be a sub-permittee on her bird banding license. Thank you to Michigan State University Comparative Medicine and Integrative Biology Department for continual support.
Funding:
This work was supported by Merial-National Institutes of Health Veterinary Scholars Summer Research Program, National Institutes of Health Predoctoral T32 Fellowship (NIHT32RR018411, NIHT32OD011167), the National Science Foundation (NSFEF-0914476), Michigan State University Graduate Office Fellowships, National Park Service Wildlife Health Branch, Colorado State University, Michigan Department of Health and Human Services (MDCH/CDC U50-CI000895-02S3), and Michigan Lyme Disease Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agresti A, Coull BA, 1998. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat 52, 119–26. [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI, 2009. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg 81, 1120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Folsom-O’Keefe CM, Tsao K, Diuk-Wasser MA, 2011. Do birds affect Lyme disease risk? Range expansion of the vector-borne pathogen Borrelia burgdorferi. Front. Ecol. Environ 9, 103–10. [Google Scholar]
- Dunford C, 1972. Summer activity of eastern chipmunks. J. Mammal 53, 176–80. [Google Scholar]
- Durden LA, Keirans JE, 1996. Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: taxonomy, identification key, distribution, hosts, and medical/veterinary importance. Thomas Say Publications in Entomology, Entomological Society of America. [Google Scholar]
- Elias SP, Smith RP Jr., Morris SR, Rand PW, Lubelczyk C, Lacombe EH, 2011. Density of Ixodes scapularis ticks on Monhegan Island after complete deer removal: a question of avian importation. J. Vector. Ecol 36, 11–23. [DOI] [PubMed] [Google Scholar]
- Foster ES, 2004. Ixodes scapularis (Acari: Ixodidae) and Borrelia burgdorferi in southwest Michigan: population ecology and verification of a geographic risk model. Masters Thesis. Michigan State University, East Lansing. [Google Scholar]
- Frame PF Meier TJ, 2007. Field-assessed injury to wolves captured in rubber-padded traps. J. Wildl. Manage 71, 2074–76. [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, Barbour AG, Fish D, Diuk-Wasser MA, 2009. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl. Environ. Microbiol 75, 2476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrott RA, 1995. Effective management of free-ranging ungulate populations using contraception. Wildl. Soc. Bull 23, 445–52. [Google Scholar]
- Ginsberg HS, Zhioua E, 1999. Influence of deer abundance on the abundance of questing adult Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 36, 376–81. [DOI] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ, 2016. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick. Dis 7, 1230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CB, Maes SE, Hojgaard A, Fleshman AC, Sheldon SW, Eisen RJ, 2018. A molecular algorithm to detect and differentiate human pathogens infecting Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick. Dis 9, 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Tsao JI, Walker ED, Hickling GJ, 2010. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. Ecohealth. 7, 47–63. [DOI] [PubMed] [Google Scholar]
- Hamer SA, Hickling GJ, Sidge JL, Walker ED, Tsao JI, 2012a. Synchronous phenology of juvenile Ixodes scapularis, vertebrate host relationships, and associated patterns of Borrelia burgdorferi ribotypes in the midwestern United States. Ticks Tick. Dis 3, 65–74. [DOI] [PubMed] [Google Scholar]
- Hamer SA, Hickling GJ, Keith R, Sidge JL, Walker ED, Tsao JI, 2012b. Associations of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan, U.S.A. Parasit. Vectors. 5, 10.1186/1756-3305-5-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Hickling GJ, Walker ED, Tsao JI, 2014. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the midwestern United States. Infect. Genet. Evol 27, 531–42. [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J, 2014. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick. Dis 5, 349–51. [DOI] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Boegler KA, Cherry CC, Maes SE, Pilgard MA, Hojgaard A, Buttke DE, Eisen RJ, 2017a. Prevalence and diversity of tick-borne pathogens in nymphal Ixodes scapularis (Acari: Ixodidae) in eastern national parks. J. Med. Entomol 54, 742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Hojgaard A, Breuner NE, Maes SE, Boegler KA, Replogle AJ, Kingry LC, Petersen JM, Eisen L, Eisen RJ, 2017b. Isolation of the Lyme disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J. Med. Entomol 54, 1088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Maes SE, Hojgaard A, Fleshman A, Boegler KA, Delory MJ, Slater KS, Karpathy SE, Bjork JK, Neitzel DF, Schiffman EK, Eisen RJ, 2018. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick. Dis 9, 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Clifford CM, 1978. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. J. Med. Entomol Suppl. 2, 1–149. [DOI] [PubMed] [Google Scholar]
- Kugeler KJ, Jordan RA, Schulze TL, Griffith KS, Mead PS, 2016. Will culling white-tailed deer prevent Lyme disease? Zoonoses Public Health. 63, 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli A, Kitron U, Jones CJ, Slajchert TL, 1993. Role of the eastern chipmunk as a host for immature Ixodes dammini (Acari: Ixodidae) in northwestern Illinois. J. Med. Entomol 30, 87–93. [DOI] [PubMed] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, Ribeiro JM, Spielman A, 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am. J. Epidemiol 130, 143–50. [DOI] [PubMed] [Google Scholar]
- McLean RG, Ubico SR, Cooksey LM, 1993. Experimental infection of the eastern chipmunk (Tamias striatus) with the Lyme disease spirochete (Borrelia burgdorferi). J. Wildl. Dis 29, 527–32. [DOI] [PubMed] [Google Scholar]
- Mead PS, 2015. Epidemiology of Lyme disease. Infect. Dis. Clin. North. Am 29, 187–210. [DOI] [PubMed] [Google Scholar]
- Messmer TA, Cornicelli L, Decker DJ, Hewitt DG, 1997. Stakeholder acceptance of urban deer management techniques. Wildl. Soc. Bull 25, 360–66. [Google Scholar]
- Michigan Department of Health and Human Services (MDHHS)., 2016. Michigan Disease Surveillance System weekly disease report. MMWR Week 41. [Google Scholar]
- National Park Service., 2016a. U.S. Department of the Interior. Sleeping Bear Dunes. North Manitou Island hunting. www.nps.gov/slbe/planyourvisit/nmihunting. [Google Scholar]
- National Park Service., 2016b. U.S. Department of the Interior. Sleeping Bear Dunes. North Manitou Island and South Manitou Island. www.nps.gov/slbe/planyourvisit/ northmanitouisland and www.nps.gov/slbe/planyourvisit/southmanitouisland. [Google Scholar]
- National Park Service., 2018. U.S. Department of the Interior. Sleeping Bear Dunes. Animals www.nps.gov/slbe/learn/nature/animals. [Google Scholar]
- Ogden NH, St-Onge L, Barker IK, Brazeau S, Bigras-Poulin M, Charron DF, Francis CM, Heagy A, Lindsay L, Maarouf A, Pascal M, Milord F, O’Callaghan CJ, Trudel L, Thompson R, 2008. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int. J. Health Geogr 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GH, Linhart SB, Holmes RA, Dasch GJ, Male CB, 1986. Injuries to coyotes caught in padded and unpadded steel foothold traps. Wildl. Soc. Bull 14, 219–23. [Google Scholar]
- Piesman J, Spielman A, Etkind P, Ruebush TK II, Juranek DD, 1979. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J. Med. Entomol 15, 537–40. [DOI] [PubMed] [Google Scholar]
- Phillips RL, 1996. Evaluation of 3 types of snares for capturing coyotes. Wildl. Soc. Bull 24, 107–10. [Google Scholar]
- Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM, 2016. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet. Infect. Dis 16, 556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand PW, Lubelczyk C, Holman MS, Lacombe EH, Smith RP Jr., 2004. Abundance of Ixodes scapularis (Acari: Ixodidae) after the complete removal of deer from an isolated offshore island, endemic for Lyme disease. J. Med. Entomol 41, 779–84. [DOI] [PubMed] [Google Scholar]
- Schmidt KA, Ostfeld RS, Schauber EM, 1999. Infestation of Peromyscus leucopus and Tamias striatus by Ixodes scapularis (Acari: Ixodidae) in relation to the abundance of hosts and parasites. J. Med. Entomol 36, 749–57. [DOI] [PubMed] [Google Scholar]
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ, 2017. Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveillance Summaries. 66, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidge JL, 2016. Investigating the importance of vertebrate hosts for Lyme disease ecology: a natural experiment presented by Lake Michigan islands at Sleeping Bear Dunes National Lakeshore; chapter 1. Ph.D. Dissertation. Michigan State University, East Lansing. [Google Scholar]
- Slajchert T, Kitron UD, Jones CJ, Mannelli A, 1997. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in northwestern Illinois, USA. J. Wildl. Dis 33, 40–6. [DOI] [PubMed] [Google Scholar]
- Sleeping Bear Dunes National Lakeshore., 2000. Resources management plan. National Park Service, U.S. Department of the Interior. [Google Scholar]
- Sonenshine DE, 1979. Ticks of Virginia, Blacksburg, VA: Virginia Polytechnic Institute and State University. College of Agriculture and Life Sciences. [Google Scholar]
- Stangroom J, 2016. Social science statistics. www.socscistatistics.com. [Google Scholar]
- Telford III SR, 2002. Deer tick-transmitted zoonosis in the eastern United States. Conservation Medicine: Ecological Health in Practice, Aguirre AA, et al., Editors. Oxford University Press: New York. 310–24. [Google Scholar]
- Telford SR, Mather TN, Moore SI, Wilson ML, Spielman A, 1988. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg 39, 105–9. [DOI] [PubMed] [Google Scholar]
- Telford SR, Spielman A, 1989a. Enzootic transmission of the agent of Lyme disease in rabbits. Am. J. Trop. Med. Hyg 41, 482–90. [DOI] [PubMed] [Google Scholar]
- Telford SR, Spielman A, 1989b. Competence of a rabbit-feeding Ixodes (Acari: Ixodidae) as a vector of the Lyme disease spirochete. J. Med. Entomol 26, 118–21. [DOI] [PubMed] [Google Scholar]
- Tryon CA, Snyder DP, 1973. Biology of the eastern chipmunk, Tamias striatus: life tables, age distributions, and trends in population numbers. J. of Mammal 54, 145–168. [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG, 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. U. S. A 101, 18159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JO, Lundy KI, Baccus R, 1988. Dispersal, inbreeding avoidance and reproductive success in white-footed mice. Anim. Behav 36, 456–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.