Abstract
Background
Acute lymphoblastic leukemia is the most common pediatric malignancy, characterized by fever, anemia, hemorrhage, and symptoms brought on by blasts infiltrating organs.
Case presentation
This is a case report of a 9-year-old Asian patient with acute lymphoblastic leukemia who presented with polyuria alone as a presenting feature without any other clinical manifestation; primary renal disease or inherited metabolic disease was highly suspected. However, the water deprivation test and water deprivation pressurization test suggested nephrogenic diabetes insipidus, and the renal biopsy displayed diffuse lymphocytic infiltration in the renal interstitium. Bone marrow aspiration was performed immediately, and a comprehensive diagnosis of B-lymphoblastic leukemia was finally made.
Conclusions
Renal infiltration with leukemic blasts mostly remains asymptomatic, but our case suggests that it can present with nephrogenic diabetes insipidus. This case fully demonstrates that the presentation of extramedullary infiltration in acute lymphoblastic leukemia is varied. When the patient has renal diabetes insipidus as the first symptom, the possibility of hematological tumor infiltration should be considered when finding the cause, and timely bone marrow cytology should be performed.
Keywords: Acute lymphoblastic leukemia, Nephrogenic diabetes insipidus, Children, Case report
Introduction
Leukemias are a group of life-threatening blood and bone marrow malignancies, while blasts have a characteristic ability to infiltrate and proliferate into various tissues and organs of the body. However, organ infiltration by blasts is associated with poor prognosis in leukemia [1]. The main localizations of extramedullary involvement are the central nervous system (CNS) and the testis [2]. The incidence of blasts directly infiltrating the kidney is high (up to 50%), but it is rare to develop obvious clinical symptoms [3]. Leukemia-related kidney damage can cause proteinuria, hematuria or even gross hematuria, hypertension, renal insufficiency, acute tubulointerstitial nephritis, renal tubular acidosis, acute uric acid nephropathy, low back pain, and acute tumor lysis syndrome [4–7]. Here, we report a case of ALL initially presenting with nephrogenic diabetes insipidus.
Case report
This case report involves a 9-year-old Asian boy who was admitted to our hospital with polyuria, vomiting, and generalized malaise for 3 months. He had no obvious medical history, including kidney or blood diseases. He also had no special family history, social history, intravenous drug history, or travel history. Three months before coming to our hospital, the child had experienced an increase in urination for no apparent reason. In the beginning, the child had a urine output of about 9.7 ml/kg/hour. He visited a local hospital and underwent some relevant examinations. Complete hemogram was normal except for mild anemia; serum potassium was 1.64 mmol/L, serum chlorine was 110 mmol/L, and other electrolytes were normal. Serum lactate dehydrogenase (LDH) was 155 U/L. Ultrasound examination showed no hepatosplenomegaly or lymphadenopathy but suggested bilateral renal enlargement with diffuse lesions (right kidney 9.2 cm × 4.9 cm × 3.7 cm, left kidney 9.4 cm × 5.2 cm × 3.9 cm). The local hospital made the following diagnoses: severe hypokalemia, diabetes insipidus, and renal tubular acidosis. The patient received treatments such as correcting acidosis, supplementing potassium, protecting the kidney, and protecting gastric mucosa. The patient was treated in the local hospital for 1 week, but the child’s polyuria still did not improve, and his urine output was still 9 ml/kg/hour, so he was then transferred to our hospital for further treatment. When he came to our hospital, his body temperature was 36.5 ℃, pulse was 78 beats per minute, respiratory rate was 20 breaths per minute, blood pressure was 128/76 mmHg, weight was 30 kg, height was 144 cm, muscle strength was 4 grade in the whole-body examination with no lymphadenopathy, and no abnormalities were found in the nervous system and other systems during the examination. Laboratory tests proved no significant abnormalities in complete blood counts, severe hypokalemia, metabolic acidosis, and renal tubular dysfunction. Critical laboratory findings are presented in Table 1. Head + whole-spine magnetic resonance imaging (MRI) showed the following results: (1) no obvious abnormality in the head, cervical spine, thoracic spine, or lumbar spine; (2) renal volume was markedly elevated; (3) pituitary gland without suggestive lesion. Water deprivation test and water deprivation pressurization test (Table 2) excluded central diabetes insipidus. In summary, the primary diagnoses were nephrogenic diabetes insipidus, renal tubular acidosis, and severe hypokalemia. The following treatments were then given immediately: intravenous drip and oral potassium chloride supplements (up to 500 mg/kg/hour); hydrochlorothiazide 3 mg/(kg·day) combined with indomethacin 1 mg/(kg·day); oral desmopressin 0.4 mg (time, q8h); intravenous infusion of methylprednisolone 1 mg/(kg·day) to improve renal interstitial lesions. We initially believed it to be primary renal disease because the child only had bilateral renal enlargement and nephrogenic diabetes insipidus, therefore the child underwent a renal biopsy. After 1 month of treatment, the electrolyte level of the child was stable and the limb muscle strength was improved. Therefore, the child was discharged from hospital awaiting the results of renal biopsy and fully penetrant genetic testing. After his discharge, the following protocol for treatment was advised: potassium citrate extended-release tablets to supplement potassium, a bailing capsule to protect the kidney, and prednisone to improve kidney function. Prednisone dosage was 15 mg/day, taken in the morning. After 15 days of oral administration, the patient’s renal pathological results returned as follows: (1) diffuse lymphoid cell infiltration in the renal interstitium, which we considered to be derived from the lymphatic and hematopoietic system tumors; (2) mild-to-moderate renal tubular atrophy and interstitial fibrosis (Fig. 1); (3) sections of the kidney immunofluorescently stained (Table 3). Whole-exome sequencing suggested SCN4A gene mutation: hypokalemic periodic paralysis type 2 (OMIM: 613345) (Fig. 2). He was immediately notified by phone to follow up in the hospital and stop taking the prednisone. The family members of this child were contacted to inform them about the examination results and the need for hospitalization again. At that time, the child had polyuria again, and the urine volume was about 5–7 L. He was brought to our hospital again. We performed bone marrow aspiration immediately. Bone marrow morphology demonstrated that primitive lymphocytes + immature lymphocytes accounted for 35.5%. Bone marrow cytological staining results were as follows: myeloperoxidase (MPO) positive rate was negative; periodic acid−Schiff (PAS) stain positive rate was 4%; nonspecific esterase (NSE) was negative. Immunophenotypic of leukemia was HLA-DR, CD10, CD19, CD22, CD38, CD58, CD71, CD123, cCD79a, TdT expressed, consistent with acute B-lymphoblastic leukemia (B-ALL) immunophenotype (Fig. 3). ALL fusion gene screening detected ETV6/RUNX1 fusion gene positivity. Karyotype was 46, XY. Therefore, the final diagnosis was B-lymphoblastic leukemia with positive ETV6/RUNX1 fusion gene. Then, vincristine + daunorubicin + lasparaginase + dexamethasone (VDLD) remission induction chemotherapy was started immediately, and bone marrow morphology was repeated on day 33 of chemotherapy, which confirmed complete remission (CR). Comprehensive assessment was in the intermediate-risk group. The child was then successively given: cyclophosphamide, cytarabine, mercaptopurine, pegaspargase (CAML), high-dose methotrexate (HDMTX), and delayed intensive VDLD regimen chemotherapy. Regular intrathecal triple therapy was also administered to prevent CNS leukemia. The child had sustained CR of bone marrow on reexamination, and cerebrospinal fluid biochemistry, routine, and cell morphology were normal; he is still undergoing follow-up HDMTX intensive chemotherapy (Table 4). As leukemia gradually resolved, the child recovered from severe hypokalemia and acidosis, and his vital signs were stable, and he was in good general condition. There was no evidence of blast infiltration into other organs.
Table 1.
Important laboratory test results
| Laboratory study | Patient’s result | Normal values |
|---|---|---|
| White blood cell count (109/L) | 4.78 (109/L) | 4.3–11.3 |
| Neutrophil count (109/L) | 2.12 (109/L) | 1.6–7.8 |
| Lymphocyte count (109/L) | 2.05 (109/L) | 1.5–4.6 |
| Hemoglobin (g/L) | 95 (g/L) ↓ | 118–156 |
| Platelet count (109/L) | 187 (109/L) | 167–453 |
| Serum potassium (mmol/L) | 1.30 (mmol/L) L) ↓↓↓ | 3.7–5.2 |
| Serum sodium (mmol/L) | 153.77 (mmol/L)↑ | 135–145 |
| Serum chlorine (mmol/L) | 125.6 (mmol/L)↑ | 98–110 |
| Serum bicarbonate (mmol/L) | 12.97 (mmol/L) ↓↓↓ | 21–25 |
| Anion gap | 16 | 8–16 |
| Serum urea (mmol/L) | 0.71 mmol/L | 2.7–7.0 |
| Serum creatinine (mmol/L) | 74.38 mmol/L↑ | 27–66 |
| Plasma osmolality (mOsm/kg·H2O) | 284 | 280–310 |
| Potential of hydrogen (pH) | 7.235 | 7.35–7.45 |
| Urine specific gravity | 1.005 | 1.010–1.025 |
| Urinary pH | 6.0 | 4.6–8.0 |
| Urinary Na | 290.6 | < 5 mmol·kg−1/24 hour |
| Urinary K | 130.6 | 25–125 mmol/24 hour |
| Urinary Cl | 166.8 | 170–250 mmol/24 hour |
| Urine osmolality (mOsm/kg·H2O) | 177 | > 600 |
| Urine occult blood | Positive (1 +) | Negative (−) |
| Urine protein | Positive (1 +) | Negative (−) |
| Urine beta2-macroglobulin (mg/L) | 0.96 mg/L↑↑↑ | < 0.2 mg/L |
| Urine β1-microglobulin (mg/L) | 85.00 mg/L↑↑↑ | 10–20 mg/L |
Table 2.
Water-deprivation test results and water-deprivation-vasopressin test results
| Time | Weight | Urine output | Blood pressure | Serum sodium | Plasma osmolality | Urine osmolality | Urine specific gravity |
|---|---|---|---|---|---|---|---|
| (kg) | (ml) | (mmHg) | (mmol/L) | (mOsm/kg·H2O) | (mOsm/kg·H2O) | ||
| 10:00 | 31.8 | 250 | 121/85 | 136.5 | 287.9 | 150 | 1.002 |
| 11:00 | 31.5 | 350 | 126/93 | 136.8 | 291.1 | 160 | 1.002 |
| 12:00 | 31.5 | 300 | 114/89 | 135.3 | 295.1 | 160 | 1.003 |
| 13:00 | 31.1 | 250 | 123/89 | 136.4 | 295.0 | 140 | 1.004 |
| 14:00 | 31.1 | 200 | 115/75 | 137.0 | 290.4 | 160 | 1.004 |
| 15:00 | 31.1 | 200 | 124/86 | 137.9 | 290.1 | 140 | 1.004 |
| 16:00 | 30.7 | 150 | 121/84 | 139.8 | 295.7 | 160 | 1.005 |
| Timing after arginine vasopressin injection (hours) | Weight | Urine output | Blood pressure | Serum sodium | Plasma osmolality | Urine osmolality | Urine specific gravity |
|---|---|---|---|---|---|---|---|
| (kg) | (ml) | (mmHg) | (mmol/L) | (mOsm/kg·H2O) | (mOsm/kg·H2O) | ||
| 0 h | 31.8 | 0 | 107/74 | 137.8 | 296.4 | 160 | 1.002 |
| 0.5 h | 31.7 | 0 | 118/87 | 160 | 1.003 | ||
| 1 h | 31.5 | 300 | 105/80 | 150 | 1.003 | ||
| 1.5 h | 31.5 | 0 | 117/80 | 130 | 1.003 | ||
| 2 h | 31.5 | 200 | 108/71 | 130 | 1.002 | ||
| 2.5 h | 31.3 | 200 | 121/89 | 140 | 1.003 | ||
| 3 h | 31.1 | 350 | 119/83 | 160 | 1.002 | ||
| 3.5 h | 31.1 | 0 | 115/81 | 160 | 1.003 | ||
| 4 h | 31.1 | 250 | 110/77 | 139.9 | 297.7 | 140 | 1.003 |
Fig. 1.
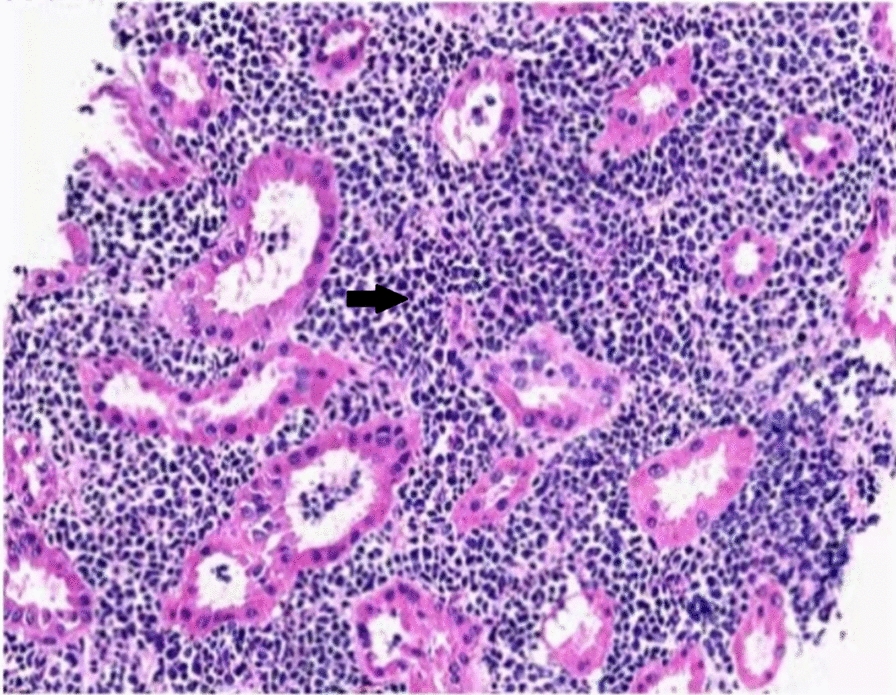
Kidney pathology image of the patient. Black arrows point to diffuse lymphocytic infiltrates in the renal interstitium
Table 3.
Fluorescence immunoassay results of kidney
| Immunofluorescence | Glomerular positive distribution | Glomerulus positive strength | Other positive | ||||
|---|---|---|---|---|---|---|---|
| Diffuse | Focal | Spherical | Segment | Mesentery | Blood vessels | ||
| IgG | Negative | ||||||
| IgM | Negative | ||||||
| IgA | √ | + | |||||
| C3d | √ | + | |||||
| C4d | √ | Small amount | |||||
| C1q | Negative | ||||||
| Fib | √ | Small amount | |||||
| TdT | Nuclear + + + | ||||||
| CD3 | Plasma + | ||||||
| CD19 | Plasma + + + | ||||||
| Ki-67 | |||||||
| CD2 | Positive background T Lymphocytes | ||||||
| CD99 | Membrane/plasma + + + | ||||||
| CD79a | Plasma + + + | ||||||
| CD10 | Membrane/plasma + + + | ||||||
| Bcl-2 | Nuclear + | ||||||
| Pax-5 | Nuclear + + + + | ||||||
CD30, MPO, CD34, and CyclinD1 were all negative; in situ hybridization EBER was negative
C4d score: C4d score (range × intensity, 0−3 points each: total score 0−9 points) 0 points
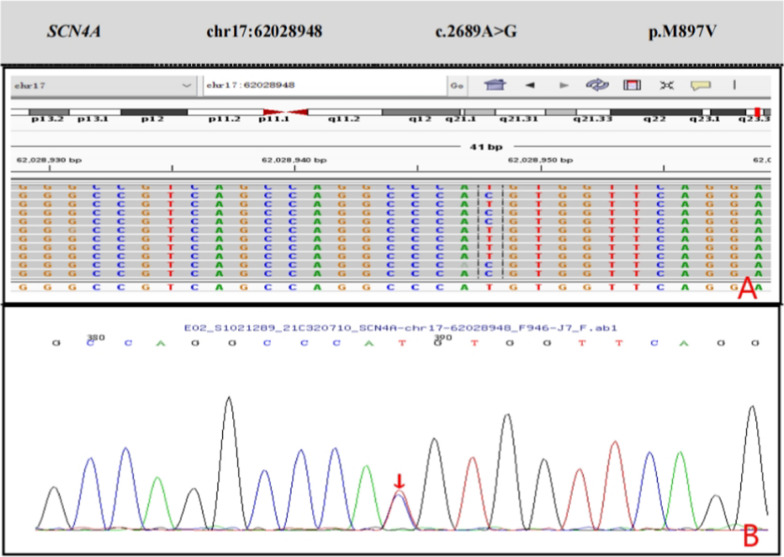
Fig. 2.
More frequently disease-causing gene mutation of Sanger sequencing verification result. A Second-generation sequencing of the gene revealed a novel mutation c.2689A > G in the SCN4A gene (the arrow shows the mutation site). B The child's father, mutations in 163 G > A, peak figure can be displayed as G > C > T A or its reverse complementary sequence
Fig. 3.
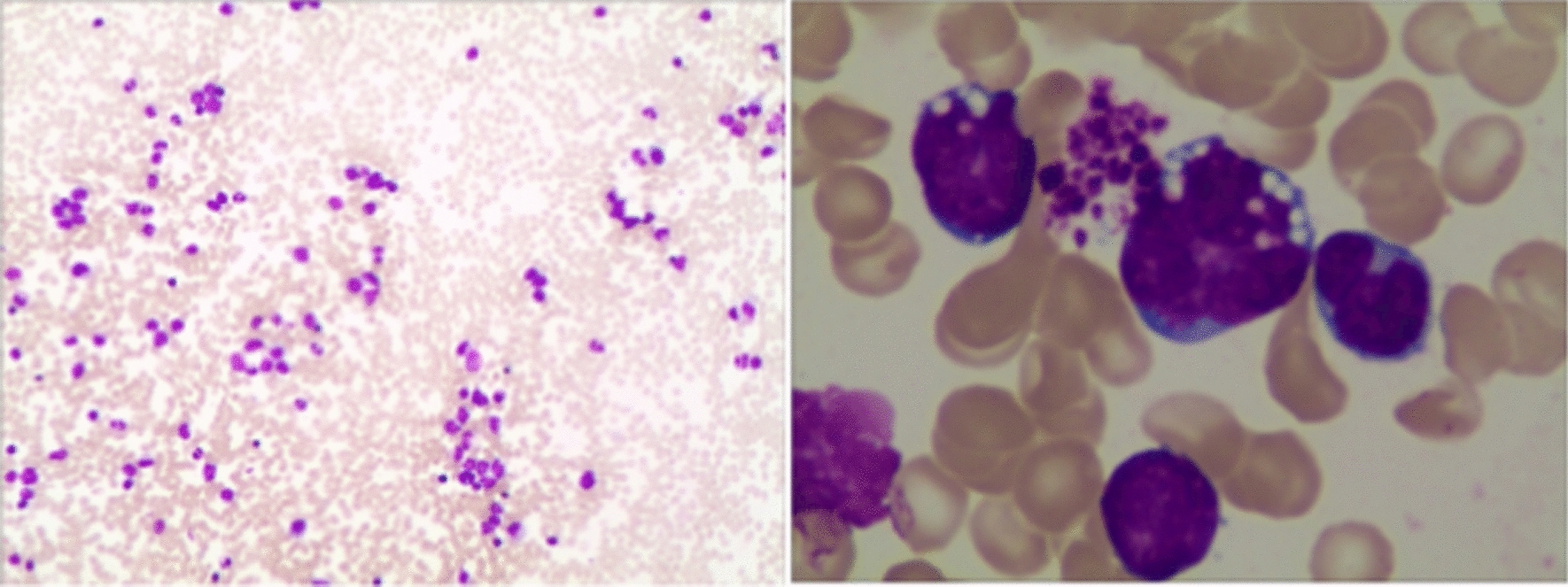
Bone marrow cell morphology analysis report
Table 4.
Patient’s historical information
| September, 2021 | He was admitted to the hospital with increased urine output, vomiting, and general weakness |
| October, 2021 | Pathological return of renal puncture: diffuse lymphoid cell infiltration of the renal interstitium |
| November, 2021 | Marrow + peripheral hematocyst report: B-lymphoblastic leukemia |
| November, 2021 | Start the induction protocol for the VDLP protocol immediately |
| December, 2021 | Such as vomiting, polyuria, and cough improve |
| January, 2022 | Continue with two courses of CAM chemotherapy regimen |
| April, 2024 | The patient is currently still in the maintenance phase of treatment and is in a continuous state |
Discussion
In this case, the patient presented with diabetes insipidus and refractory hypokalemia as the initial symptoms, and there were no positive signs of leukemia on physical examination. After a series of complex tests, the diagnosis of B-lymphoblastic leukemia/lymphoma was finally confirmed. Previous literature reports have suggested that impaired kidney function and enlarged kidneys are common initial manifestations of ALL. However, acute lymphoblastic leukemia with kidney infiltration presenting as nephrogenic diabetes insipidus is rare. This case suggests that clinicians should try to explain multiple clinical manifestations as much as possible from a holistic perspective, broadening our understanding of ALL.
At the beginning of the disease, children with ALL have a rare initial onset with renal enlargement or a urinary symptom, such as edema, hematuria, hypertension, oliguria, or polyuria [8, 9]. A study showed 24 ALL children with renal damage as the first symptom, accounting for 2.33% of the newly treated children in the same period, being very rare in clinical practice. The first symptom was edema (75.0%), and more than half of them (58.3%) were first diagnosed in a nonhematology department [10].
Meanwhile, diabetes insipidus caused by leukemia is rare and can be divided into central diabetes insipidus and nephrogenic diabetes insipidus. The former is caused by blasts infiltrating the nervous system, which is CNS leukemia. According to research by Abraham Kornberg et al., leukemia can infiltrate the pituitary gland or hypothalamus causing central diabetes insipidus, respectively [11]. Nephrogenic diabetes insipidus due to renal infiltration of blasts is rare. One case has been reported by Dezhi Li et al., which describes a 19-year-old man suffering from weakness, polydipsia, and polyuria for 1 month [12]. Nephrogenic diabetes insipidus was diagnosed by water deprivation and pressurization test. Combined with the findings of immunophenotypic of bone marrow examination, cerebrospinal fluid cytology, and abdominal ultrasonography, the final diagnosis of precursor B-cell ALL with renal infiltration was confirmed. Foresti reported on a 69-year-old male patient with 4-month history of polyuria and polydipsia. Plasma vasopressin levels were undetectable, and dehydration tests yielded abnormal results. These findings led to a diagnosis of central diabetes insipidus. Additionally, hematological assessments revealed acute monocytic leukemia. A potential link between the hematological and endocrine disturbances was proposed, with post mortem histological examinations revealing leukemic infiltration of the pituitary stalk [13]. In this case, the initial symptoms of the patient were diabetes insipidus and refractory hypokalemia, and there were no leukemia-related positive signs from the physical examination. At that time, acute glomerulopathy was highly suspected, but renal biopsy showed lymphocyte infiltrates. Central diabetes insipidus was excluded by diagnostic examinations, and the diagnosis of nephrogenic diabetes insipidus with renal tubular acidosis was then confirmed, while bone marrow aspiration and renal biopsy confirmed the diagnosis of ALL. This case bears resemblance to the study conducted by Dezhi Li, but the child’s examination detailed in this report is notably more comprehensive, as renal biopsy vividly demonstrated tumor cell infiltration in the kidneys. Research indicates that diabetes insipidus stemming from central nervous system leukemia is more prevalent than that caused by renal involvement in leukemia. Foresti elaborated on the association between acute monocytic leukemia and central diabetes insipidus. Although the initial symptoms of acute lymphoblastic leukemia (ALL) are varied, inaccuracies in diagnosis can exacerbate the condition, underscoring the importance of prompt and precise diagnosis and treatment. This case offers a novel perspective on the urinary system as an initial indicator of the disease. ALL should be considered in the differential of unexplained renal injury, even if blood investigations are normal. Special attention should also be paid to examination of the liver, spleen, and lymph nodes. The peripheral blood cells should be submitted for morphological analysis, and bone marrow biopsy or renal biopsy should be performed as soon as possible to confirm the diagnosis. Nevertheless, this study has its limitations. It documents a rare instance of acute lymphoblastic leukemia presenting primarily with renal diabetes insipidus. The generalizability of findings from a single case is minimal, necessitating further case studies for validation.
Conclusion
This paper reports a case with nephrogenic diabetes insipidus as the initial symptoms and analyzes the details of the case. Clinicians’ understanding of ALL is widened by the evidence that ALL renal infiltrates can cause nephrogenic diabetic insipidus.
Acknowledgements
Not applicable.
Author contributions
Ning Qu analyzed and interpreted the patient data regarding the hematological disease and the transplant. Hongtao Zhu performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported in part by grants from Study on the correlation between FKBP4 gene polymorphism and the therapeutic effect of glucocorticoid in children with systemic lupus erythematosus (2022D01C466).
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee. Written informed consent was obtained from individual or guardian participants.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14(6):e205–17. 10.1016/S1470-2045(12)70580-6 [DOI] [PubMed] [Google Scholar]
- 2.Levinsen M, Taskinen M, Abrahamsson J, Forestier E, Frandsen TL, Harila-Saari A, et al. Clinical features and early treatment response of central nervous system involvement in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(8):1416–21. 10.1002/pbc.24981 [DOI] [PubMed] [Google Scholar]
- 3.Kirshbaum JD. Leukemia: a clinical and pathologic study of one hundred and twenty-three fatal cases in a series of 14,400 necropsies. JAMA Internal Med. 1943;71(6):777–92. 10.1001/ARCHINTE.1943.00210060038003. 10.1001/ARCHINTE.1943.00210060038003 [DOI] [Google Scholar]
- 4.Suriya OM, Aleem A. Frank hematuria as the presentation feature of acute leukemia. Saudi J Kidney Dis Transplant. 2010;21(5):940–2. [PubMed] [Google Scholar]
- 5.Suh WM, Wainberg ZA, de Vos S, Cohen AH, Kurtz I, Nguyen MK. Acute lymphoblastic leukemia presenting as acute renal failure. Nat Clin Pract Nephrol. 2007;3(2):106–10. 10.1038/ncpneph0400 [DOI] [PubMed] [Google Scholar]
- 6.Elsurer R, Afsar B, Ozdemir HB, Ozdemir NF, Sezer S, Haberal M. Acute tubulointerstitial nephritis and acute leukemia: report of 2 cases. J Nephrol. 2006;19(4):521–4. [PubMed] [Google Scholar]
- 7.Laing CM, Toye AM, Capasso G, Unwin RJ. Renal tubular acidosis: developments in our understanding of the molecular basis. Int J Biochem Cell Biol. 2005;37(6):1151–61. 10.1016/j.biocel.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Arora SK, Swarnim S, Hemal A, Bidhuri N. Acute lymphoblastic leukemia presenting as nephromegaly in a child: a rare case report. Turk J Pediatr. 2019;61(1):97–101. 10.24953/turkjped.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 9.Sherief LM, Azab SF, Zakaria MM, Kamal M, Elbasset Aly MA, Ali A, et al. Renal presentation in pediatric acute leukemia: report of 2 cases. Medicine (Baltimore). 2015;94(37): e1461. 10.1097/MD.0000000000001461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiantian X, Yufeng L, Huixia W, Linlin W, Shufang S, Bai L, et al. Clinical analysis of 24 cases of pediatric acute lymphoblastic leukemia with renal involvement as the initial manifestation. Chin J Appl Clin Pediatrics. 2021;36(23):1796–800. [Google Scholar]
- 11.Kornberg A, Zimmerman J, Matzner Y, Polliack A. Acute lymphoblastic leukemia. Association with vasopressin-responsive diabetes insipidus. Arch Internal Med. 1980;140(9):1236. 10.1001/archinte.1980.00330200112030 [DOI] [PubMed] [Google Scholar]
- 12.Li D, Liu Q, Feng Z, Zhang Q, Feng S. Nephrogenic diabetes insipidus in initial stage of acute lymphoblastic leukemia and relapse after haploidentical hematopoietic stem-cell transplantation: a case report. Medicine (Baltimore). 2018;97(24): e11157. 10.1097/MD.0000000000011157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foresti V, Casati O, Villa A, et al. Central diabetes insipidus due to acute monocytic leukemia: case report and review of the literature. J Endocrinol Invest. 1992;15(2):127–30. 10.1007/BF03348677 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.