Abstract
Background
Opioid-related fatalities are a leading cause of death in Ohio and nationally, with an increasing number of overdoses attributable to fentanyl. Rapid fentanyl test strips can identify fentanyl and some fentanyl analogs in urine samples and are increasingly being used to check illicit drugs for fentanyl before they are used. Fentanyl test strips are a promising harm reduction strategy; however, little is known about the real-world acceptability and impact of fentanyl test strip use. This study investigates fentanyl test strip distribution and education as a harm reduction strategy to prevent overdoses among people who use drugs.
Methods
The research team will recruit 2400 individuals ≥ 18 years with self-reported use of illicit drugs or drugs purchased on the street within the past 6 months. Recruitment will occur at opioid overdose education and naloxone distribution programs in 16 urban and 12 rural Ohio counties. Participating sites will be randomized at the county level to the intervention or non-intervention study arm. A brief fentanyl test strip educational intervention and fentanyl test strips will be provided to participants recruited from sites in the intervention arm. These participants will be eligible to receive additional fentanyl test strips for 2 years post-enrollment. Participants recruited from sites in the non-intervention arm will not receive fentanyl test strip education or fentanyl test strips. All participants will be followed for 2 years post-enrollment using biweekly, quarterly, and 6-month surveys. Primary outcomes include (1) identification of perceived barriers and facilitating factors associated with incorporating fentanyl test strip education and distribution into opioid overdose education and naloxone distribution programs; (2) differences in knowledge and self-efficacy regarding how to test drugs for fentanyl and strategies for reducing overdose risk between the intervention and non-intervention groups; and (3) differences in non-fatal and fatal overdose rates between the intervention and non-intervention groups.
Discussion
Findings from this cluster randomized controlled trial will contribute valuable information about the feasibility, acceptability, and impact of integrating fentanyl test strip drug checking in rural and urban communities in Ohio and help guide future overdose prevention interventions.
Trial registration
ClinicalTrials.gov NCT05463341. Registered on July 19, 2022. https://clinicaltrials.gov/study/NCT05463341
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08440-y.
Keywords: Overdose, Harm reduction, Fentanyl, Fentanyl test strips, Study protocol, Randomized controlled trial, Substance use
Background
The United States (US) is experiencing an opioid-related public health crisis. In 2021, Ohio ranked 7th among all states for the highest age-adjusted drug overdose death rate, 48.1 per 100,000 population, which was 48.5% higher than that of the overall US (32.4 per 100,000 population) [1, 2]. This drug overdose fatality rate is driven by the use of opioids [1–3]. An increasing number of opioid-related deaths in the US are attributable to fentanyl, a highly potent synthetic opioid pain medication [3–5]. Illicit fentanyl and its analogs may be manufactured and sold alone or added to other drugs, such as heroin, cocaine, and counterfeit prescription pills, with or without the user’s knowledge [3–5].
The increasing pervasiveness of highly lethal fentanyl and fentanyl analogs in the illicit drug supply in the US, including Ohio, has posed a substantial challenge for public health officials looking for strategies to reduce overdoses. While some effective harm reduction strategies, such as opioid overdose education and naloxone distribution (OEND) programs, are becoming more available and widely accepted, they may not be sufficient for preventing overdose deaths due to fentanyl.
Rapid fentanyl test strips (FTS), designed to test for the presence of fentanyl and some fentanyl analogs in urine samples, are increasingly being used off-label to test illicit drugs for fentanyl before they are consumed and are highly sensitive and specific in detecting fentanyl [6–9]. Research indicates that when people who use drugs (PWUD) receive a positive result from a fentanyl test strip, they are more likely to perform overdose risk reduction behaviors [9]. These behaviors (e.g., using less of the drug; using in the presence of someone else) may help to prevent an overdose, or ensure that assistance is nearby, if needed. In the US, access to FTS for home use is variable and is primarily being supported by public health departments and community-based harm reduction organizations. Because access to FTS is limited, little is known about (1) the feasibility and acceptability of this intervention among public health workers, community-based organizations, and PWUD and (2) how outcomes from this intervention compare with OEND-only programs.
This study protocol is designed to test an intervention to prevent drug overdoses among PWUD in rural and urban counties of Ohio. Rural populations are disproportionately burdened by the opioid crisis and face serious health disparities related to their ability to access substance use disorder treatment and emergency care, making them an important population for this research [10, 11]. The proposed intervention will incorporate FTS education and distribution into a subset of OEND sites in Ohio. The long-term goal of this research is the reduction of overdose-related morbidity and mortality in Ohio and nationally.
Study objectives and aims
The research objectives of this study are:
Determine the feasibility and acceptability of providing FTS education and testing materials distribution in existing OEND programs.
Determine if adding FTS education and distribution to OEND programs decreases opioid overdose rates among PWUD.
Using a two-arm cluster-randomized trial design, we will answer the research objectives by testing the following specific aims:
Specific aim #1. Determine the perceived barriers and facilitating factors associated with incorporating FTS education and distribution in existing OEND programs in rural and urban counties.
Specific aim #2. Test the hypothesis that PWUD who receive FTS education and testing materials as part of an OEND program will have improved knowledge and self-efficacy regarding how to test drugs for fentanyl and strategies for lowering their risk of an opioid overdose.
Specific aim #3. Test the hypothesis that individuals who receive FTS education and testing materials as part of an OEND program will have a lower opioid overdose rate than individuals who receive OEND only (“usual practice”).
Methods/design
Study setting
Ohio has an established infrastructure to streamline OEND that can aid in opioid overdose prevention. In an effort to prevent opioid overdose fatalities in the state, the Ohio Department of Health has partnered with local public health departments and community organizations in the state to establish a network of OEND sites. The initiative, called Project DAWN (Deaths Avoided With Naloxone), has more than 200 sites throughout the state, with many counties having multiple sites (https://odh.ohio.gov/know-our-programs/project-dawn/project-dawn-programs). Project DAWN sites use trained overdose prevention educators to provide OEND at no cost to clients. Recruitment for this study will occur at Project DAWN sites in Ohio.
Eligibility
Inclusion criteria for the study are (1) age 18 years or older; (2) visitor to a Project DAWN site in Ohio that has agreed to participate in the study; (3) self-reported use of illicit drugs or any drugs purchased on the street within the past 6 months; (4) has a phone number or email address to allow for follow-up contact; and (5) able to participate in study activities in English. Individuals will be excluded from the study if they are unwilling or unable to give informed consent due to altered mental status or other reasons or if they are currently incarcerated.
Study recruitment
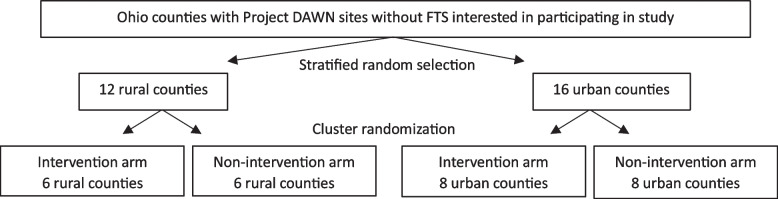
Study recruitment will happen on a rolling basis. Periodically throughout the recruitment period, our study partner, Ohio Department of Health, will send recruitment invitations to Project DAWN sites on our behalf. A stratified sampling process will be used to select 12 rural and 16 urban counties from among those with Project DAWN sites that indicate interest in participating in the study and that are not distributing FTS at the time of study enrollment.
Participating counties will then be randomized into the intervention and non-intervention arms of the study, stratified by rural/urban status (Fig. 1). Randomization will occur at the county level, and all Project DAWN sites in the same county will be assigned to the same study arm (either intervention or non-intervention). Therefore, all participants enrolled in the same county will be in the same study arm. County randomization will be conducted by the biostatistician on the project (SAF), without influence by study principal investigators (PIs) or staff.
Fig. 1.
Method of assignment of counties to intervention and non-intervention study arms
To achieve our recruitment goal of 2400 participants, study staff will be on-site to enroll Project DAWN clients who wish to participate in the study. Informed written or e-consent and baseline data, contact information (i.e., locator form), and demographics will be obtained. Project DAWN sites vary in size and operate according to different schedules, but generally provide OEND at least once per week, and frequently see clients more than once. Research study staff will coordinate with each site to ensure rotating coverage of all study counties. Enrollment will begin upon Institutional Review Board (IRB) approval and follow-up will continue with each participant for 2 years. Six-month follow-up questionnaires will be administered to each participating client based on their date of enrollment. Clients who decline to participate will not be included in the study. Individuals who decline to participate but indicate they are interested in obtaining FTS will be offered a printed list of alternate sources of FTS.
Intervention
Fentanyl test strip intervention
A brief 20-min FTS educational intervention will be provided by the study team to participants at Project DAWN sites in the intervention arm of the study following enrollment and collection of baseline data (Table 1). The education will be offered one-on-one with participants in the intervention arm using a curriculum developed by the study team. The curriculum contains the following components: education on the purpose, benefits, and limitations of FTS testing; a hands-on demonstration of how to use FTS for drug testing prior to consumption; diagrams explaining how to interpret FTS results; and what to do if the FTS is positive. Education will be provided on how to use FTS for different drug delivery methods (e.g., injection, snorted, pills). A brief video will be developed by the study team to demonstrate how to use and interpret the FTS. The video will be shown during the educational intervention and will also be accessible to participants in the intervention arm following enrollment. Participants will be advised of the possibility of false positive/negative results, as well as the possibility that their drugs could be mixed with other harmful and/or unanticipated substances not detectable with FTS. Participants will be encouraged to practice other harm reduction strategies (e.g., having someone with them when using drugs, keeping naloxone nearby).
Table 1.
Schedule of enrollment and follow-up assessments
| Activity | Time point | |||
|---|---|---|---|---|
| Screening | Enrollment | Biweekly for 2 years post-enrollment | 6 months post-enrollment | |
| − 1 | 0 | 1 | 2 | |
| Enrollment | ||||
| Eligibility screening | X | |||
| Informed consent | X | |||
| Intervention | ||||
| FTS training (intervention group) | X | |||
| Receive 10 FTS (intervention group) | X | |||
| Additional FTS upon request (intervention group) | X | X | ||
| Usual practice (non-intervention group) | X | X | X | X |
| Assessment | ||||
| Demographics | X | |||
| History of overdose | X | |||
| Substance use experiences | X | X | ||
| FTS knowledge | X | X | ||
| FTS attitudes | X | X | ||
| FTS self-efficacy | X | X | ||
| Drug testing behaviors | X | X | ||
| Recent overdose (past 2 weeks) | X | |||
| Drug use (past 2 weeks) | X | |||
| FTS use (past 2 weeks) | X | |||
| Protocol deviation/adverse event reporting | As needed throughout study | |||
FTS fentanyl test strip
Each study participant will be given 10 FTS upon enrollment. The strips will be packaged with instructions on how to use FTS, a QR code link to the video, harm reduction strategies, and contact information for the study team. Replacement FTS will be available to study participants in the intervention arm upon request throughout their 2-year follow-up period and participants will be asked if they need additional FTS during their biweekly surveys. Replacement FTS can be mailed to participants via US Postal Service or obtained from study staff during subsequent visits to the Project DAWN sites.
Non-intervention group
Participants in the non-intervention arm of the study will not receive FTS education or test strips upon enrollment, but will receive OEND from Project DAWN staff according to their usual practice. During the latter half of year 5, after data collection is complete, participants in the non-intervention arm will be offered the FTS educational intervention and a supply of FTS.
Data collection
Questionnaires
Baseline and 6-month follow-up questionnaires will consist of true/false knowledge questions, 5-point Likert scale attitude and self-efficacy questions, and multiple-choice questions related to participant behaviors and characteristics. Participants will be asked to indicate their degree of interest in using or avoiding drugs containing fentanyl. Baseline questionnaires will be administered to Project DAWN clients who enroll in the study using iPads and the REDCap (Research Electronic Data Capture) data collection platform. Follow-up questionnaires will be administered at 6 months post-enrollment to study participants via email/text, in-person at Project DAWN sites, or via telephone. Data from paper questionnaires will be entered into REDCap by the research team upon completion and double-entry verification will be used.
Qualitative data
In the second quarter of year 5, qualitative data will be collected through interviews with Project DAWN personnel in the intervention arm to examine the feasibility and acceptability of offering FTS at Project DAWN sites. Topics of discussion will include (1) attitudes about the use of FTS; (2) perceptions of the FTS intervention that was offered at their Project DAWN site, including perceived benefits and harms; (3) barriers and enabling/reinforcing factors related to offering FTS at Project DAWN sites; and (4) interest in continuing to offer FTS at Project DAWN sites. Interviews will be conducted by study staff using an interview guide and will be audio recorded and transcribed. Coding and analysis of the transcripts will be conducted by the study team.
Statistical analyses
Study data will be analyzed using an intention-to-treat approach.
Specific aim #1: To determine the feasibility and acceptability of incorporating FTS education and distribution into existing OEND programs, a questionnaire will be administered to Project DAWN personnel, site supervisors, health commissioners, and other key intervention site personnel in year 5. Project DAWN clients in the intervention arm will be asked about the acceptability of the program as part of their 6-month follow-up questionnaire. Process measures will be collected throughout the study as another source of data on the feasibility and acceptability of the intervention. Quantitative data on process measures will include, but is not limited to, number of Project DAWN sites that express interest in participating in the study; number of Project DAWN sites that enroll; number of potential participants who request to enroll in the study; number of participants successfully enrolled; number of replacement FTS requested and distributed; proportion of participants who complete the brief biweekly surveys; and the proportion of participants who complete the 6-month follow-up questionnaire. Descriptive statistics will be calculated, including overall mean estimates with 95% confidence intervals. Analyses will also be performed by subgroups (e.g., rural/urban, Project DAWN site personnel/client, and demographic subgroups).
Specific aim #2: Measures will be taken at two time points (baseline and 6 months) to test specific aim #2. The instrument to test change in knowledge is composed of true/false items. Correct responses for each of the items will be assigned a value of 1, and incorrect responses will be given a value of 0. Total scores will be calculated. There are also items to test changes in attitudes and self-efficacy that use a 5-point Likert scale. We will also compute total scores for these items. We will fit a linear mixed model using total scores for knowledge as the response variable to test change in knowledge and will fit another linear mixed model using total scores for attitudes/self-efficacy as the response variable to test change in self-efficacy.
Random effects for county (rural/urban), Project DAWN site within county, and participant within county will be included, as well as fixed effects for time (baseline or 6 months) and treatment (intervention/non-intervention). A random variable for secular time will also be included to account for changes across time (e.g., new opioid overdose prevention initiatives, changes in drug supply). To test the hypothesis of change differences between the two time points between the two arms, we will include an interaction term “treatment × time (baseline, 6 months)” in the model. This model will include demographic variables (gender, age, and race) and other relevant covariates or confounding factors, such as education level completed, employment, previous receipt of FTS education and testing materials (Y/N), and previously experienced an overdose (Y/N). Other relevant interaction terms, such as “treatment × gender” and “treatment × race,” will be evaluated. Holm’s method will be used to adjust for ad hoc multiple comparisons. In addition, general linear mixed models (GLMM) with a logit link function will be used to study change differences for specific Y/N items or questions.
Specific aim #3: Non-fatal overdose measures will be taken every 2 weeks and fatal overdose measures will be taken quarterly to test specific aim #3. To study the odds of an experienced overdose (Y/N), a GLMM with logit function will be used. This model will include the same random and fixed effects as identified for the model used to test specific aim #2. At the end of the study period, the overall non-fatal overdose rate (with a 95% confidence interval) will be compared between intervention and non-intervention arms. The same comparison will be done separately for the overall fatal overdose rate (with a 95% confidence interval) between the intervention and non-intervention arms.
Missing data
The analyses will be conducted using an intention-to-treat approach. For the primary and secondary outcomes, every effort will be made to minimize missing data; however, in the event that data are missing, we will document the process that resulted in the missing data and consider model-based imputation methods to account for the missing data. Guidelines for handing missing data in clinical trials will be followed [12].
Sample size
We expect an enrollment rate of 65% of eligible participants and 30% attrition, a conservative estimate based on research with similar populations [13]. For the power calculations, we conservatively assumed one Project DAWN site per county. We also assumed that non-fatal overdose rates in the non-intervention and intervention groups are 20% and 10%, respectively, and the annual fatal overdose rate in the non-intervention group is 0.65% [14]. Based on our sample size calculations, we will enroll 1200 participants in each study arm for a total of 2400 participants. Assuming a 30% attrition rate, 840 participants in each arm will complete 2 years of follow-up, for a total of 1680 participants. This will permit detection of an effect size range of 0.3–0.4 for specific aim #2 and rate differences of 0.1 and 0.14, respectively, for non-fatal and fatal rates for specific aim #3, given the assumptions identified above.
Participant retention
Participant retention will be managed in a series of ongoing steps (Table 2). First, participants will receive “thank you” messages via email through an automated REDCap system or letter via US mail, if email is not available. Next, participants’ locator form contact information will be verified by the enrolling research assistant (RA) within 2 weeks of enrollment.
Table 2.
Participant follow-up procedures
| Task | When | How | Who | |
|---|---|---|---|---|
| New participant “thank you” messages | Within 3 business days of enrollment | Email (if available) | Automated via REDCap | |
| Within 2 weeks of enrollment | US mail | Enrolling RA | ||
| Locator form contact verification | Within 2 weeks of enrollment | Email/phone | Enrolling RA | |
| Biweekly survey follow-up | 1 | Every 3 days—up to 4 reminders for each biweekly survey | Email/text | Automated via REDCap default survey delivery method |
| Within 2 weeks of missing second survey in a row | Email and/or text, US mail, social media | RAs | ||
| Within 3 weeks of missing second survey in a row | Locator form friend/family contacts, internet search | |||
| 2 | After completing monthly follow-up series above (step 1) | Repeat step 1 process monthly until participant successfully contacted | ||
| 6-month survey follow-up | 1 | Every 5 days—up to 5 reminders | Email/text | Automated via REDCap default survey delivery method |
| Within 2 weeks of missing survey | Email and/or text, US mail, social media | RAs | ||
| Within 3 weeks of missing survey | Locator form friend/family contacts, internet search | |||
| 2 | After completing monthly follow-up series above (step 1) | Repeat step 1 process monthly for up to 2 months | ||
| Participant engagement cards and newsletters | By the 5th day of each month | Birthday cards | Administrative assistant | |
| January: New Year card | Holiday/seasonal cards | RAs | ||
| August: participant newsletter | ||||
After this, participants will receive follow-up communication from the study team if they miss biweekly surveys or their 6-month survey. Participants receive automated reminders via REDCap every 3 days for up to four reminders for each biweekly survey via email or text, depending on the participant’s preference. Within 2 weeks of missing a second consecutive biweekly survey, RAs will send an email, call/voicemail, letter, and/or social media message depending on the participant’s preferences. Within 3 weeks of missing a second consecutive biweekly survey, the RAs will use the participant’s locator form to contact the participant’s friend or family. This process will be completed monthly until the individual has been successfully contacted or begins surveys. Participants also will receive automated reminders for the 6-month survey every 5 days with up to five reminders via their default survey delivery method through REDCap. Within 2 weeks of missing their 6-month survey, RAs will send an email, call/voicemail, letter, and/or message through social media. Within 3 weeks of missing the 6-month survey, RAs will use the participant’s locator form to contact the participant’s friends or family. This process will be completed for up to 2 months. Participants will also receive a participant newsletter and annual “New Year” and birthday cards.
Study timeline
We expect it will take approximately 24 months to enroll all study participants. After enrollment, study participants will complete follow-up activities for 2 years. Participants may choose to withdraw from the study at any time and will not receive further contact from the study team.
Data management
A Certificate of Confidentiality is in place for this study. Confidentiality will be promoted by assigning an identification number to each study participant. We will use only these identification numbers (and not participants' names) in the database used for study analyses. Study materials containing identifiers, including signed consent forms and gift card receipts, will be scanned and then paper copies will be shredded. Research records will be stored in a password protected computer file. Only study team members with a research need to view the data, appropriate research certifications, and IRB approval will have access. Identifiable data will be retained for 6 years after the research is complete. Upon acceptance of all study manuscripts, any electronic files with participant identifiers will be deleted.
Study team
The study team consists of two principal investigators who collaborate closely to oversee the day-to-day work of the study team and are responsible for all aspects of this research, as well as 3 co-investigators, a research coordinator, and 4 research associates. Study co-investigators assisted the principal investigators with the development of study protocols and processes in year 1 and contribute their expertise as needed throughout the study. The research coordinator oversees the implementation of participant recruitment, retention, and consent processes as well as data collection procedures conducted by the research associates. A principal investigator meets with the research coordinator at least weekly and meets with the research associates at least biweekly. The study also utilizes a community advisory board that consists of representatives from non-profit organizations active in the harm reduction community, representatives from government agencies, such as health departments and mental health services, and individuals with lived experience of drug use. The advisory committee meets approximately every 6 months.
Data safety and monitoring
A data safety and monitoring board (DSMB) will be used for this study. Collectively, the DSMB has expertise in medicine, harm reduction, behavioral science (including qualitative research expertise), biostatistics, and public health. The DSMB will review study protocols to identify whether appropriate safeguards are in place to prevent adverse events, such as fatal drug overdoses, and to determine whether the observed frequency and type of events exceed those expected in the study population. The DSMB will review the frequency of adverse events reported among intervention and non-intervention participants to identify any unanticipated problems that may increase the risk of harm among study participants or others and will make recommendations for additional safety measures, or in the case of severe unanticipated negative outcomes, stopping the trial. The DSMB will meet twice a year to review study progress and may convene additional meetings as necessary. Further details about the DSMB charter are available upon request.
Dissemination of study findings
Study findings will be shared with study participants and partnering Project DAWN sites. This study is registered on www.ClinicalTrials.gov, and summaries of study findings will be available on the website upon study completion. Study findings will also be shared through publication in peer-reviewed journals and presentation at scientific meetings and conferences. Publication authorship will be determined using International Committee of Medical Journal Editors guidelines.
Discussion
Because opioid overdose is a tremendous problem in Ohio and nationally, more studies on the primary and secondary prevention of overdose are needed. Collaborating on this research project with public health officials at the state and local levels, as well as community-based harm reduction organizations, will give us insight into the real-world benefits, challenges, and unanswered questions associated with implementing FTS education and distribution programs and guide future studies.
We expect that the findings of this study will be used to inform decisions by public health leaders and policy makers on whether to support the continuation and expansion of fentanyl test strip education and distribution in Ohio. Using improved scientific rigor compared with previous research, the findings of this study will provide missing, fundamental information to our base of knowledge regarding the feasibility, acceptability, and associated benefits and harms of this emerging strategy for preventing opioid overdoses. In addition to Ohio, we believe that this program could serve as a model for other states.
Trial status
Protocol version 5, approved on October 11, 2023. Study recruitment began on September 9, 2022, and is ongoing. We anticipate study recruitment will be complete by December 31, 2024.
Supplementary Information
Acknowledgements
We would like to thank our colleagues Alexandra Antonova, Aleah Cumberbatch, Jacob Holycross, and Spencer Long for their contributions to this research.
Abbreviations
- FTS
Fentanyl test strips
- DSMB
Data safety and monitoring board
- GLMM
General linear mixed models
- IRB
Institutional Review Board
- OEND
Opioid overdose education and naloxone distribution
- PI
Principal investigator
- Project DAWN
Project Deaths Avoided With Naloxone
- PWUD
People who use drugs
- RA
Research assistant
- REDCap
Research Electronic Data Capture
- US
United States
Authors’ contributions
ASM led manuscript preparation. The research was conceptualized and the original study protocol was developed by NLM, GAS, and SAF. All authors provided feedback on the manuscript and approved the final draft of the manuscript for publication.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01DA052580. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design of this study and will not have any role in the collection, management, analysis, and interpretation of data; writing of the report; or decision to submit the report for publication.
Availability of data and materials
The study PIs will oversee the management of all aspects of this research study and will determine access to the final trial dataset. Unique study resources and data will be made available for research purposes to qualified individuals within the scientific community after publication. Upon written request to the study contact PI (NLM), de-identified data used in publications will be made available to users under a data-sharing agreement. Along with the data, we also will make available the data instruments used to collect the data, methods of collection, variable definitions, and potential limitations for use.
Declarations
Ethics approval and consent to participate
The Nationwide Children’s Hospital Institutional Review Board has given ethical approval for this study (STUDY00001919). Any modifications to the study protocol are subject to approval by the IRB of record. Relevant modifications will be reported to the trial registry and other parties as necessary. Informed consent will be obtained from all study participants according to the IRB-approved study protocol. The consent process will take place on-site at the recruitment location and will be completed prior to any data collection. At the time of enrollment, a member of the research team will review the contents of the informed consent document with the potential participant and answer any questions they may have prior to obtaining written consent via e-signature or paper consent form. Consent will be documented in REDCap and a pdf file of the signed e-consent form will be emailed to the participant. If the participant does not have an email address, a paper copy will be signed and distributed to them. Paper consent forms will be used to obtain written consent in person at the time of enrollment only as necessary (e.g., due to technology failure). Participants will be able to leave the study or stop participating in study activities at any time.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Sponsor
As the primary organization conducting this study and associated data analysis, Nationwide Children’s Hospital is the sponsor of this research. They can be contacted at 700 Children’s Drive, Columbus, OH 43205, USA; phone number: 614–722-2000.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. Ohio priority topic investments. Ohio overdose investment snapshot. CDC. https://www.cdc.gov/injury/budget-funding/ohio-aces-and-overdose-prevention-funding.html?CDC_AAref_Val=https://www.cdc.gov/injury/budget/policystatesnapshots/ohio.html. Accessed 27 Jul 2024.
- 2.Centers for Disease Control and Prevention. Drug overdose mortality by state. CDC/National Center for Health Statistics. 2021. https://www.cdc.gov/nchs/pressroom/sosmap/drug_poisoning_mortality/drug_poisoning.htm. Accessed 27 Jul 2024.
- 3.Centers for Disease Control and Prevention. Preventing opioid overdoses. 2024. https://www.cdc.gov/overdose-prevention/prevention/index.html. Accessed 27 Jul 2024.
- 4.Peterson AB, Gladden RM, Delcher C, Spies E, Garcia-Williams A, Wang Y, Halpin J, Zibbell J, McCarty CL, DeFiore-Hyrmer J, DiOrio M, Goldberger BA. Increases in fentanyl-related overdose deaths - Florida and Ohio, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):844–9. 10.15585/mmwr.mm6533a3. 10.15585/mmwr.mm6533a3 [DOI] [PubMed] [Google Scholar]
- 5.Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2017. NCHS Data Brief. 2018(329):1-7. Hyattsville: National Center for Health Statistics. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf. Accessed 16 Jun 2024.
- 6.Goldman JE, Waye KM, Periera KA, Krieger MS, Yedinak JL, Marshall BDL. Perspectives on rapid fentanyl test strips as a harm reduction practice among young adults who use drugs: a qualitative study. Harm Reduct J. 2019;16(1):3. 10.1186/s12954-018-0276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieger MS, Yedinak JL, Buxton JA, Lysyshyn M, Bernstein E, Rich JD, Green TC, Hadland SE, Marshall BDL. High willingness to use rapid fentanyl test strips among young adults who use drugs. Harm Reduct J. 2018;15(1):7. 10.1186/s12954-018-0213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger MS, Goedel WC, Buxton JA, Lysyshyn M, Bernstein E, Sherman SG, Rich JD, Hadland SE, Green TC, Marshall BDL. Use of rapid fentanyl test strips among young adults who use drugs. Int J Drug Policy. 2018;61:52–8. 10.1016/j.drugpo.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiper NC, Clarke SD, Vincent LB, Ciccarone D, Kral AH, Zibbell JE. Fentanyl test strips as an opioid overdose prevention strategy: findings from a syringe services program in the Southeastern United States. Int J Drug Policy. 2019;63:122–8. 10.1016/j.drugpo.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 10.Rembert M, Betz M, Feng B, Partridge M. Taking measure of Ohio’s opioid crisis. Swank program in rural-urban policy. The Ohio State University. 2017. https://cpb-us-w2.wpmucdn.com/u.osu.edu/dist/2/14548/files/2017/10/SWANK-Taking-Measure-of-Ohios-Opioid-Crisis-1vtx548.pdf. Accessed 27 Jul 2024.
- 11.Joudrey PJ, Edelman EJ, Wang EA. Drive times to opioid treatment programs in urban and rural counties in 5 US states. JAMA. 2019;322(13):1310–2. 10.1001/jama.2019.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Research Council (US) Panel on Handling Missing Data in Clinical Trials. The prevention and treatment of missing data in clinical trials. Washington, DC: National Academies Press; 2010. 10.17226/12955. [PubMed]
- 13.Karno MP, Rawson R, Rogers B, Spear S, Grella C, Mooney LJ, Saitz R, Kagan B, Glasner S. Effect of screening, brief intervention and referral to treatment for unhealthy alcohol and other drug use in mental health treatment settings: a randomized controlled trial. Addiction. 2021;116(1):159–69. 10.1111/add.15114. 10.1111/add.15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Management of substance abuse: information sheet on opioid overdose. August 2018. https://www.who.int/substance_abuse/information-sheet/en/ Accessed 28 August 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study PIs will oversee the management of all aspects of this research study and will determine access to the final trial dataset. Unique study resources and data will be made available for research purposes to qualified individuals within the scientific community after publication. Upon written request to the study contact PI (NLM), de-identified data used in publications will be made available to users under a data-sharing agreement. Along with the data, we also will make available the data instruments used to collect the data, methods of collection, variable definitions, and potential limitations for use.