Abstract
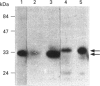
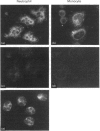
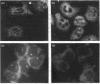
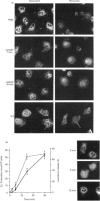
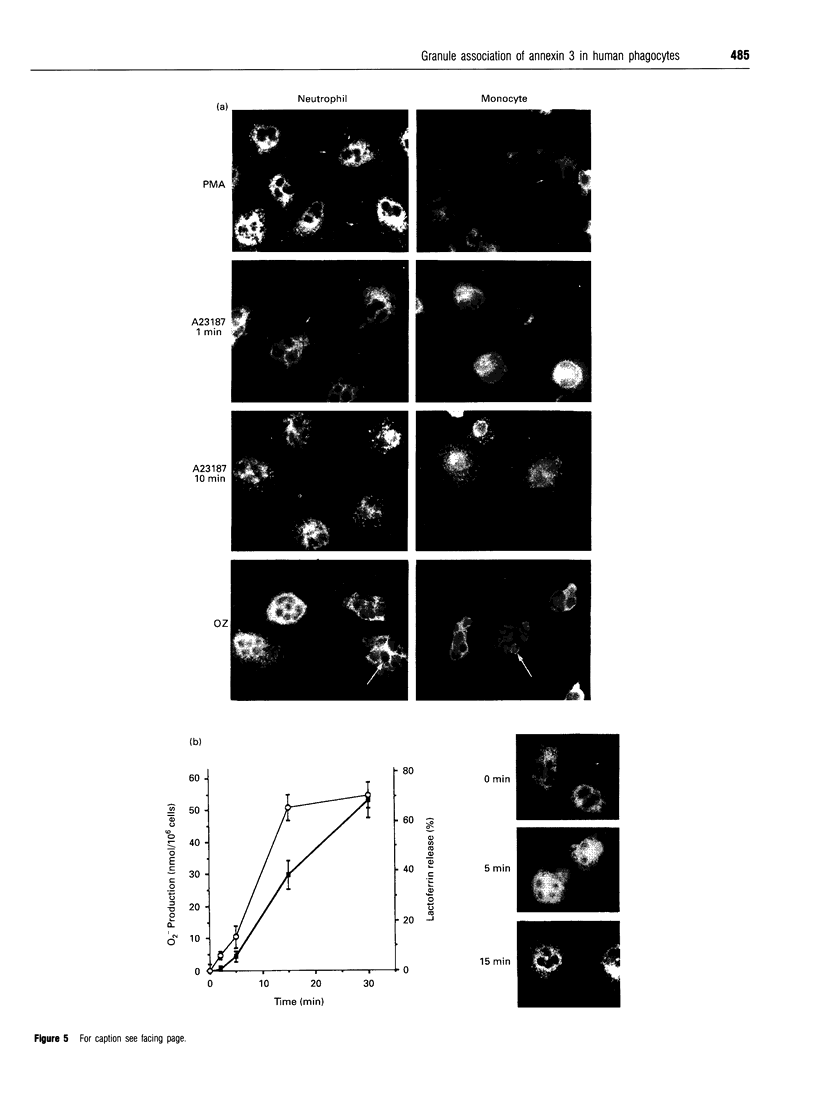
Annexins are soluble proteins capable of binding to phospholipid membranes in a calcium-dependent manner. Annexin 3, a 33 kDa protein mainly expressed in neutrophils, aggregates granules in cell-free assays, and a 36 kDa variant of this protein, specifically expressed in monocytes, has recently been identified. To obtain further information on these proteins, we defined their subcellular localization in resting and activated cells by immunofluorescence microscopy. Both proteins were associated with cytoplasmic granules in resting cells. We obtained evidence to indicate that, in neutrophils which possess a heterogenous granule population, annexin 3 was more likely to be associated with the specific granules. In cells activated with phorbol 12-myristate 13-acetate or opsonized zymosan, the 33 kDa and 36 kDa proteins translocated to the plasma or the phagosome membrane. Upon stimulation with A23187, annexin 3 translocated to the plasma membrane only in neutrophils. We also report that while annexin 3 was associated with restricted membranes in intact cells, it binds indiscriminately to every membrane fraction in cell-free assay. In conclusion, association of both forms of annexin 3 with granules suggests that these proteins could be implicated in processes of granule fusion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Burgoyne R. D. The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell Signal. 1990;2(3):265–276. doi: 10.1016/0898-6568(90)90054-e. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. W., Borregaard N. Mixed enzyme-linked immunosorbent assay (MELISA) for HLA class I antigen: a plasma membrane marker. Scand J Immunol. 1990 Mar;31(3):305–313. doi: 10.1111/j.1365-3083.1990.tb02773.x. [DOI] [PubMed] [Google Scholar]
- Blackwood R. A., Ernst J. D. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990 Feb 15;266(1):195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. The annexin family of calcium-binding proteins. Review article. Cell Calcium. 1989 Jan;10(1):1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Burns A. L., Magendzo K., Shirvan A., Srivastava M., Rojas E., Alijani M. R., Pollard H. B. Calcium channel activity of purified human synexin and structure of the human synexin gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3798–3802. doi: 10.1073/pnas.86.10.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coméra C., Rothhut B., Cavadore J. C., Vilgrain I., Cochet C., Chambaz E., Russo-Marie F. Further characterization of four lipocortins from human peripheral blood mononuclear cells. J Cell Biochem. 1989 Jul;40(3):361–370. doi: 10.1002/jcb.240400312. [DOI] [PubMed] [Google Scholar]
- Coméra C., Rothhut B., Russo-Marie F. Identification and characterization of phospholipase A2 inhibitory proteins in human mononuclear cells. Eur J Biochem. 1990 Feb 22;188(1):139–146. doi: 10.1111/j.1432-1033.1990.tb15381.x. [DOI] [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J Biol Chem. 1978 Apr 25;253(8):2858–2866. [PubMed] [Google Scholar]
- Creutz C. E., Sterner D. C. Calcium dependence of the binding of synexin to isolated chromaffin granules. Biochem Biophys Res Commun. 1983 Jul 18;114(1):355–364. doi: 10.1016/0006-291x(83)91635-2. [DOI] [PubMed] [Google Scholar]
- Creutz C. E. cis-Unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J Cell Biol. 1981 Oct;91(1):247–256. doi: 10.1083/jcb.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. R., Moss S. E., Crumpton M. J. Diversity in the lipocortin/calpactin family. Cell. 1988 Oct 7;55(1):1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Ernst J. D. Annexin III translocates to the periphagosomal region when neutrophils ingest opsonized yeast. J Immunol. 1991 May 1;146(9):3110–3114. [PubMed] [Google Scholar]
- Goud B., Zahraoui A., Tavitian A., Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990 Jun 7;345(6275):553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Fitch J. M., Jones J. M., Schlaepfer D. D. Two lipocortin-like proteins, endonexin II and anchorin CII, may be alternate splices of the same gene. Trends Biochem Sci. 1989 Feb;14(2):48–50. doi: 10.1016/0968-0004(89)90041-8. [DOI] [PubMed] [Google Scholar]
- Hauptmann R., Maurer-Fogy I., Krystek E., Bodo G., Andree H., Reutelingsperger C. P. Vascular anticoagulant beta: a novel human Ca2+/phospholipid binding protein that inhibits coagulation and phospholipase A2 activity. Its molecular cloning, expression and comparison with VAC-alpha. Eur J Biochem. 1989 Oct 20;185(1):63–71. doi: 10.1111/j.1432-1033.1989.tb15082.x. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Jaconi M. E., Lew D. P., Carpentier J. L., Magnusson K. E., Sjögren M., Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990 May;110(5):1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. A., Perin M. S., Reynolds G. A., Wasserman S. A., Südhof T. C. Two novel annexins from Drosophila melanogaster. Cloning, characterization, and differential expression in development. J Biol Chem. 1990 Jul 5;265(19):11382–11388. [PubMed] [Google Scholar]
- Joiner K. A., Ganz T., Albert J., Rotrosen D. The opsonizing ligand on Salmonella typhimurium influences incorporation of specific, but not azurophil, granule constituents into neutrophil phagosomes. J Cell Biol. 1989 Dec;109(6 Pt 1):2771–2782. doi: 10.1083/jcb.109.6.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska K., Khrebtukova I. A., Gudkova D. A., Pinaev G. P., Sobota A. Actin-binding proteins involved in the capping of epidermal growth factor receptors in A431 cells. Exp Cell Res. 1991 Oct;196(2):255–263. doi: 10.1016/0014-4827(91)90259-w. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
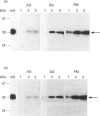
- Le Cabec V., Russo-Marie F., Maridonneau-Parini I. Differential expression of two forms of annexin 3 in human neutrophils and monocytes and along their differentiation. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1471–1476. doi: 10.1016/0006-291x(92)90240-l. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Errasfa M., Russo-Marie F. Inhibition of O2- generation by dexamethasone is mimicked by lipocortin I in alveolar macrophages. J Clin Invest. 1989 Jun;83(6):1936–1940. doi: 10.1172/JCI114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Malawista S. E., Stubbe H., Russo-Marie F., Polla B. S. Heat shock in human neutrophils: superoxide generation is inhibited by a mechanism distinct from heat-denaturation of NADPH oxidase and is protected by heat shock proteins in thermotolerant cells. J Cell Physiol. 1993 Jul;156(1):204–211. doi: 10.1002/jcp.1041560127. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Tringale S. M., Tauber A. I. Identification of distinct activation pathways of the human neutrophil NADPH-oxidase. J Immunol. 1986 Nov 1;137(9):2925–2929. [PubMed] [Google Scholar]
- Maridonneau-Parini I., Yang C. Z., Bornens M., Goud B. Increase in the expression of a family of small guanosine triphosphate-binding proteins, rab proteins, during induced phagocyte differentiation. J Clin Invest. 1991 Mar;87(3):901–907. doi: 10.1172/JCI115096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridonneau-Parini I., de Gunzburg J. Association of rap1 and rap2 proteins with the specific granules of human neutrophils. Translocation to the plasma membrane during cell activation. J Biol Chem. 1992 Mar 25;267(9):6396–6402. [PubMed] [Google Scholar]
- Marie-Cardine A., Maridonneau-Parini I., Ferrer M., Danielian S., Rothhut B., Fagard R., Dautry-Varsat A., Fischer S. The lymphocyte-specific tyrosine protein kinase p56lck is endocytosed in Jurkat cells stimulated via CD2. J Immunol. 1992 Jun 15;148(12):3879–3884. [PubMed] [Google Scholar]
- Meers P., Bentz J., Alford D., Nir S., Papahadjopoulos D., Hong K. Synexin enhances the aggregation rate but not the fusion rate of liposomes. Biochemistry. 1988 Jun 14;27(12):4430–4439. doi: 10.1021/bi00412a033. [DOI] [PubMed] [Google Scholar]
- Meers P., Mealy T., Pavlotsky N., Tauber A. I. Annexin I-mediated vesicular aggregation: mechanism and role in human neutrophils. Biochemistry. 1992 Jul 21;31(28):6372–6382. doi: 10.1021/bi00143a003. [DOI] [PubMed] [Google Scholar]
- Meers P., Mealy T., Tauber A. I. Annexin I interactions with human neutrophil specific granules: fusogenicity and coaggregation with plasma membrane vesicles. Biochim Biophys Acta. 1993 Apr 22;1147(2):177–184. doi: 10.1016/0005-2736(93)90002-h. [DOI] [PubMed] [Google Scholar]
- Melan M. A., Sluder G. Redistribution and differential extraction of soluble proteins in permeabilized cultured cells. Implications for immunofluorescence microscopy. J Cell Sci. 1992 Apr;101(Pt 4):731–743. doi: 10.1242/jcs.101.4.731. [DOI] [PubMed] [Google Scholar]
- Nakata T., Sobue K., Hirokawa N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J Cell Biol. 1990 Jan;110(1):13–25. doi: 10.1083/jcb.110.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshry L., Meers P., Mealy T., Tauber A. I. Annexin-mediated membrane fusion of human neutrophil plasma membranes and phospholipid vesicles. Biochim Biophys Acta. 1991 Jul 22;1066(2):239–244. doi: 10.1016/0005-2736(91)90192-b. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Pryzwansky K. B., Breton-Gorius J. Identification of a subpopulation of primary granules in human neutrophils based upon maturation and distribution. Study by transmission electron microscopy cytochemistry and high voltage electron microscopy of whole cell preparations. Lab Invest. 1985 Dec;53(6):664–671. [PubMed] [Google Scholar]
- Ross T. S., Tait J. F., Majerus P. W. Identity of inositol 1,2-cyclic phosphate 2-phosphohydrolase with lipocortin III. Science. 1990 May 4;248(4955):605–607. doi: 10.1126/science.2159184. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Sawyer D. W., Sullivan J. A., Mandell G. L. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985 Nov 8;230(4726):663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- Sekimoto S., Tashiro T., Komiya Y. Two 68-kDa proteins in slow axonal transport belong to the 70-kDa heat shock protein family and the annexin family. J Neurochem. 1991 May;56(5):1774–1782. doi: 10.1111/j.1471-4159.1991.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Sengeløv H., Kjeldsen L., Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993 Feb 15;150(4):1535–1543. [PubMed] [Google Scholar]
- Zaks W. J., Creutz C. E. Ca(2+)-dependent annexin self-association on membrane surfaces. Biochemistry. 1991 Oct 8;30(40):9607–9615. doi: 10.1021/bi00104a007. [DOI] [PubMed] [Google Scholar]