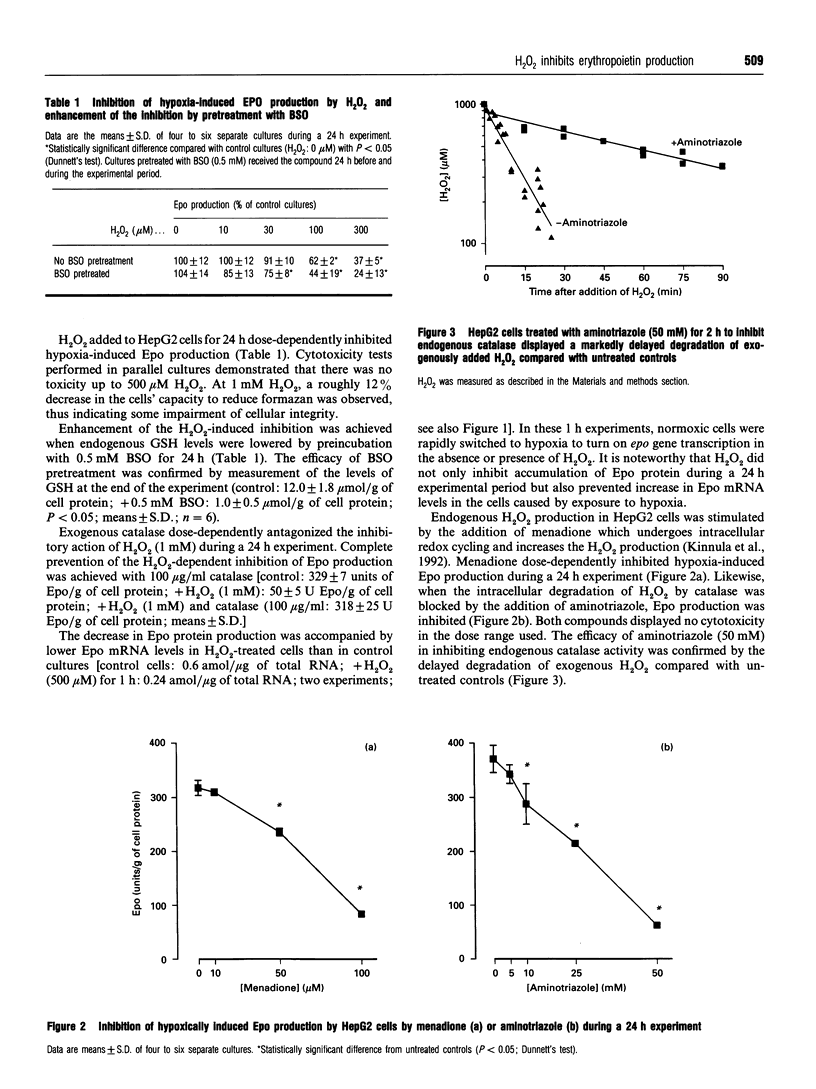
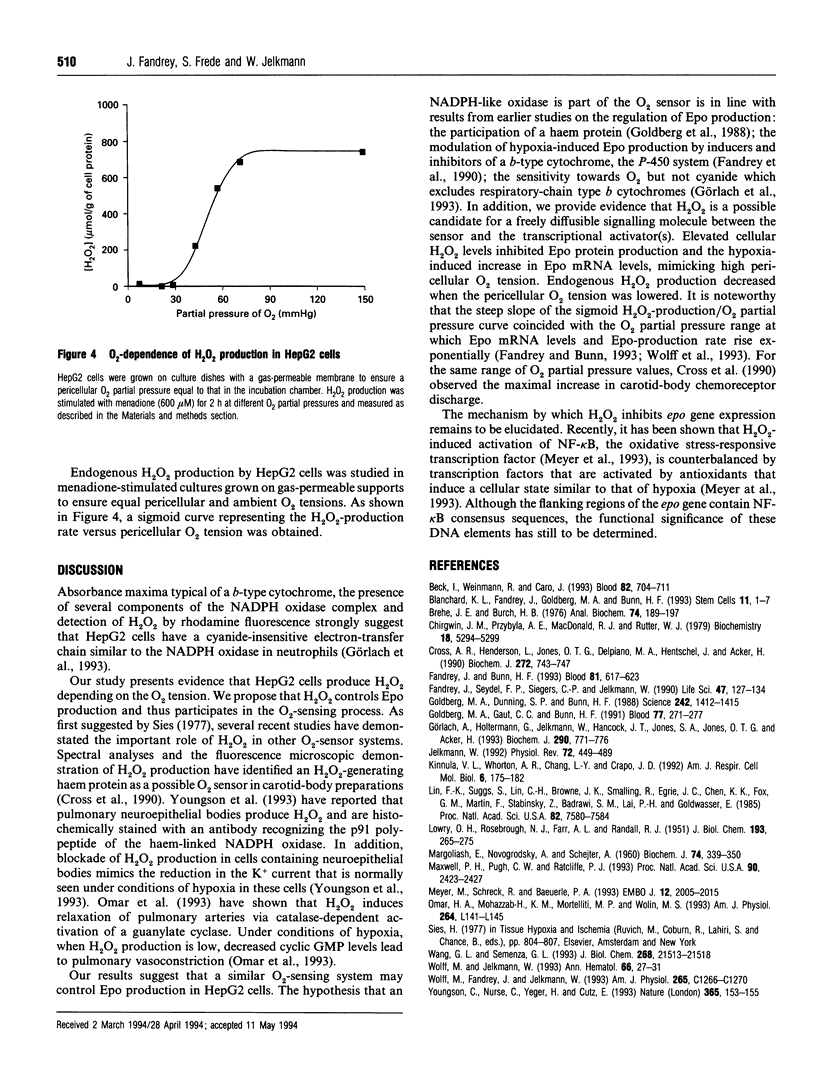
Abstract
The addition of exogenous H2O2 inhibited hypoxia-induced erythropoietin (Epo) production in the human hepatoma cell line HepG2. Likewise, elevation of endogenous H2O2 levels by the addition of menadione or the catalase inhibitor, aminotriazole, dose-dependently lowered Epo production. The inhibitory effect of exogenous H2O2 on Epo formation could be completely overcome by co-incubation with catalase. When GSH levels in HepG2 cells were lowered, Epo production was more susceptible to H2O2-induced inhibition, indicating that H2O2 might affect thiol groups in regulatory proteins. Endogenous production of H2O2 in HepG2 cells was dependent on the pericellular O2 tension, being lowest under conditions of hypoxia. Our results support the hypothesis that an H2O2-generating haem protein might be part of the O2 sensor that controls Epo production. High H2O2 levels under conditions of normoxia suppress, whereas lower levels in hypoxic cells allow epo gene expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck I., Weinmann R., Caro J. Characterization of hypoxia-responsive enhancer in the human erythropoietin gene shows presence of hypoxia-inducible 120-Kd nuclear DNA-binding protein in erythropoietin-producing and nonproducing cells. Blood. 1993 Aug 1;82(3):704–711. [PubMed] [Google Scholar]
- Blanchard K. L., Fandrey J., Goldberg M. A., Bunn H. F. Regulation of the erythropoietin gene. Stem Cells. 1993 May;11 (Suppl 1):1–7. doi: 10.1002/stem.5530110604. [DOI] [PubMed] [Google Scholar]
- Brehe J. E., Burch H. B. Enzymatic assay for glutathione. Anal Biochem. 1976 Jul;74(1):189–197. doi: 10.1016/0003-2697(76)90323-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Henderson L., Jones O. T., Delpiano M. A., Hentschel J., Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J. 1990 Dec 15;272(3):743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandrey J., Bunn H. F. In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood. 1993 Feb 1;81(3):617–623. [PubMed] [Google Scholar]
- Fandrey J., Seydel F. P., Siegers C. P., Jelkmann W. Role of cytochrome P450 in the control of the production of erythropoietin. Life Sci. 1990;47(2):127–134. doi: 10.1016/0024-3205(90)90225-g. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Dunning S. P., Bunn H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988 Dec 9;242(4884):1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Gaut C. C., Bunn H. F. Erythropoietin mRNA levels are governed by both the rate of gene transcription and posttranscriptional events. Blood. 1991 Jan 15;77(2):271–277. [PubMed] [Google Scholar]
- Görlach A., Holtermann G., Jelkmann W., Hancock J. T., Jones S. A., Jones O. T., Acker H. Photometric characteristics of haem proteins in erythropoietin-producing hepatoma cells (HepG2). Biochem J. 1993 Mar 15;290(Pt 3):771–776. doi: 10.1042/bj2900771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992 Apr;72(2):449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Kinnula V. L., Whorton A. R., Chang L. Y., Crapo J. D. Regulation of hydrogen peroxide generation in cultured endothelial cells. Am J Respir Cell Mol Biol. 1992 Feb;6(2):175–182. doi: 10.1165/ajrcmb/6.2.175. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin F. K., Suggs S., Lin C. H., Browne J. K., Smalling R., Egrie J. C., Chen K. K., Fox G. M., Martin F., Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell P. H., Pugh C. W., Ratcliffe P. J. Inducible operation of the erythropoietin 3' enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. L., Semenza G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993 Oct 15;268(29):21513–21518. [PubMed] [Google Scholar]
- Wolff M., Fandrey J., Jelkmann W. Microelectrode measurements of pericellular PO2 in erythropoietin-producing human hepatoma cell cultures. Am J Physiol. 1993 Nov;265(5 Pt 1):C1266–C1270. doi: 10.1152/ajpcell.1993.265.5.C1266. [DOI] [PubMed] [Google Scholar]
- Wolff M., Jelkmann W. Effects of chemotherapeutic and immunosuppressive drugs on the production of erythropoietin in human hepatoma cultures. Ann Hematol. 1993 Jan;66(1):27–31. doi: 10.1007/BF01737686. [DOI] [PubMed] [Google Scholar]
- Youngson C., Nurse C., Yeger H., Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993 Sep 9;365(6442):153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]



