Abstract
Mutations in voltage‐gated sodium (Nav) channels, which are essential for generating and propagating action potentials, can lead to serious neurological disorders, such as epilepsy. However, disease‐causing Nav channel mutations do not always result in severe symptoms, suggesting that the disease conditions are significantly affected by other genetic factors and various environmental exposures, collectively known as the “exposome”. Notably, recent research emphasizes the pivotal role of commensal bacteria in neural development and function. Although these bacteria typically benefit the nervous system under normal conditions, their impact during pathological states remains largely unknown. Here, we investigated the influence of commensal microbes on seizure‐like phenotypes exhibited by para Shu —a gain‐of‐function mutant of the Drosophila Nav channel gene, paralytic. Remarkably, the elimination of endogenous bacteria considerably ameliorated neurological impairments in para Shu . Consistently, reintroducing bacteria, specifically from the Lactobacillus or Acetobacter genera, heightened the phenotypic severity in the bacteria‐deprived mutants. These findings posit that particular native bacteria contribute to the severity of seizure‐like phenotypes in para Shu . We further uncovered that treating para Shu with antibiotics boosted Nrf2 signaling in the gut, and that global Nrf2 activation mirrored the effects of removing bacteria from para Shu . This raises the possibility that the removal of commensal bacteria suppresses the seizure‐like manifestations through augmented antioxidant responses. Since bacterial removal during development was critical for suppression of adult para Shu phenotypes, our research sets the stage for subsequent studies, aiming to elucidate the interplay between commensal bacteria and the developing nervous system in conditions predisposed to the hyperexcitable nervous system.
Keywords: commensal bacteria, Drosophila melanogaster, gut‐brain connection, Nrf2, seizure, voltage‐gated sodium channel
Removing commensal bacteria from Drosophila mutants with a gain‐of‐function voltage‐gated sodium channel mutation significantly reduces their seizure‐like symptoms, while reintroducing bacteria reverses the phenotypic severity. In these bacteria‐deprived mutants, Nrf2 signaling in the gut is enhanced, and activating Nrf2 globally in the naïve mutants replicates the effects of removing bacteria.
1. INTRODUCTION
Voltage‐gated sodium (Nav) channels play crucial roles in the generation and propagation of action potentials in neurons. Consequently, mutations in the Nav channels have been linked to numerous neurological and psychiatric disorders. 1 Notably, over 700, such mutations have been identified as causes of various epilepsy syndromes, 2 underscoring the significant relationship between Nav channel malfunctions and epilepsy. However, these disease‐causing mutations do not always lead to severe symptoms. In fact, a comprehensive exome sequencing study found that pathogenic Nav channel mutations were present in both epilepsy patients and unaffected individuals at comparable rates. 3 This surprisingly low disease penetrance suggests that the epileptic manifestations stemming from Nav channel mutations are greatly influenced by genetic enhancers and suppressors as well as the non‐genetic “exposome”, which refers to the totality of environmental exposures that individuals encounter throughout their lifetime. 4 Identifying these phenotypic modifiers and understanding their underlying mechanisms are crucial to fully grasp how Nav channel mutations lead to neurological symptoms.
The metazoan gut harbors a myriad of indigenous microbes on its mucosal surface. These resident microbes, collectively referred to as commensal gut microbiota, play vital roles in regulating the host organism's metabolism and immunity. 5 , 6 , 7 As such, they are regarded as a “non‐self‐organ” crucial for the host's health and well‐being. 8 , 9 , 10 Studies have also revealed that the gut microbiota has a significant impact on the development and function of the nervous system. 11 , 12 , 13 This suggests that commensal microbes might act as environmental modifiers of Nav channel mutations. Intriguingly, germ‐free mice, produced either through aseptic techniques or antibiotic treatments, demonstrate behaviors distinct from those of conventionally raised mice. Moreover, these mice exhibit neural abnormalities akin to those seen in humans with neurological and psychiatric conditions. 13 Yet, we still do not understand the degree to which the gut microbiota influences a dysfunctional nervous system in Nav channel mutants and, in turn, affects the animal's physiology and behavior. Considering that the interactions between the brain and gut microbiota are likely intricate and bidirectional, exploring the effects of commensal microbiota on Nav channel mutants demands a model system that is straightforward, well‐characterized and subject to thorough experimental interventions.
The fruit fly, Drosophila melanogaster, is an organism that is easy to manipulate genetically and has a relatively simple microbial community, predominantly within the gut. 14 , 15 , 16 , 17 , 18 The intestinal microbiota of laboratory‐reared Drosophila consists of 20 to 30 bacterial species present in the crop, midgut and hindgut, with most belonging to the genera Lactobacillus and Acetobacter. 19 Eukaryotic microorganisms in the Drosophila microbiota are from the Saccharomycetaceae family. The specific taxa present in the gut depend on the Drosophila species, its food sources and laboratory conditions. 20 This relative simplicity in the prokaryotic and eukaryotic microbial community makes Drosophila an effective genetic model for exploring the functional interactions between a host and its microbiota. 21 , 22 In our study, we explored how commensal microbiota affects the seizure‐like characteristics of para Shu , a gain‐of‐function Nav channel mutant in Drosophila. We show that, when the commensal bacteria were removed from para Shu mutants, specifically during the larval stage, their neurological symptoms were significantly suppressed. Furthermore, upon removal of commensal bacteria from para Shu mutants, we observed activation of the Nrf2 antioxidant signaling pathway in the gut, along with a suppression of the neurological phenotypes. Experimentally boosting this Nrf2 signaling pathway mirrored the effects of bacterial removal on para Shu phenotypes. These results imply that the gut environment in para Shu mutants diverges from that in wild‐type flies and that the mitigation of the phenotype after eliminating gut bacteria appears to stem from an amplified antioxidant response in the para Shu mutants.
2. MATERIALS AND METHODS
2.1. Fly stocks and culture conditions
Flies were raised in a 12‐h light/dark cycle, at 25 °C and 65% humidity on the diet originating from a recipe developed by Edward Lewis 23 and modified by Rodney Williamson (Beckman Research Institute of the City of Hope, Duarte, CA): a cornmeal/glucose/yeast/agar medium supplemented with the mold inhibitor methyl 4‐hydroxybenzoate (0.05%), propionic acid and phosphoric acid. The exact composition of the fly food used in this study was described in Kasuya et al.. 24 Shudderer (para Shu ) was a gift from Rodney Williamson with Canton‐S 2202U used as a background control. GEFS+ (w para GEFS+ ; UAS‐GFP), Dravet (w para DS ; UAS‐GFP) and control (w; UAS‐GFP) flies were obtained from Dr. Diane O'Dowd (University of California, Irvine). GstD1‐GFP flies were acquired from Dr. Subhabrata Sanyal (Calico, San Francisco, CA) and Keap1 null (w;; Keap1 036 /TM3) and control flies (w 1118 ) were obtained from Dr. Heinrich Jasper (Buck Institute for Research on Aging, Novato, CA).
2.2. Identification of culturable gut microbiota
Morphologically distinct colonies were isolated from adult gut homogenates plated on MRS agar (Sigma Aldrich, St. Louis, MO). Colonies were plated on either MRS agar plates containing the antibiotics tetracycline (50 μg/mL), ampicillin (100 μg/mL) and kanamycin (30 μg/mL) (Research Products International, Mount Prospect, IL) or plates containing 0.0007% cycloheximide (Sigma Aldrich, St. Louis, MO), an inhibitor of eukaryotic translation. Prokaryotic microbes were identified as sensitive to antibiotics but resistant to cycloheximide, while eukaryotic microbes were deemed resistant to antibiotics but sensitive to cycloheximide. For prokaryotic microbes, 10 replicates of each distinct colony were re‐streaked on MRS agar for phylogenetic identification. Briefly, DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) and 16S ribosomal DNA sequences were amplified with 27F and 1492R universal 16S rDNA primers (Integrated DNA Technologies, Coralville, IA): (27F forward primer: 5′‐ AGAGTTTGATCCTGGCTCAG‐3′; 1492R reverse primer: 5′‐ GGTTACCTTGTTACGACTT‐3′). PCR products were sequenced using Sanger Sequencing and sequences were aligned to the bacterial genome with NCBI BLAST. To identify eukaryotic microbes, colonies were sent to Nelson Laboratories (Salt Lake City, UT) where the D2 region of large subunit ribosomal DNA was amplified, sequenced and aligned to the fungal genome. Following phylogenetic identification, gut microbes were distinguished based on colony morphology.
2.3. Scoring of morphological defects
Newly eclosed para Shu female heterozygotes (para Shu /+) and Canton‐S 2202U (CS) flies were collected and scored for cuticular defects and abnormal wing posturing either 24 h or 5 days later. As described previously, 24 , 25 , 26 , 27 cuticular defects were defined as any indentation in the back of the thorax. Abnormal wing posture was defined by non‐overlapping (downturned) wings.
2.4. Behavioral analyses
Video‐tracking locomotor analysis: para Shu mutants display spontaneous tremors at room temperature. 26 , 28 The severity of this behavioral phenotype was indirectly evaluated using the “time‐inside” locomotor assay. 24 , 25 , 26 , 27 For this assay, virgin female flies were collected post‐eclosion and aged for 5 days. Flies were individually transferred to a plastic well (15 mm diameter × 3 mm depth) and allowed to acclimate for 5 min. Following acclimation, fly behavior was recorded at 30 frames per second (fps) using a web camera (Agama V‐1352R) at 320 × 240 resolution for 5 min. Videos were analyzed using pySolo 29 to track fly locomotion and calculate the x, y coordinates of an individual fly during each frame of the 5‐min video. The locomotion defect was defined as the percentage of time the fly remained within a circle whose radius was 70% of the radius of the entire chamber. The distance between the fly and the center of the chamber was calculated using the distance formula (X F – X C )2 + (Y F – Y C )2 < 132 where X F and Y F are the coordinates of the fly, X C and Y C are the coordinates of the chamber center, and 13 is 70% of the chamber radius.
Assay for heat‐induced behavioral abnormalities in adult flies: 5‐day old virgin females were individually transferred to empty glass vials (15 mm × 45 mm). After 10–15 min of acclimation, vials were submerged in a water bath for 2 min. Presence or absence of seizing for individual flies was determined every 5 s and the proportion of flies seizing at each time point was calculated. Heat‐induced seizures were defined as a loss of posture accompanied by leg twitches, wing flapping or abdominal curling as previously described. 30 para Shu , para GEFS+ and para DS flies were assayed at 37 °C, 26 40 °C 30 and 38 °C, 31 respectively, as previously described.
2.5. Removal of bacteria
Antibiotic Food: Antibiotic‐supplemented food was generated by adding a solution of tetracycline, ampicillin and kanamycin (Research Products International, Mount Prospect, IL) to liquefied food at 55–60 °C. The food was mixed thoroughly and allowed to solidify. Final concentrations of antibiotics were: tetracycline (50 μg/mL), ampicillin (100 μg/mL) and kanamycin (30 μg/mL). Water was used as a vehicle control.
Embryo Sterilization: Eggs laid overnight by mated females were collected from grape juice agar plates, immersed in an 8% hypochlorite solution for 5 min, rinsed twice in sterile water, and transferred to a sterile diet. Control embryos were rinsed in sterile water and transferred to a sterile diet. All embryonic manipulations were performed under a laminar‐flow hood with sterile technique.
2.6. Mono‐association of gut microbes
Single, morphologically distinct colonies were selected from MRS agar plates and grown up overnight in MRS broth at 30 °C. OD600 values of serially diluted cultures (1:10, 1:20, 1:50, 1:100) were measured with a spectrophotometer. OD600 values between 0.1 and 0.2 were recorded along with their corresponding dilutions. To normalize cell density, the equation [(O × V × D)/C = 1 mL] was used, where O = observed OD600, D = fold dilution, C = OD600 of predetermined constant and V = μL of overnight culture. 32 For Lactobacillus and Acetobacter, predetermined constants (OD600 normalized to 1 × 107 CFU/mL) of 0.077 and 0.052, respectively were used. For Candida, cell density was normalized to OD600 = 0.1 since no CFU/OD600 constant was available. To normalize cell density the equation was solved for “V”, yielding the amount, in microliters, of overnight cultured to be resuspended in 1 mL of MRS broth. Germ‐free embryos, sterilized as described above, were inoculated with 50 μL of cell density‐normalized culture and raised in a 12‐h light/dark cycle at 25 °C and 65% humidity prior to scoring morphological defects and performing behavioral analyses on eclosed flies.
2.7. Quantification of Nrf2 activity
The gastrointestinal tract, including both midgut and hindgut, was dissected out of five‐day‐old virgin females (10 per assay) in 1× PBS at room temperature and homogenized in 200 μL Schneider's Drosophila Medium (Life Technologies, Carlsbad, CA). Gut homogenate was centrifuged at 12 k RPM for 5 min. 50 μL of supernatant was pipetted into a 96‐well plate and 50 μL of Schneider's Medium was added to the well for a total volume of 100 μL (performed in triplicate). The relative fluorescent intensity (arbitrary units) was measured at 485 nm excitation/540 nm emission on a Synergy4 microplate reader (BioTek Instruments, Winooski, VT). The relative fluorescent intensity of guts dissected from animals without the GstD1‐GFP transgene was subtracted from animals with the transgene to account for tissue autofluorescence. For representative images, guts were dissected from animals with the GstD1‐GFP transgene, fixed overnight at 4 °C, washed, and mounted with SlowFade™ Diamond Antifade Mountant containing DAPI. Guts were imaged at 5× magnification with a Zeiss LSM 710 confocal microscope and z‐stack images were recomposed to generate a single z‐stack projection of the whole midgut using the Stitching plugin in ImageJ.
2.8. Statistics
All data presented in this study were generated from independent sets of experiments. Unless stated otherwise, “n” represents number of flies. Statistical analysis was performed using SigmaPlot 13.0 (Systat Software, Inc., Point Richmond, CA). Statistical comparisons between two groups were performed using the two‐tailed student's t‐test assuming unequal variance or, for non‐normally distributed data, the Mann–Whitney Rank Sum test. For multiple groups displaying a normal distribution, statistical significance was determined using one‐way ANOVA, with Bonferroni t‐test comparisons between control and treatment groups post hoc. For data exhibiting non‐normal distributions, the Kruskal‐Wallis one‐way ANOVA on ranks test was performed, with Dunn's method for post hoc comparison among groups. Fisher's exact test was used to analyze morphological phenotypes. Temperature‐induced behavioral phenotypes were analyzed using two‐way repeated measures ANOVA with the Holm‐Sidak method for post hoc analysis. Data not conforming to a normal distribution are presented as box‐and‐whisker plots showing values of the first, second and third quartiles (box), as well as the 10th and 90th percentiles (whisker).
3. RESULTS
3.1. Elimination of commensal bacteria suppresses seizure‐like phenotypes in para Shu mutants
para Shu , originally referred to as Shudderer or Shu, 28 is a dominant, gain‐of‐function mutant allele of the paralytic (para) gene, which represents the only voltage‐gated sodium (Nav) channel gene in Drosophila. 26 The para gene is X‐linked. Both hemizygous males (para Shu /Y) and heterozygous females (para Shu /+) manifest pronounced seizure‐like phenotypes. These include neuronal hyperactivity, spontaneous spasms, leg shaking induced by ether and heat‐triggered convulsions. 26 , 28 Moreover, para Shu mutants display an unusual wing posture characterized by downturned wings and an indented thorax, possibly due to abnormal muscle activity resulting from neuronal hyperexcitability. 26 While homozygous para Shu females (para Shu /para Shu ) are viable and show more pronounced symptoms, they are rare in para Shu mutant stocks (para Shu /FM7). This scarcity is because para Shu /Y males seldom mate, largely due to their marked lethargy. For our study, we used heterozygous para Shu females (para Shu /+), which were bred by pairing para Shu /FM7 females with wild‐type males. Throughout this document, unless otherwise noted, we'll refer to these heterozygous females simply as para Shu females or para Shu mutants.
The guts of larval and adult flies are home to both prokaryotic and eukaryotic microbes. 33 Identity of microbes in para Shu mutants was determined by isolating gut homogenates from adult para Shu mutants and culturing them on agar media infused with or without a cocktail of antibiotics (tetracycline, ampicillin and kanamycin, hereafter termed as TAK) or cycloheximide, an inhibitor of eukaryotic translation. In Figure 1A, the TAK‐sensitive bacterial colonies and the TAK‐insensitive eukaryotic colonies are indicated by the arrow and the arrowhead, respectively. DNA from prokaryotic microbes, identified as sensitive to antibiotics but resistant to cycloheximide, was amplified with 27F and 1492R universal 16S rDNA primers, 34 sequenced, and aligned to the bacterial genome with NCBI BLAST. On the other hand, DNA from eukaryotic microbes, resistant to antibiotics but sensitive to cycloheximide, was amplified with primers corresponding to the D2 region of large subunit ribosomal DNA, sequenced, and aligned to the fungal genome. These analyses revealed that the primary components of the para Shu gut microbiota are the prokaryotic Lactobacillus and Acetobacter species and the eukaryotic yeast species, Candida freyschussii (Lansdon et al., manuscript in preparation). To study the influence of commensal microbiota on the neurological phenotypes of para Shu mutants, we raised the mutants on fly food containing TAK or with just the vehicle as controls.
FIGURE 1.
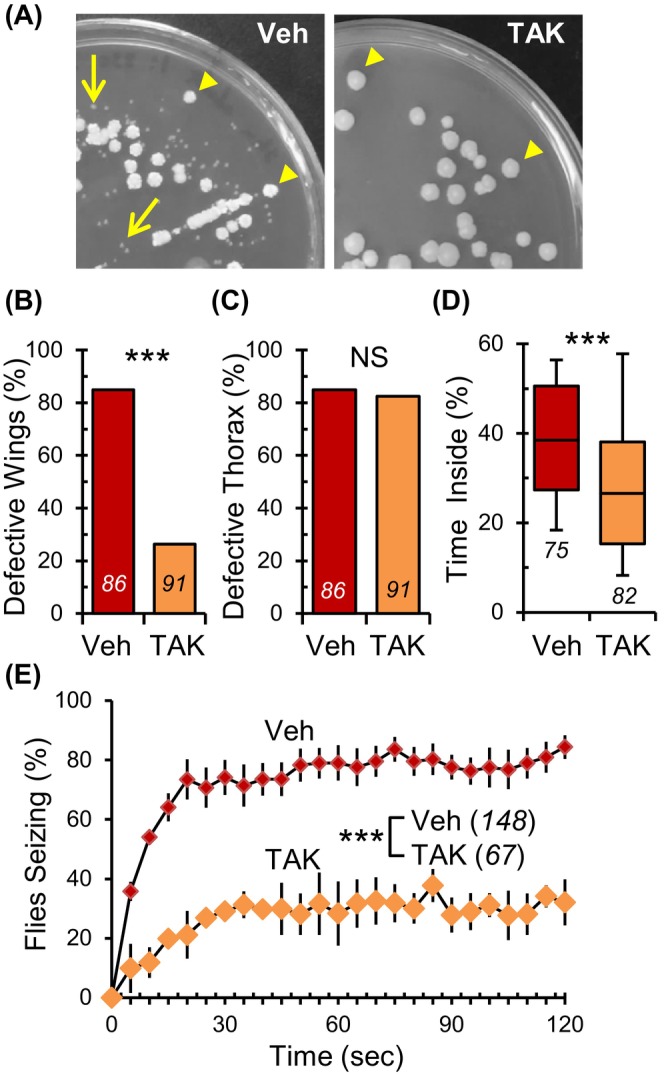
Administration of antibiotics to para Shu mutants suppresses morphological and behavioral defects. (A) Representative images of vehicle‐treated (left) and TAK‐treated (right) para Shu gut homogenates cultured on MRS agar. Incidence of (B) downturned wings and (C) indented thorax in para Shu mutants treated with vehicle or antibiotics (TAK). (D) Percentage of time spent in the center area of the chamber for para Shu mutants treated with vehicle or TAK. (E) The average percentages of flies that seized at each time point (±SEM) at 37 °C following treatment with vehicle or TAK. The number of flies scored under each condition is indicated in italics. ***p < 0.001; NS, not significant (p > 0.05).
As documented in previous studies, 24 , 25 , 26 , 27 para Shu mutants on vehicle‐treated standard food consistently displayed distinct morphological traits, such as downturned wings (n = 86, 85%; Figure 1B, Veh, Table S1) and an indented thorax (n = 86, 85%; Figure 1C, Veh, Table S1). However, when these mutants were raised on TAK‐infused food, the prevalence of downturned wings dropped significantly to 26% (n = 91, X 2 = 58.771, df = 1, p < 0.001, Fisher's Exact Test; Figure 1B, TAK, Table S1). The indented thorax characteristic, on the other hand, was largely consistent at 82% (n = 91, X 2 = 0.0575, df = 1, p > 0.05, Fisher's Exact Test; Figure 1C, TAK, Table S1). The introduction of TAK also had a noticeably positive impact on the behavioral characteristics of para Shu mutants in a “time‐inside” locomotor test. 24 , 25 , 26 , 27 This assay is based on the observation that para Shu mutants, when placed in a small circular chamber, spend significantly more time in the central area compared with control flies. This behavior is primarily due to their sporadic tremors and associated uncoordinated movements, which serve as an indirect measure of spontaneous tremor levels. In the test setting, wild‐type flies typically traveled around the edges of the chamber, spending roughly 3% of their time in the central section. 26 In stark contrast, para Shu mutants on standard food spent about 38% (n = 75) of their time in the central section due to frequent uncontrolled spasms and diminished coordination (Figure 1D, Veh). However, when fed TAK, these mutants only spent 27% (n = 82, U = 1934.0, p < 0.001, Mann–Whitney Rank Sum Test) of their time centrally (Figure 1D, TAK), suggesting that the TAK diet reduced the severity of involuntary shaking and uncoordinated movement. Additionally, para Shu mutants have a pronounced susceptibility to heat‐triggered seizure‐like phenotypes when exposed to higher temperatures, such as 37 °C. 26 Our observations showed that after 30‐s, 1 and 2 min of exposure to 37 °C, 74%, 79% and 84% (n = 148) of para Shu mutants on standard food experienced seizure activity, respectively (Figure 1E, Veh). There was a statistically significant effect of TAK‐diet on heat‐induced seizure behavior (F(1,6) = 40.643, p < 0.001, Two‐Way Repeated Measures ANOVA). 30%, 25% and 31% of TAK‐treated animals (n = 67) exhibited seizure activities at the above‐mentioned timepoints (Figure 1E, TAK). Importantly, the TAK treatment did not negatively impact the genetic control strain, Canton‐S (CS). Regardless of whether they were on the TAK diet or not, CS flies did not present any morphological anomalies (0%, n = 69 for vehicle; 0%, n = 61 for TAK) and were not prone to heat‐induced seizures at 37 °C (0%, n = 90 for vehicle; 0%, n = 90 for TAK) (Figure S1). Moreover, the movement patterns of these control flies, whether on the standard diet or TAK‐infused diet, were virtually identical, with both groups spending 7.3% (n = 69) and 6.7% (n = 61, U = 1951.5, p > 0.05, Mann–Whitney Rank Sum Test) of their time in the central zone, respectively (Figure S1).
Given that antibiotics can exert effects beyond simply eliminating endogenous bacteria, 35 , 36 we decided to assess the impact of bacterial removal using an alternative approach. Specifically, we dechorionated embryos and raised them in germ‐free environments. Dechorionation is the process of removing the chorion membrane, the outermost layer of Drosophila eggs, which is often laden with microbes. This well‐established method allows for the generation of germ‐free embryos without the use of antibiotics. 32 By applying this technique, we managed to fully eradicate all culturable microbes from the adult gut, including prokaryotes such as Lactobacillus sp. And Acetobacter sp., as well as the eukaryote Candida freyschussii (Figure 2A, Mock and Dch).
FIGURE 2.
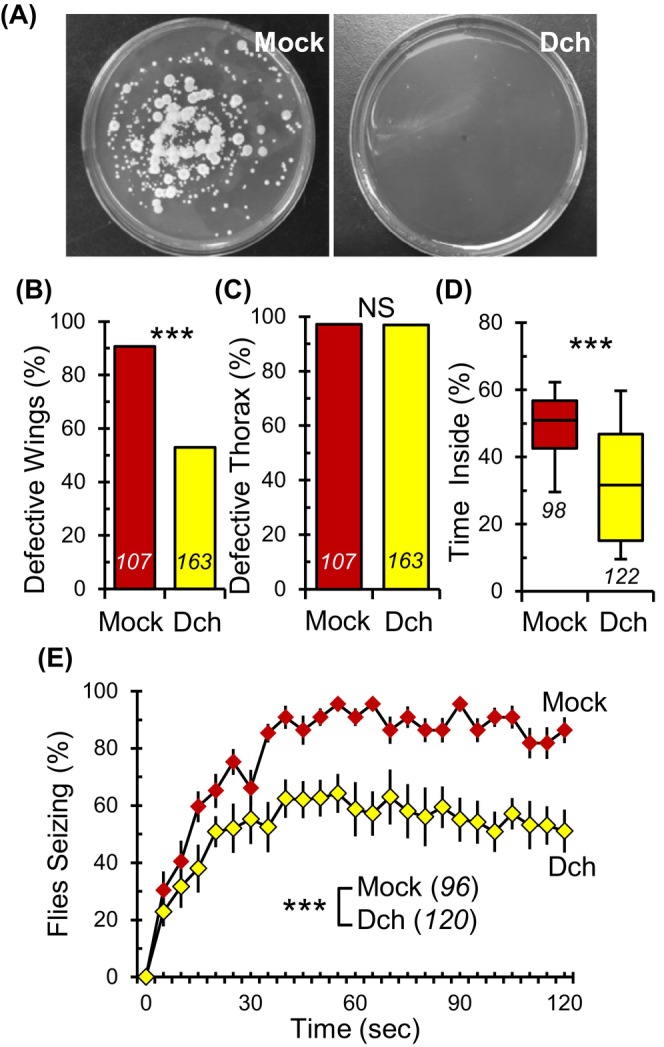
Germ‐free para Shu mutants exhibit improvements in wing morphology and seizure behavior. (A) Representative images of mock‐treated (Mock, left) and dechorionated germ‐free (Dch, right) para Shu gut homogenates cultured on MRS agar shows complete removal of the gut microbiota. (B) Incidence of downturned wings and (C) indented thorax in germ‐free para Shu mutants compared with mock‐treated mutants. (D) Percentage of time spent in the center area of the chamber for mock‐treated and germ‐free para Shu females. (E) The average percentages of flies seizing at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics. ***p < 0.001; NS, not significant (p > 0.05).
Adult para Shu mutants derived from mock‐treated embryos displayed severe morphological abnormalities, with 91% (n = 107) manifesting downturned wings (Figure 2B, Mock, Table S1). In contrast, germ‐free para Shu mutants, produced from dechorionated embryos, showed a substantial decline in this trait, with only 55% (n = 163, X 2 = 36.459, df = 1, p < 0.001, Fisher's Exact Test) having downturned wings (Figure 2B, Dch, Table S1). Mirroring the results from antibiotic treatments, dechorionation had no effect on the indented thorax phenotype; a vast majority of the mock‐treated and dechorionated mutants, 97% (n = 107) and 97% (n = 163, X 2 = 0.0585, df = 1, p > 0.05, Fisher's Exact Test) respectively, presented this anomaly regardless of treatment (Figure 2C, Mock and Dch, Table S1). Dechorionation also notably improved the behavioral phenotypes of para Shu mutants. In a locomotor test, dechorionated para Shu mutants spent only 32% (n = 122) of their time in the center (Figure 2D, Dch), whereas their mock‐treated counterparts spent 51% (n = 98, U = 2911.0, p < 0.001, Mann–Whitney Rank Sum Test) (Figure 2D, Mock). Furthermore, there was a statistically significant effect of dechorionation (F(1,10) = 15.57, p < 0.01, Two‐Way Repeated Measures ANOVA) on heat‐induced seizure behavior. After 30‐s, 1 and 2‐min of exposure to 37 °C, 83%, 93% and 89% (n = 96) of mock‐treated para Shu mutants showed seizure phenotypes, respectively (Figure 2E, Mock). In contrast, only 56%, 58% and 51% (n = 120) of germ‐free para Shu mutants exhibited seizures at the same timepoints (Figure 2E, Dch). This dechorionation procedure had little to no discernible effects on our genetic controls, as evidenced by the morphology and behavior of the CS flies. Whether dechorionated or mock‐treated, CS flies did not display any morphological anomalies (0%, n = 338 for dechorionated; 0%, n = 176 for mock‐treated) or heat‐triggered seizure phenotypes at 37 °C (0%, n = 216 for dechorionated; 0%, n = 107 for mock‐treated). Although we did observe a statistical difference in the locomotor patterns between mock‐treated and dechorionated CS flies, they dedicated only 4.4% (n = 109) and 6.8% (n = 261, U = 9153.5, p < 0.001, Mann–Whitney Rank Sum Test) of their activity to the center area, respectively (Figure S2). In essence, our findings underscore the potent role of sterile interventions in tempering the seizure‐like behaviors observed in para Shu mutants.
3.2. The removal of bacteria affects behavioral phenotypes of different Nav channel mutants in distinct ways
We next explored if the phenotypic suppression observed with antibiotic treatment could be replicated in other Drosophila Nav channel mutants. Generalized epilepsy with febrile seizures plus (GEFS+) and Dravet syndrome (DS) are common early‐onset genetic epilepsy syndromes often linked to mutations in the SCN1A gene. This gene codes for the human Nav channel α‐subunit Nav 1.1. 37 , 38 para GEFS+ and para DS serve as Drosophila knock‐in models for GEFS+ and DS, respectively. Each model possesses a para mutation corresponding to one found in human epilepsy patients with SCN1A mutations. 30 , 31 para GEFS+ is a gain‐of‐function mutation akin to para Shu and para DS is a loss‐of‐function mutation resembling para ts1 . Although neither para GEFS+ nor para DS mutants display the downturned wing posture or spontaneous tremors observed in para Shu mutants, each mutant does exhibit distinct behavioral abnormalities in response to heat. 30 , 31 For our study, we provided para GEFS+ and para DS homozygous females with either TAK‐infused or control diets and observed the changes in their heat‐induced behavior. Vehicle‐fed para GEFS+ mutants, when subjected to 40 °C, quickly exhibited seizure‐like symptoms, mirroring the behavior of para Shu mutants. There was a statistically significant effect of diet on para GEFS+ mutant seizures (F(1,4) = 21.674, p < 0.01, Two‐Way Repeated Measures ANOVA). Specifically, after 30 s, 1 and 2‐min at this temperature, 67%, 92% and 96% (n = 75) of para GEFS+ mutants respectively showed intense heat‐induced seizures, while only 21%, 56% and 81% (n = 70) of TAK‐treated para GEFS+ mutants displayed seizures at the same timepoints (Figure 3A). However, the story was different for para DS mutants. Unlike para GEFS+ and para Shu , antibiotic intervention did not alleviate the para DS symptoms (F(1,4) = 0.485, p = 0.524, Two‐Way Repeated ANOVA). When exposed to 38 °C, para DS mutants on both the TAK and control diets quickly lost their balance and struggled to regain their posture. Regardless of the TAK treatment, over 95% (n = 100 for vehicle; n = 85 for TAK) of para DS mutants displayed this loss of control within 30 s of the temperature elevation (Figure 3B).
FIGURE 3.
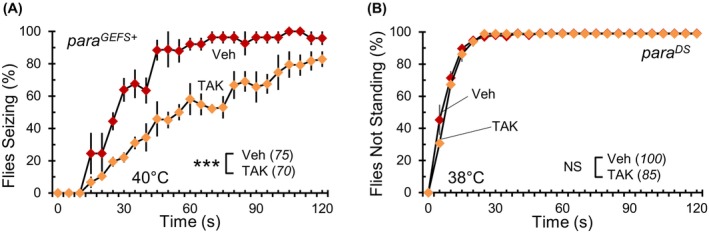
The removal of bacteria has different effects on neurological phenotypes in other Nav channel mutants. (A) The average percentages of para GEFS+ or (B) para DS Nav channel mutants not standing at each time point (±SEM) at the indicated temperatures following vehicle or TAK treatment. The number of flies scored under each condition is indicated in italics. ***p < 0.001; NS, not significant (p > 0.05).
3.3. Mono‐association with Lactobacillus or Acetobacter, but not Candida, exacerbates para Shu phenotypes
Two distinct methods of removing endogenous bacteria from para Shu mutants consistently led to a significant reduction in their seizure‐like adult phenotypes (Figures 1 and 2). This pattern suggests that the bacterial component of commensal microbes might amplify these neurological phenotypes. To verify this notion, we reintroduced specific gut microbes to germ‐free para Shu mutants and examined their effects. We created gnotobiotic flies with a defined gut microbe composition by introducing a culture of either Candida, Lactobacillus, or Acetobacter to dechorionated embryos. As mentioned earlier, these three gut microbes were identified in para Shu using microbial culture and DNA sequencing. Each of the three gnotobiotic fly groups, harboring one specific microorganism, was successfully generated without any observed cross‐contamination (Figure 4A).
FIGURE 4.
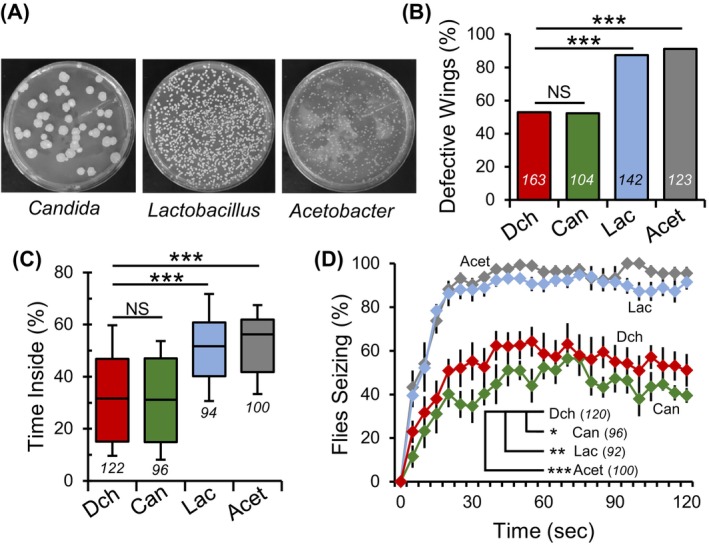
Mono‐association of Lactobacillus or Acetobacter with para Shu mutants increases the severity of seizure‐like phenotypes. (A) Representative images of gut homogenates from gnotobiotic flies mono‐associated with specific gut microbes. (B) Incidence of downturned wings in germ‐free mutants and gnotobiotic para Shu flies mono‐associated with Lactobacillus (Lac), Acetobacter (Acet), or Candida (Can). (C) Percentage of time spent in the center area of the chamber for gnotobiotic and germ‐free flies. (D) The average percentages of flies seizing at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics. *p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant (p > 0.05).
Germ‐free para Shu mutants showed a downturned wing incidence of 55% (n = 163) and gnotobiotic para Shu mutants inoculated with Candida displayed a similar incidence at 54% (n = 104) (X 2 = 0.009, df = 1, p = 0.926, Fisher's Exact Test; Figure 4B, Table S1). In stark contrast, para Shu mutants associated with Lactobacillus or Acetobacter showed an elevated incidence of this phenotype, closely resembling that of conventionally raised mutants. Specifically, 88% (n = 142) and 92% (n = 123) of these mutants exhibited downturned wings (Figure 4B, Table S1). In a locomotor test, germ‐free para Shu mutants and those associated solely with Candida showed comparable behavior, occupying the central zone 32% (n = 122) and 31% (n = 96) of the time, respectively (Q = 0.0013, p > 0.05, Kruskal‐Wallis One‐way ANOVA on ranks with Dunn's Method post hoc; Figure 4C). Yet, those associated with either Lactobacillus or Acetobacter displayed a markedly worse locomotor pattern, spending more time in the central area at 52% (n = 91) and 56% (n = 98), respectively (for Lactobacillus; Q = 6.395, p < 0.001, for Acetobacter; Q = 7.3, p < 0.001, Kruskal‐Wallis One‐way ANOVA on ranks with Dunn's Method post hoc; Figure 4C).
After being subjected to 37 °C for 30‐s, 1 and 2‐min, 41%, 56% and 44% (n = 96) of para Shu mutants associated with Candida showed seizure phenotypes, respectively (Figure 4D, +Can). However, para Shu mutants associated with either Lactobacillus or Acetobacter demonstrated a higher incidence of heat‐induced seizures. At the same timepoints, 85%, 88% and 89% of Lactobacillus‐associated mutants (n = 92) and 88%, 93% and 90% of Acetobacter‐associated mutants (n = 100) exhibited similar phenotypes (Figure 4D, +Lac, + Acet). In comparison, only 56%, 58% and 51% (n = 120) of germ‐free para Shu mutants exhibited heat‐induced seizures at 30‐s, 1 and 2‐min. There was a statistically significant effect of bacterial association on para Shu mutant behavior in the heat‐induced seizure assay compared with dechorionated animals (F(3,16) = 20.944, p < 0.001, Two‐Way Repeated Measures ANOVA). Post‐hoc analysis revealed a significant difference in heat‐induced seizure behavior of para Shu mutants associated with either Lactobacillus (t = 4.38, p < 0.001, Holm‐Sidak Method) or Acetobacter (t = 5.31, p < 0.001, Holm‐Sidak Method), but not Candida (t = 1.59, p > 0.05, Holm‐Sidak Method) when compared with germ‐free para Shu mutants. Notably, gnotobiotic CS flies did not display any morphological abnormalities when associated with Candida (0%, n = 224), Lactobacillus (0%, n = 299), or Acetobacter (0%, n = 200), nor did they exhibit heat‐induced seizures at 37 °C (Candida, n = 90; Lactobacillus, n = 90; Acetobacter, n = 102). During the locomotor test, CS flies associated with Candida (n = 90), Lactobacillus (n = 95) and Acetobacter (n = 93) spent 8.0%, 8.2% and 6.0% of their total activity in the central zone, respectively (Figure S3). This was not significantly different from germ‐free CS flies which spent 6.8% (n = 261) of time in the central area (H = 6.191, df = 3, Kruskal‐Wallis One‐way ANOVA on ranks with Dunn's Method post hoc, p > 0.05).
3.4. Nrf2 signaling is increased in the para Shu mutant midgut, following antibiotic treatment
Oxidative stress has been implicated in the progression of numerous neurological disorders, including epilepsy. 39 Moreover, the endogenous gut microbiota is known to play a significant role in the production of reactive oxygen species (ROS) by the host. 40 Therefore, we hypothesized that changes in oxidative stress conditions could contribute to the bacteria‐driven alterations observed in para Shu phenotypes. Nuclear factor erythroid 2‐related factor 2 (Nrf2) is a transcription factor activated in response to oxidative stress. When activated, it induces the expression of various antioxidant genes, providing protection against oxidative damage. 41 To probe the potential role of oxidative stress‐related signaling pathways in the observed phenotypic shifts of para Shu mutants after bacterial removal, we studied the effect of antibiotics on Nrf2 activity in the gut. For this purpose, we utilized an Nrf2 reporter transgene, GstD1‐GFP. This transgene features the GFP reporter gene positioned after the Nrf2 binding sequence, known as the antioxidant response element (ARE), localized in the Glutathione S‐transferase D1 (GstD1) promoter. When Nrf2 is activated, it binds to the ARE, triggering GFP gene transcription. 42 Through confocal microscopy of adult midgut samples, we observed that the antibiotic treatment amplified the GFP signal in para Shu mutants, especially in the anterior midgut region. This increase wasn't seen in wild‐type flies (Ctrl) (Figure 5A). Quantifying the GFP fluorescence intensity corroborated our observations (n = 7 replicates, 10 guts/replicate). It showed that the Nrf2‐driven antioxidant response was notably elevated in the intestines of antibiotic‐treated para Shu mutants (t = −9.014, df = 12, p < 0.001, Student's t‐test), but not in similarly treated wild‐type flies (t = −0.340, df = 12, p > 0.05, Student's t‐test). Interestingly, GFP reporter expression was naturally higher in wild‐type flies compared with para Shu mutants when both were fed a standard diet (t = 3.221, df = 12, p < 0.01, Student's t‐test) (Figure 5B). These findings suggest that, in comparison to their wild‐type counterparts, para Shu mutants exhibit suppressed Nrf2 signaling under normal conditions. However, this signaling is enhanced when commensal bacteria are eliminated.
FIGURE 5.
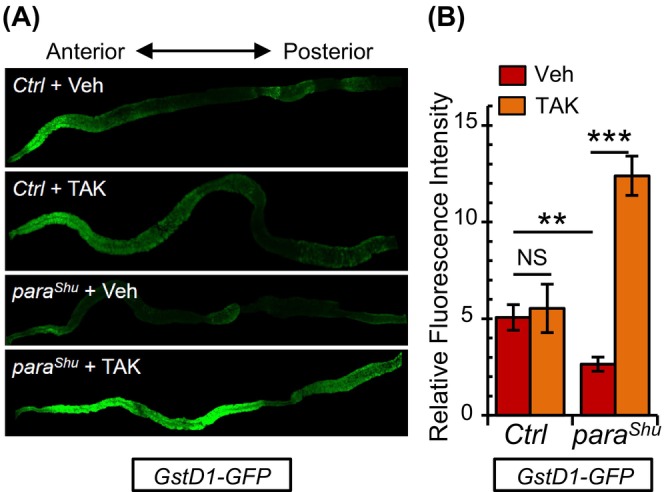
GstD1‐GFP fluorescence is increased in the gut of para Shu mutants fed antibiotics. (A) Representative images of GstD1‐GFP fluorescence in adult midguts dissected from CS (Ctrl) and para Shu flies cultured in vehicle or antibiotic‐containing food. (B) Relative fluorescence intensity in midguts dissected from CS (Ctrl) and para Shu females carrying the GstD1‐GFP transgene following treatment with vehicle or TAK. n = 7 replicates, 10 guts/replicate. *p < 0.05; ***p < 0.001; NS, not significant (p > 0.05).
3.5. Enhanced Nrf2 antioxidant pathway mitigates para Shu phenotypes
Nrf2's antioxidant response is typically restrained by its cytoplasmic inhibitor, Keap1. Keap1 curtails Nrf2 activity by sequestering it in the cytoplasm and targeting it for proteasomal degradation. 43 Thus, by diminishing Keap1 function, one can genetically enhance Nrf2 activity and the subsequent antioxidant responses. To gage the effects of intensified antioxidant responses on the phenotypes of para Shu , we incorporated a null allele of Keap1 into the para Shu mutant background. This modification reduced Keap1 levels, amplifying Nrf2 activity. Specifically, the Keap1 036 allele possesses a 1500 base pair deletion within Keap1. This deletion eliminates the initial coding exon and a segment of the second coding exon (Figure 6A). 42
FIGURE 6.
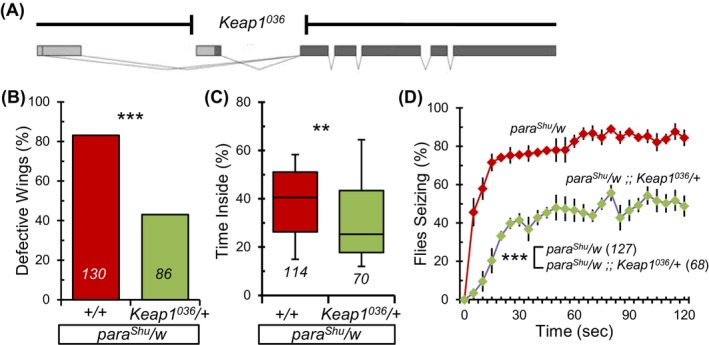
Morphological and behavioral phenotypes in para Shu mutants are suppressed by a Keap1 null allele. (A) Schematic illustration of Drosophila Keap1 locus with exons depicted as rectangles and coding segments in dark color (adapted from 42 ). A null allele (Keap1 036 ) removes 1500 bp of the Keap1 locus. (B) Incidence of downturned wings in para Shu mutants with a copy of the Keap1 036 allele compared with mutant controls (para Shu /w). (C) Percentage of time spent in the center area of the chamber for para Shu /w and para Shu /w;; Keap1 036 /+. (D) Average percentages of flies seizing at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics. **p < 0.01; ***p < 0.001.
The introduction of a single copy of Keap1 036 (para Shu /w;; Keap1 036 /+) significantly reduced the prevalence of downturned wings in para Shu mutants compared with mutant controls (para Shu /w;; +/+) with a decrease from 83% (n = 130) to 43% (n = 86) (X 2 = 35.838, df = 1, p < 0.001, Fisher's Exact Test; Figure 6B, Table S1). Additionally, activating the Nrf2 pathway significantly improved both the locomotor activity and heat‐induced seizure behavior in para Shu mutants. Specifically, the para Shu /w flies spent 41% (n = 114) of their time in the central area, compared with just 25% (n = 70) for para Shu /w;; Keap1 036 /+ flies (U = 2820.0, p < 0.01, Mann–Whitney Rank Sum Test; Figure 6C). When exposed to 37 °C for 30‐s, 1 and 2‐min, 76%, 83% and 84% (n = 127) of the control para Shu /w mutants displayed seizure behavior, respectively. In contrast, only 43%, 47% and 46% (n = 68) of the para Shu /w;; Keap1 036 /+ flies exhibited similar phenotypes at the same timepoints (F(1,6) = 71.527, p < 0.001, Two‐Way Repeated Measures ANOVA) (Figure 6D). The Keap1 036 allele, when introduced into the CS background, did not influence wing posture or behavior. Flies with the +/w;; Keap1 036 /+ genotype did not show any morphological defects (0%, n = 115) and were unaffected by heat‐induced seizures at 37 °C (0%, n = 63). In the locomotor test, the +/w;; Keap1 036 /+ flies spent 4.6% (n = 57) of their time in the central area, whereas the genetic control (+/w) spent 6.8% (n = 109) of time in the central zone (U = 2452.0, p > 0.05, Mann–Whitney Rank Sum Test) (Figure S4).
3.6. The suppression of para Shu phenotypes requires the removal of bacteria during the larval stage
To gain mechanistic insights into how bacterial eradication reduces the severity of neurological phenotypes in adult para Shu mutants, we aimed to determine the timing required for eliminating commensal bacteria to achieve phenotypic suppression. The life cycle of Drosophila includes two feeding periods: the larval stage and the adult stage. For the first group, we cultured flies in food containing TAK during the larval and pupal stages and transferred them to vehicle‐containing food upon eclosion. For the second group, we raised larvae and pupae in vehicle food and then transferred newly eclosed adult flies to TAK food. The phenotypes of adult mutants were assessed 5 days after eclosion.
para Shu mutants reared on TAK food only during the larval stage showed a significant decrease in the incidence of downturned wings, compared with mutants reared on vehicle food (n = 110, larval; n = 86, vehicle) (38% vs. 84%, X 2 = 41.510, df = 1, p < 0.001, Fisher's exact Test; Figure 7A, Table S1). The incidence of downturned wings in mutants with larval TAK treatment was not significantly different from that of mutants administered TAK throughout life (X 2 = 2.636, df = 1, p = 0.104, Fisher's exact Test; Figure 1B and Figure 7A, Table S1). Compared with vehicle controls, para Shu mutants treated with TAK only during adulthood did not show improvement in the wing phenotype; 76% of the adult TAK‐fed mutants (n = 109) exhibited downturned wings (X 2 = 1.780, df = 1, p = 0.182, Fisher's exact Test; Figure 7A, Table S1). Larval administration of TAK likewise suppressed behavioral phenotypes of adult para Shu mutants. In the locomotor assay, para Shu mutants raised on TAK food only during the larval stage spent 29% of their time in the center area (n = 73). In contrast, mutants fed TAK food only during the adult stage and those fed vehicle food throughout life spent 42% and 38%, respectively of their time in the center area (n = 93, adult; n = 75, vehicle) (for larval TAK feeding; Q = 3.372, p < 0.001, for adult TAK feeding; Q = 1.256, p = 0.418, Kruskal‐Wallis One‐way ANOVA on ranks with Dunn's Method post hoc; Figure 7B). Locomotor defects between mutants administered antibiotics during the larval stage and throughout life did not differ significantly (Q = 0.792, p = 1, Kruskal Wallis one‐way ANOVA on ranks, Dunn's multiple comparisons; Figures 1D and 7B).
FIGURE 7.
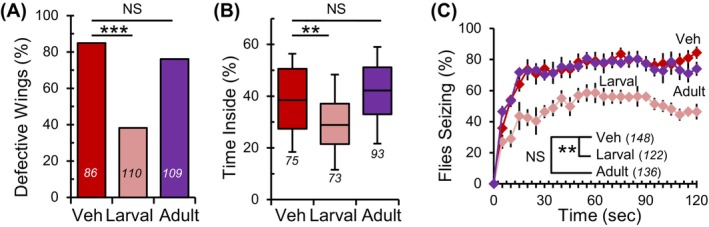
Suppression of para Shu phenotypes requires antibiotic treatment during the larval stage. (A) Incidence of down‐turned wings in para Shu flies administered antibiotics during the larval stage or adult stage compared with vehicle‐fed controls. (B) Percentage of time spent in the center area of the chamber for mutants fed vehicle, TAK during the larval stage, or TAK during the adult stage. (C) The average percentages of flies seizing at each time point (±SEM) at 37°C. The total number of flies scored under each condition is indicated in italics. **p < 0.01; ***p < 0.001; NS, not significant (p > 0.05).
Consistently, there was a statistically significant effect of developmental stage‐specific TAK‐treatment on para Shu mutant seizures (F(2,10) = 11.304, p = 0.003, Two‐Way Repeated Measures ANOVA) (Figure 7C). Post‐hoc analysis revealed that para Shu mutants fed TAK food only during the larval stage exhibited reduced heat‐induced seizure activity compared with vehicle‐fed mutants (t = 4.32, p = 0.003, Holm‐Sidak Method). In contrast, no phenotypic suppression was observed in para Shu mutants fed TAK food only during adulthood (t = 0.151, p = 0.883, Holm‐Sidak Method). For comparison, 46.4%, 58.5% and 45.4% of flies with larval TAK treatment (n = 122) showed seizures after 30‐s, 1 and 2‐min of exposure to 37 °C, respectively. In contrast, 70.2%, 77.8% and 74.0% of flies with adult TAK treatment (n = 136) exhibited seizures at the same time points. It should be noted that, although larval TAK feeding reduced the severity of para Shu adult phenotypes, it was less effective than TAK treatment throughout life (t = 3.196, p = 0.015, Two‐way repeated‐measures ANOVA, Holm‐Sidak multiple comparisons; Figures 1E and 7C). As observed when antibiotics were administered throughout life, CS flies with either larval or adult TAK treatment (n = 207, larval; n = 215, adult) did not show morphological defects nor heat‐induced seizure behavior at 37 °C (n = 80, larval; n = 89, adult) (Figure S5). In the locomotor test, CS flies experiencing either larval or adult TAK treatment (n = 96, larval; n = 133, adult) spent 11.3% and 7.9% of their time in the central area, respectively. This was not significantly different than CS flies reared on vehicle‐containing food, which spent 7.3% of time in the central zone (n = 69) (Q = 2.589, p > 0.05, Mann–Whitney Rank Sum Test with Dunn's Method post hoc) (Figure S5).
4. DISCUSSION
4.1. The presence of commensal bacteria exacerbates the neurological phenotypes in para Shu mutants: possible involvement of the immune‐inflammatory response
In this study, we explored the impact of host‐associated microbes on the neurological phenotypes of para Shu , a gain‐of‐function mutant form of the Drosophila Nav channel gene. Sequence analysis of microbial DNA revealed that the gut microbiota in para Shu mutants primarily consists of prokaryotic Lactobacillus and Acetobacter species, along with eukaryotic yeast species. This composition aligns with that of other laboratory‐reared Drosophila. 19 , 20 , 44 Our findings demonstrated the following: (1) removing commensal bacteria from para Shu mutants decreased the severity of their seizure‐like phenotypes (Figures 1 and 2), and (2) gnotobiotic para Shu mutants associated with either Lactobacillus or Acetobacter species exhibited more pronounced phenotypes than germ‐free mutants (Figure 4). Overall, these results suggest that commensal bacteria amplify the neurological defects observed in para Shu mutants. Unlike commensal bacteria, the commensal yeast Candida does not appear to affect the mutant phenotypes (Figure 5). Host responses to commensal bacteria and commensal yeast differ in several ways, including distinct immune signaling pathways, different antimicrobial peptides and variations in metabolic and physiological interactions. 45 , 46 , 47 These differences, or combinations thereof, may contribute to the contrasting effects of bacteria and yeast on para Shu phenotypes. For example, metabolites produced by commensal bacteria in the gut, such as short‐chain fatty acids, lactic acid and a variety of vitamins, may impact neural development and function, thereby enhancing the mutant phenotypes.
Commensal bacteria typically benefit the host under normal physiological conditions. Removing them can result in a range of negative outcomes. 11 , 12 , 13 However, under specific pathological circumstances, these ordinarily beneficial or harmless bacteria can negatively impact the host's neurological conditions. For instance, in a mouse model studying Parkinson's disease (PD), removing commensal microbes lessened motor deficits in adult animals, while reintroducing them worsened such deficits. 48 In a Drosophila model of Alzheimer's disease (AD), an oral infection with non‐pathogenic enterobacteria (Ecc15) intensified neurodegeneration. 49 Under these disease conditions, bacterial species that are harmless to healthy animals can trigger unusual immune responses, heightening inflammation and worsening brain defects. 48 , 49 Notably, genes associated with innate immunity, such as those encoding antimicrobial peptides and peptidoglycan recognition proteins, were significantly elevated in para Shu mutants. 26 Given that commensal bacteria can stimulate innate immune responses and increase the severity of symptoms in both PD and AD animal models, the typically harmless endogenous bacteria in para Shu might also contribute to the amplified expression of innate immune genes, thereby exacerbating the mutant phenotype.
Our previous studies suggest a possible involvement of the immune‐inflammatory response in the manifestation of para Shu phenotypes. 24 , 25 , 27 Specifically, an unbiased forward genetic screen revealed that the reduction of glutathione S‐transferase S1 (GstS1) function has a similar effect to bacterial removal, leading to significant suppression of para Shu phenotypes. 25 GstS1 is an insect ortholog of mammalian hematopoietic prostaglandin D synthase (HPGDS), 50 , 51 which catalyzes the production of prostaglandin D2 (PGD2). This lipid mediator plays an critical role in immune and inflammatory responses in mammals. 52 In Drosophila, overexpression of GstS1 leads to an increase in the number of macrophage‐like immune cells known as hemocytes. 53 Moreover, the presence of GstS1 transcripts in hemocytes surges tenfold during the onset of metamorphosis, 54 underscoring the importance of GstS1 in immunity.
Additionally, we serendipitously found that a diet containing milk whey significantly reduced the seizure‐like phenotypes in para Shu mutants. 24 Subsequent studies identified alpha‐linolenic acid (ALA) as a key dietary component responsible for this diet‐dependent suppression of seizures. 27 ALA is an omega‐3 polyunsaturated fatty acid (PUFA) with 18 carbons (18:3; n‐3) and serves as the primary precursor for longer‐chain omega‐3 PUFAs, including eicosapentaenoic acid (EPA, 20:5; n‐3) and docosahexaenoic acid (DHA, 22:6, n‐3). Both EPA and DHA are recognized for their immunomodulatory and anti‐inflammatory effects in mammals. 55 , 56 GstS1 and ALA may influence the severity of para Shu symptoms through a shared mechanism by modulating immune responses and regulating inflammation. To better understand the role of commensal bacteria in seizure‐like mutant phenotypes, it is vital to examine how the removal of these bacteria impacts neuroimmune interactions, especially where GstS1 and ALA could play a part.
4.2. Oxidative stress and Nrf2 signaling in the modulation of para Shu phenotypes by commensal bacteria
Oxidative stress is known to play an important role in the onset and development of multiple neurological conditions, including epilepsy. 39 Oxidative stress and neuronal excitability mutually reinforce each other through various mechanisms. On one hand, neuronal hyperexcitability can induce oxidative stress by increasing metabolic demand and enhancing the production of reactive oxygen species (ROS), 57 elevating intracellular Ca2+ levels, which activates enzymes that generate ROS, 58 impairing mitochondrial function and increasing electron leakage from the electron transport chain, 59 or triggering neuroinflammatory responses and activating immune cells that release ROS. 60 On the other hand, oxidative stress can increase neuronal excitability by modulating ion channel function, 61 impairing mitochondrial function and compromising the energy supply needed for maintaining ion gradients, 62 or affecting the synthesis, release and reuptake of neurotransmitters, leading to an excitatory/inhibitory imbalance. 63 , 64 In para Shu mutants, the primary defect is neuronal hyperexcitability due to abnormal Nav channels resulting from a single amino acid substitution. 26 Through the above‐mentioned complex interplay between hyperexcitability and oxidative stress, this primary defect can create a positive feedback loop that exacerbates neural dysfunction, leading to the severe seizure‐like phenotypes observed in adult para Shu mutants. Since the severity of para Shu phenotypes is reduced by bacterial eradication, it is likely that commensal bacteria enhance this positive feedback loop, resulting in severe neurological symptoms. This hypothesis is further supported by the allele‐specific effects observed with bacterial eradication in other para mutant alleles. Similar to para Shu , phenotypic suppression was also evident in another gain‐of‐function para mutant, para GEFS+ , following antibiotic treatment. However, suppression was not observed in the loss‐of‐function mutant para DS (Figure 3). This finding suggests that eliminating bacteria may mitigate the negative developmental effects caused by increased neuronal excitability in gain‐of‐function Nav channel mutants.
Nrf2 signaling serves as a key cellular defense mechanism against oxidative stress by upregulating the expression of various antioxidant and anti‐inflammatory genes (Suzuki et al., 2013). Previous studies have shown that spontaneous seizures are reduced by Nrf2 activation in a rat model of epilepsy (Mazzuferi et al., 2013). Consistently, we found that activation of the Nrf2 pathway reduced the severity of para Shu phenotypes, underscoring the potential role of Nrf2 signaling in the bacteria‐dependent modulation of these mutant phenotypes (Figure 6). An interesting finding is that the eradication of commensal bacteria enhanced, rather than suppressed, Nrf2 signaling in para Shu mutants (Figure 5). This seems contradictory to a previous report that the bacterial taxon Lactobacilli activates Nrf2 signaling in response to ROS generated in the gut epithelial cells of wild‐type flies (Jones et al., 2015). The different oxidative stress statuses in wild‐type flies and para Shu mutants may contribute to this apparent discrepancy in the effects of commensal bacteria on Nrf2 signaling. In para Shu mutants, the enhanced positive feedback loop between neuronal hyperexcitability and oxidative stress may involve chronical activation of Nrf2 signaling, followed by homeostatic regulation resulting in the downregulation of this pathway. Since commensal bacteria contribute to the exacerbation of para Shu phenotypes, their eradication may counteract the neuronal activity‐oxidative feedback loop and normalize the responsiveness of the Nrf2 pathway. To fully understand the presumed changes in Nrf2 signaling responsiveness, a thorough future analysis of the gut at the molecular and cellular levels is essential for both wild‐type flies and para Shu mutants.
4.3. Potential effects of commensal bacteria on neural development and function in para Shu mutants
Prior research with germ‐free mice has shown that early‐life microbiota is instrumental in the development of the nervous system, with its influence on neural function and behavior persisting into adulthood. 65 , 66 , 67 We previously found that providing a diet enriched with milk whey or ALA during the larval stages effectively suppressed the seizure‐like phenotypes in adult para Shu mutants. 24 , 27 Notably, the current study has demonstrated that removal of the commensal bacteria during the larval stage is critical for reducing the severity of para Shu adult phenotypes (Figure 7). These findings suggest that the suppression of adult para Shu phenotypes through dietary adjustments or eradication of commensal bacteria primarily results from alterations in neural development.
The proper formation of mature neural circuits within the adult CNS hinges on meticulously coordinated processes that occur at specific developmental stages. Aberrant neural activity and resultant alterations in molecular and cellular processes during these pivotal moments can interfere with development of the nervous system, subsequently impacting the architecture and operation of neuronal circuits and potentially leading to neurological issues in later life. 68 , 69 , 70 Our recent study revealed developmental abnormalities in the para Shu nervous system. Specifically, class IV dendritic arborization (C4da) neurons in the larval peripheral nervous system (PNS) exhibit increased dendritic complexity in para Shu larvae compared with control larvae. 27 Notably, dietary adjustments that lessen the severity of adult para Shu phenotypes fully rectified this neurodevelopmental anomaly in the mutant. 27 While these defects in the larval PNS have little to do with seizure‐like phenotypes in adult para Shu mutants, analogous developmental irregularities might occur in the para Shu CNS neurons responsible for motor control. Future research aims to determine if such developmental irregularities exist in the para Shu brain areas associated with motor functions and whether these potential CNS neuronal defects can be mitigated by dietary modifications or eliminating bacteria during the developmental phase. It is crucial to determine how the diet‐ and bacteria‐mediated processes functionally interact in affecting neural development and subsequently modifying the neurological phenotypes in adult mutants.
In summary, our research underscores the interplay between the nervous system and the commensal gut microbiota, highlighting their significance in the manifestation of seizure‐like phenotypes in the para Shu Nav channel mutant. Investigating deeper into the molecular and cellular foundations of these bacteria‐brain interactions in Drosophila promises to shed light on the pivotal roles and mechanisms of commensal bacteria in influencing the nervous system, especially under conditions prone to seizures. This understanding is expected to have implications for a range of evolutionary diverse species, including humans.
FUNDING INFORMATION
This study was supported by National Institutes of Health grants (R21 NS101542, R03 NS101541, R21 NS127364) for TK and an NIH training grant fellowship (T32 NS045549) for PL.
Supporting information
Figure S1. Administration of antibiotics to Canton‐S flies does not affect morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in Canton‐S (CS) flies treated with vehicle or antibiotics (TAK). (C) Percentage of time spent in the center area of the chamber for CS flies treated with vehicle or TAK. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C following treatment with vehicle or TAK. The number of flies scored under each condition is indicated in italics.
Figure S2: Germ‐free Canton‐S flies do not display differences in morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in germ‐free Canton‐S (CS) flies compared with mock‐treated animals. (C) Percentage of time spent in the center area of the chamber for mock‐treated and germ‐free CS flies. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics. ***p < 0.001.
Figure S3: Mono‐association of Candida, Lactobacillus, or Acetobacter with Canton‐S flies does not affect morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in germ‐free mutants and gnotobiotic Canton‐S (CS) flies mono‐associated with Lactobacillus (Lac), Acetobacter (Acet), or Candida (Can). (C) Percentage of time spent in the center area of the chamber for gnotobiotic and germ‐free flies. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics.
Figure S4: A null allele of Keap1 does not affect morphology or behavior in Canton‐S flies. (A) Incidence of downturned wings and (B) indented thorax in Canton‐S (CS) flies with a copy of the Keap1 036 allele compared with controls (CS/w). (C) Percentage of time spent in the center area of the chamber for CS/w and CS/w;; Keap1 036 /+. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics.
Figure S5: Antibiotic administration to either larval or adult Canton‐S flies does not affect morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in Canton‐S (CS) flies administered antibiotics during the larval stage or adult stage compared with vehicle‐fed controls. (C) Percentage of time spent in the center area of the chamber for CS flies fed vehicle, TAK during the larval stage, or TAK during the adult stage. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics.
Supplementary Table 1: Morphological Defects in para Shu Mutants.
ACKNOWLEDGMENTS
We thank Dr. Diane O'Dowd (University of California, Irvine) for providing fly strains (para GEFS+ and para DS ), Dr. Heinrich Jasper (Buck Institute for Research on Aging, Novato, CA) for providing Keap1 036 null mutants, and Ms. Morgan Lohr and Ms. Hanxi Tang (Kitamoto lab) for their technical assistance.
Lansdon P, Kasuya J, Kitamoto T. Commensal bacteria exacerbate seizure‐like phenotypes in Drosophila voltage‐gated sodium channel mutants. Genes, Brain and Behavior. 2024;23(5):e70000. doi: 10.1111/gbb.70000
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kullmann DM, Waxman SG. Neurological channelopathies: new insights into disease mechanisms and ion channel function. J Physiol. 2010;588:1823‐1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catterall WA. Sodium Channel mutations and epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado‐Escueta AV, eds. Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Oxford University Press; 2012. [Google Scholar]
- 3. Klassen T, Davis C, Goldman A, et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wild CP. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847‐1850. [DOI] [PubMed] [Google Scholar]
- 5. Backhed F, Crawford PA. Coordinated regulation of the metabolome and lipidome at the host‐microbial interface. Biochim Biophys Acta. 2010;1801:240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodman AL, Gordon JI. Our unindicted coconspirators: human metabolism from a microbial perspective. Cell Metab. 2010;12:111‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bocci V. The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect Biol Med. 1992;35:251‐260. [DOI] [PubMed] [Google Scholar]
- 9. Fraune S, Bosch TC. Why bacteria matter in animal development and evolution. Bioessays. 2010;32:571‐580. [DOI] [PubMed] [Google Scholar]
- 10. O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735‐742. [DOI] [PubMed] [Google Scholar]
- 12. Cryan JF, Dinan TG. Mind‐altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701‐712. [DOI] [PubMed] [Google Scholar]
- 13. Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corby‐Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DE. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl Environ Microbiol. 2007;73:3470‐3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox CR, Gilmore MS. Native microbial colonization of Drosophila melanogaster and its use as a model of enterococcus faecalis pathogenesis. Infect Immun. 2007;75:1565‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during drosophila aging without life‐span trade‐off. Cell Metab. 2007;6:144‐152. [DOI] [PubMed] [Google Scholar]
- 17. Ryu JH, Kim SH, Lee HY, et al. Innate immune homeostasis by the homeobox gene caudal and commensal‐gut mutualism in drosophila. Science. 2008;319:777‐782. [DOI] [PubMed] [Google Scholar]
- 18. Wong CN, Ng P, Douglas AE. Low‐diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol. 2011;13:1889‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark RI, Salazar A, Yamada R, et al. Distinct shifts in microbiota composition during drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12:1656‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chandler JA, Eisen JA, Kopp A. Yeast communities of diverse drosophila species: comparison of two symbiont groups in the same hosts. Appl Environ Microbiol. 2012;78:7327‐7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee WJ, Brey PT. How microbiomes influence metazoan development: insights from history and drosophila modeling of gut‐microbe interactions. Annu Rev Cell Dev Biol. 2013;29:571‐592. [DOI] [PubMed] [Google Scholar]
- 22. Wong AC, Dobson AJ, Douglas AE. Gut microbiota dictates the metabolic response of drosophila to diet. J Exp Biol. 2014;217:1894‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis EB. A new standard food medium. Drosoph Inf Serv. 1960;34:117‐118. [Google Scholar]
- 24. Kasuya J, Iyengar A, Chen HL, Lansdon P, Wu CF, Kitamoto T. Milk‐whey diet substantially suppresses seizure‐like phenotypes of para(Shu), a drosophila voltage‐gated sodium channel mutant. J Neurogenet. 2019;33:164‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen HL, Kasuya J, Lansdon P, et al. Reduced function of the glutathione S‐transferase S1 suppresses behavioral Hyperexcitability in drosophila expressing mutant voltage‐gated sodium. Channels. 2020;10:1327‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaas GA, Kasuya J, Lansdon P, et al. Lithium‐responsive seizure‐like Hyperexcitability is caused by a mutation in the Drosophila Voltage‐gated Sodium Channel Gene paralytic . eNeuro. 2016;3:ENEURO.0221‐16.2016. [DOI] [PMC free article] [PubMed]
- 27. Kasuya J, Johnson W, Chen HL, Kitamoto T. Dietary supplementation with Milk lipids leads to suppression of developmental and behavioral phenotypes of Hyperexcitable drosophila mutants. Neuroscience. 2023;520:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williamson RL. Lithium stops hereditary shuddering in Drosophila melanogaster. Psychopharmacology. 1982;76:265‐268. [DOI] [PubMed] [Google Scholar]
- 29. Gilestro GF, Cirelli C. pySolo: a complete suite for sleep analysis in drosophila. Bioinformatics. 2009;25:1466‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun L, Gilligan J, Staber C, et al. A knock‐in model of human epilepsy in drosophila reveals a novel cellular mechanism associated with heat‐induced seizure. J Neurosci. 2012;32:14145‐14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schutte RJ, Schutte SS, Algara J, et al. Knock‐in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J Neurophysiol. 2014;112:903‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koyle ML, Veloz M, Judd AM, et al. Rearing the fruit Fly <em>Drosophila melanogaster</em> under axenic and Gnotobiotic conditions. Rearing the Fruit Fly Drosophila Melanogaster under Axenic and Gnotobiotic Conditions. J Vis Exp; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ludington WB, Ja WW. Drosophila as a model for the gut microbiome. PLoS Pathog. 2020;16:e1008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74:2461‐2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Champagne‐Jorgensen K, Kunze WA, Forsythe P, Bienenstock J, McVey Neufeld KA. Antibiotics and the nervous system: more than just the microbes? Brain Behav Immun. 2018;77:7‐15. [DOI] [PubMed] [Google Scholar]
- 36. Singh R, Sripada L, Singh R. Side effects of antibiotics during bacterial infection: mitochondria, the main target in host cell. Mitochondrion. 2014;16:50‐54. [DOI] [PubMed] [Google Scholar]
- 37. Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588:1849‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Claes L, Del‐Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium‐channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pearson‐Smith JN, Patel M. Metabolic dysfunction and oxidative stress in epilepsy. Int J Mol Sci. 2017;18:2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jones RM, Luo L, Ardita CS, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox‐mediated generation of reactive oxygen species. EMBO J. 2013;32:3017‐3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in drosophila. Dev Cell. 2008;14:76‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motohashi H, Yamamoto M. Nrf2‐Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549‐557. [DOI] [PubMed] [Google Scholar]
- 44. Wong AC, Chaston JM, Douglas AE. The inconstant gut microbiota of drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013;7:1922‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bahuguna S, Atilano M, Glittenberg M, et al. Bacterial recognition by PGRP‐SA and downstream signalling by toll/DIF sustain commensal gut bacteria in drosophila. PLoS Genet. 2022;18:e1009992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. 2013;11:615‐626. [DOI] [PubMed] [Google Scholar]
- 47. Kuraishi T, Hori A, Kurata S. Host‐microbe interactions in the gut of Drosophila melanogaster. Front Physiol. 2013;4:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469.e12‐1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu SC, Cao ZS, Chang KM, Juang JL. Intestinal microbial dysbiosis aggravates the progression of Alzheimer's disease in drosophila. Nat Commun. 2017;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sajjadian SM, Ahmed S, Al Baki MA, Kim Y. Prostaglandin D2 synthase and its functional association with immune and reproductive processes in a lepidopteran insect spodoptera exigua. Gen Comp Endocrinol. 2019;287:113352. [DOI] [PubMed] [Google Scholar]
- 51. Scarpati M, Qi Y, Govind S, Singh S. A combined computational strategy of sequence and structural analysis predicts the existence of a functional eicosanoid pathway in Drosophila melanogaster. PLoS One. 2019;14:e0211897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ 2nd. Prostaglandin D2 generation after activation of rat and human mast cells with anti‐IgE. J Immunol. 1982;129:1627‐1631. [PubMed] [Google Scholar]
- 53. Stofanko M, Kwon SY, Badenhorst P. A misexpression screen to identify regulators of drosophila larval hemocyte development. Genetics. 2008;180:253‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Regan JC, Brandao AS, Leitao AB, et al. Steroid hormone signaling is essential to regulate innate immune cells and fight bacterial infection in drosophila. PLoS Pathog. 2013;9:e1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Al‐Khalaifah H. Modulatory effect of dietary polyunsaturated fatty acids on immunity, represented by phagocytic activity. Front Vet Sci. 2020;7:569939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gutierrez S, Svahn SL, Johansson ME. Effects of Omega‐3 fatty acids on immune cells. Int J Mol Sci. 2019;20:5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2020;467:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Waldbaum S, Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J Bioenerg Biomembr. 2010;42:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fabisiak T, Patel M. Crosstalk between neuroinflammation and oxidative stress in epilepsy. Front Cell Dev Biol. 2022;10:976953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Orfali R, Alwatban AZ, Orfali RS, et al. Oxidative stress and ion channels in neurodegenerative diseases. Front Physiol. 2024;15:1320086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360:201‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clarke G, Grenham S, Scully P, et al. The microbiome‐gut‐brain axis during early life regulates the hippocampal serotonergic system in a sex‐dependent manner. Mol Psychiatry. 2013;18:666‐673. [DOI] [PubMed] [Google Scholar]
- 66. Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047‐3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. J Physiol. 2004;558:263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Giachello CN, Baines RA. Inappropriate neural activity during a sensitive period in embryogenesis results in persistent seizure‐like behavior. Curr Biol. 2015;25:2964‐2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hunter I, Coulson B, Pettini T, et al. Balance of activity during a critical period tunes a developing network. Elife. 2023;12:RP91599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Meredith RM, Dawitz J, Kramvis I. Sensitive time‐windows for susceptibility in neurodevelopmental disorders. Trends Neurosci. 2012;35:335‐344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Administration of antibiotics to Canton‐S flies does not affect morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in Canton‐S (CS) flies treated with vehicle or antibiotics (TAK). (C) Percentage of time spent in the center area of the chamber for CS flies treated with vehicle or TAK. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C following treatment with vehicle or TAK. The number of flies scored under each condition is indicated in italics.
Figure S2: Germ‐free Canton‐S flies do not display differences in morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in germ‐free Canton‐S (CS) flies compared with mock‐treated animals. (C) Percentage of time spent in the center area of the chamber for mock‐treated and germ‐free CS flies. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics. ***p < 0.001.
Figure S3: Mono‐association of Candida, Lactobacillus, or Acetobacter with Canton‐S flies does not affect morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in germ‐free mutants and gnotobiotic Canton‐S (CS) flies mono‐associated with Lactobacillus (Lac), Acetobacter (Acet), or Candida (Can). (C) Percentage of time spent in the center area of the chamber for gnotobiotic and germ‐free flies. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics.
Figure S4: A null allele of Keap1 does not affect morphology or behavior in Canton‐S flies. (A) Incidence of downturned wings and (B) indented thorax in Canton‐S (CS) flies with a copy of the Keap1 036 allele compared with controls (CS/w). (C) Percentage of time spent in the center area of the chamber for CS/w and CS/w;; Keap1 036 /+. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics.
Figure S5: Antibiotic administration to either larval or adult Canton‐S flies does not affect morphology or behavior. (A) Incidence of downturned wings and (B) indented thorax in Canton‐S (CS) flies administered antibiotics during the larval stage or adult stage compared with vehicle‐fed controls. (C) Percentage of time spent in the center area of the chamber for CS flies fed vehicle, TAK during the larval stage, or TAK during the adult stage. (D) The average percentages of flies that seized at each time point (±SEM) at 37 °C. The number of flies scored under each condition is indicated in italics.
Supplementary Table 1: Morphological Defects in para Shu Mutants.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


