Abstract
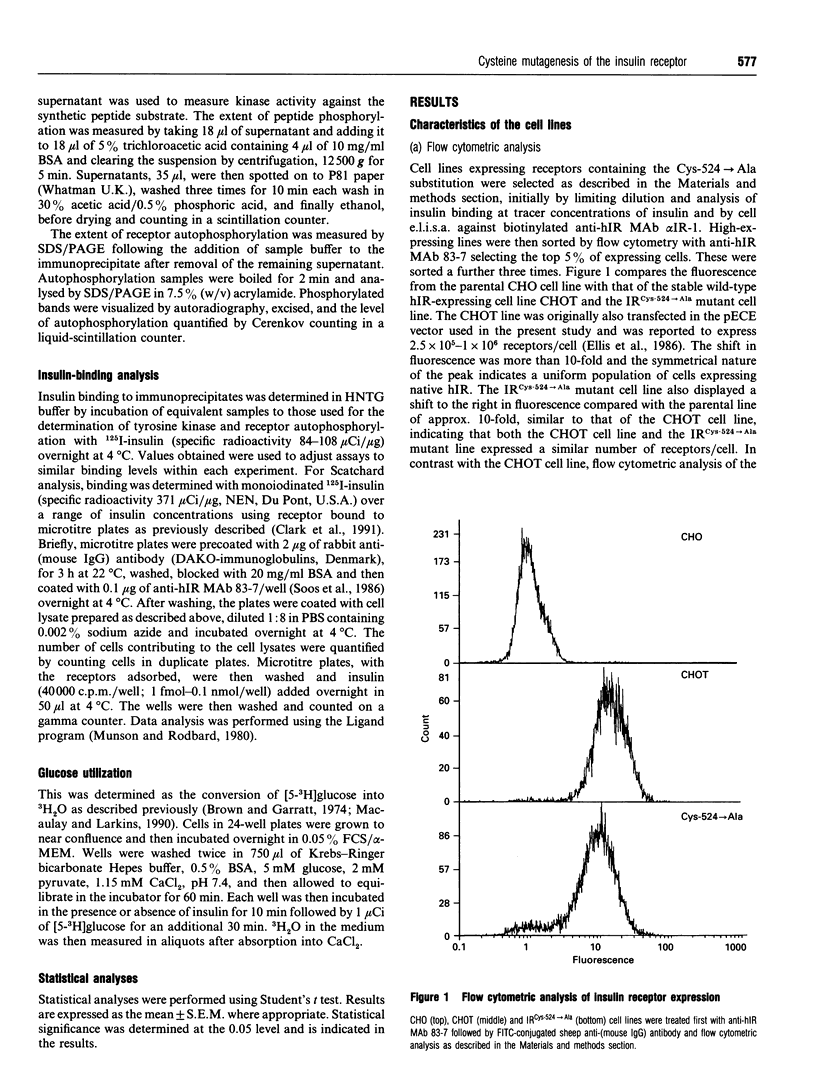
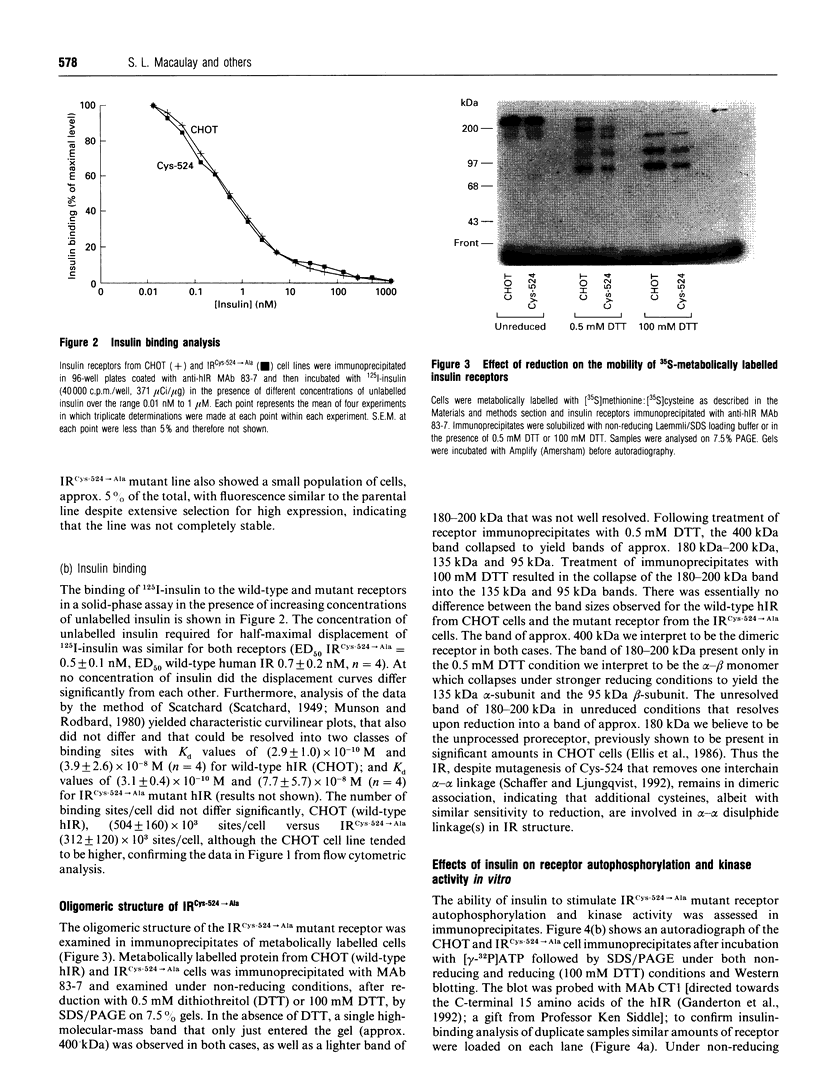
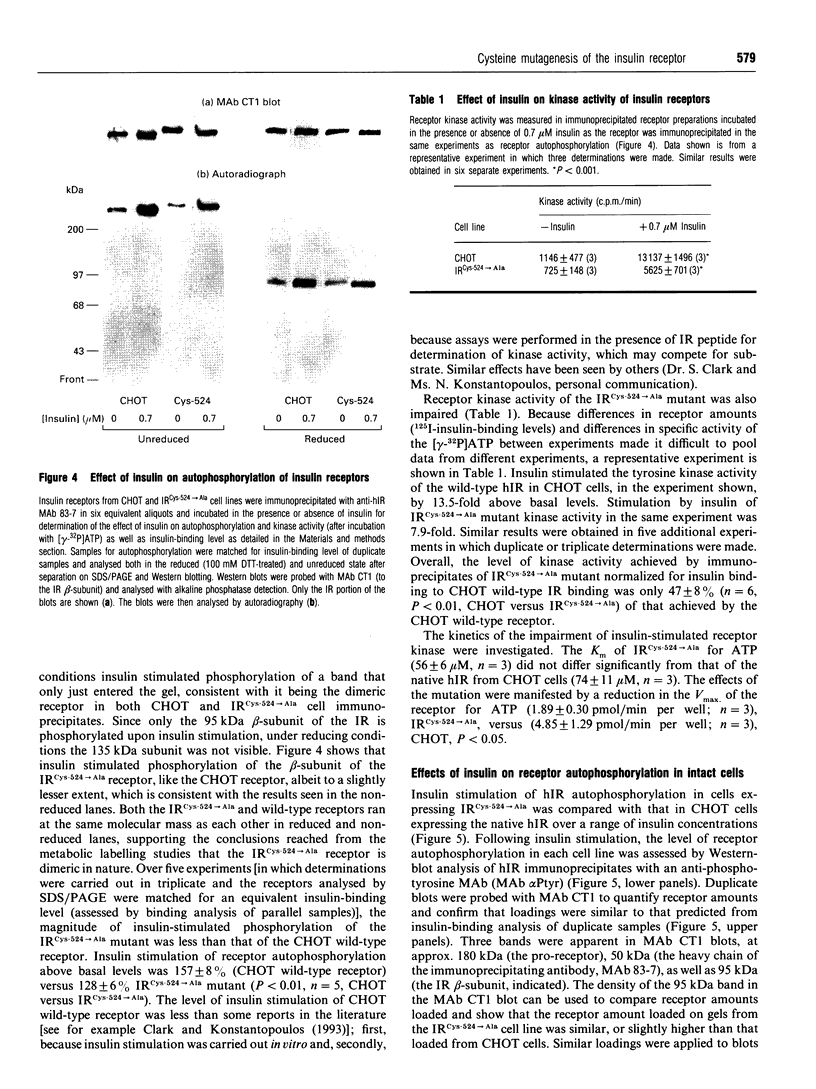
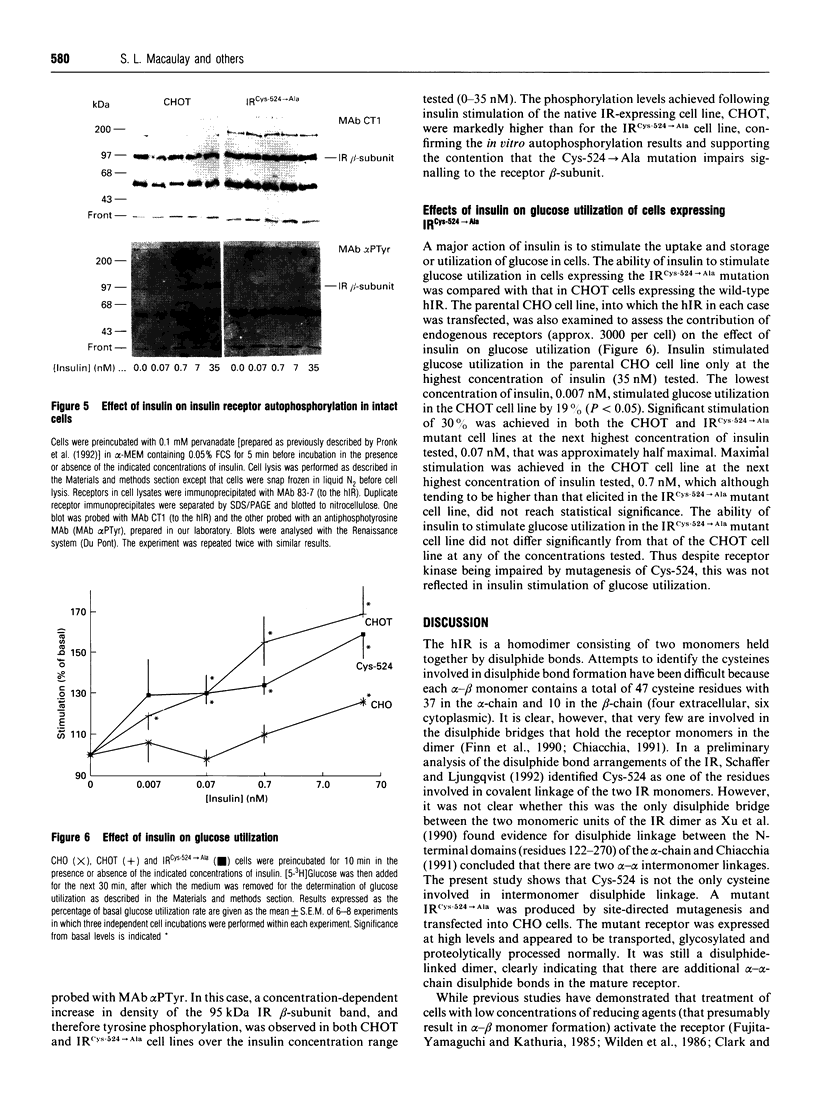
The human insulin receptor (hIR) is a member of the transmembrane tyrosine kinase receptor family. It is a disulphide-linked homodimer which can be reduced to two insulin-binding monomers by mild reduction of class-I disulphide bonds. The number of disulphide bonds between the alpha- and beta-chains within the monomer or between the monomers in the dimer is not known, although one dimer bond involving hIR Cys-524 has recently been identified [Schaffer and Ljungqvist (1992) Biochem. Biophys. Res. Commun. 189, 650-653]. In the present report hIR Cys-524 was converted into alanine by site-directed mutagenesis and expressed at high levels in Chinese hamster ovary (CHO) cells. The mutant receptor was processed normally and shown to bind insulin normally, with ED50 and KD values not different from those of the wild-type hIR. It was still a disulphide-linked dimer as judged by SDS/PAGE, indicating that there are alpha-alpha-chain disulphide bonds additional to the Cys-524 linkage in the insulin receptor dimer. Insulin-stimulated receptor autophosphorylation and kinase activity of the mutated receptor were both impaired compared with that of the wild-type receptor by 49% and 53% respectively. CHO cells overexpressing the mutant receptor, however, did not show a reduced capacity to stimulate glucose utilization, indicative that the level of receptor expression was sufficient to saturate downstream insulin action. These findings indicate that alpha-alpha disulphides additional to that provided by Cys-524 hold the receptor dimer together and that mutagenesis of Cys-524 reduces the ability of the receptor to signal insulin action subsequent to hormone binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj M., Waterfield M. D., Schlessinger J., Taylor W. R., Blundell T. On the tertiary structure of the extracellular domains of the epidermal growth factor and insulin receptors. Biochim Biophys Acta. 1987 Nov 26;916(2):220–226. doi: 10.1016/0167-4838(87)90112-9. [DOI] [PubMed] [Google Scholar]
- Brown D., Garratt C. J. A simple method for determining total glucose utilisation by isolated adipocytes using (5-3H)-glucose. Anal Biochem. 1974 Oct;61(2):492–499. doi: 10.1016/0003-2697(74)90416-3. [DOI] [PubMed] [Google Scholar]
- Chiacchia K. B. Quantitation of the class I disulfides of the insulin receptor. Biochem Biophys Res Commun. 1991 May 15;176(3):1178–1182. doi: 10.1016/0006-291x(91)90409-z. [DOI] [PubMed] [Google Scholar]
- Christiansen K., Tranum-Jensen J., Carlsen J., Vinten J. A model for the quaternary structure of human placental insulin receptor deduced from electron microscopy. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):249–252. doi: 10.1073/pnas.88.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S., Eckardt G., Siddle K., Harrison L. C. Changes in insulin-receptor structure associated with trypsin-induced activation of the receptor tyrosine kinase. Biochem J. 1991 May 15;276(Pt 1):27–33. doi: 10.1042/bj2760027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S., Konstantopoulos N. Sulphydryl agents modulate insulin- and epidermal growth factor (EGF)-receptor kinase via reaction with intracellular receptor domains: differential effects on basal versus activated receptors. Biochem J. 1993 May 15;292(Pt 1):217–223. doi: 10.1042/bj2920217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyts P., Gu J. L., Shymko R. M., Kaplan B. E., Bell G. I., Whittaker J. Identification of a ligand-binding region of the human insulin receptor encoded by the second exon of the gene. Mol Endocrinol. 1990 Mar;4(3):409–416. doi: 10.1210/mend-4-3-409. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Finn F. M., Ridge K. D., Hofmann K. Labile disulfide bonds in human placental insulin receptor. Proc Natl Acad Sci U S A. 1990 Jan;87(1):419–423. doi: 10.1073/pnas.87.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Kathuria S. The monomeric alpha beta form of the insulin receptor exhibits much higher insulin-dependent tyrosine-specific protein kinase activity than the intact alpha 2 beta 2 form of the receptor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6095–6099. doi: 10.1073/pnas.82.18.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganderton R. H., Stanley K. K., Field C. E., Coghlan M. P., Soos M. A., Siddle K. A monoclonal anti-peptide antibody reacting with the insulin receptor beta-subunit. Characterization of the antibody and its epitope and use in immunoaffinity purification of intact receptors. Biochem J. 1992 Nov 15;288(Pt 1):195–205. doi: 10.1042/bj2880195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Su Y. F., Cuatrecasas P. A monoclonal antibody to human insulin receptor. Biochem Biophys Res Commun. 1982 Jun 15;106(3):1019–1026. doi: 10.1016/0006-291x(82)91813-7. [DOI] [PubMed] [Google Scholar]
- Lammers R., Gray A., Schlessinger J., Ullrich A. Differential signalling potential of insulin- and IGF-1-receptor cytoplasmic domains. EMBO J. 1989 May;8(5):1369–1375. doi: 10.1002/j.1460-2075.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay S. L., Larkins R. G. Isolation of insulin-sensitive phosphatidylinositol-glycan from rat adipocytes. Its impaired breakdown in the streptozotocin-diabetic rat. Biochem J. 1990 Oct 15;271(2):427–435. doi: 10.1042/bj2710427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- O'Bryan J. P., Frye R. A., Cogswell P. C., Neubauer A., Kitch B., Prokop C., Espinosa R., 3rd, Le Beau M. M., Earp H. S., Liu E. T. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991 Oct;11(10):5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor. A multifunctional protein. Diabetes. 1990 Sep;39(9):1009–1016. doi: 10.2337/diab.39.9.1009. [DOI] [PubMed] [Google Scholar]
- Pronk G. J., Medema R. H., Burgering B. M., Clark R., McCormick F., Bos J. L. Interaction between the p21ras GTPase activating protein and the insulin receptor. J Biol Chem. 1992 Nov 25;267(33):24058–24063. [PubMed] [Google Scholar]
- Riedel H., Dull T. J., Schlessinger J., Ullrich A. A chimaeric receptor allows insulin to stimulate tyrosine kinase activity of epidermal growth factor receptor. Nature. 1986 Nov 6;324(6092):68–70. doi: 10.1038/324068a0. [DOI] [PubMed] [Google Scholar]
- Schaefer E. M., Erickson H. P., Federwisch M., Wollmer A., Ellis L. Structural organization of the human insulin receptor ectodomain. J Biol Chem. 1992 Nov 15;267(32):23393–23402. [PubMed] [Google Scholar]
- Schaefer E. M., Siddle K., Ellis L. Deletion analysis of the human insulin receptor ectodomain reveals independently folded soluble subdomains and insulin binding by a monomeric alpha-subunit. J Biol Chem. 1990 Aug 5;265(22):13248–13253. [PubMed] [Google Scholar]
- Schäffer L., Ljungqvist L. Identification of a disulfide bridge connecting the alpha-subunits of the extracellular domain of the insulin receptor. Biochem Biophys Res Commun. 1992 Dec 15;189(2):650–653. doi: 10.1016/0006-291x(92)92250-2. [DOI] [PubMed] [Google Scholar]
- Shoelson S. E., White M. F., Kahn C. R. Tryptic activation of the insulin receptor. Proteolytic truncation of the alpha-subunit releases the beta-subunit from inhibitory control. J Biol Chem. 1988 Apr 5;263(10):4852–4860. [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway J. L., Morrison B. D., Soos M. A., Siddle K., Olefsky J., Ullrich A., McClain D. A., Pessin J. E. Transdominant inhibition of tyrosine kinase activity in mutant insulin/insulin-like growth factor I hybrid receptors. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):214–218. doi: 10.1073/pnas.88.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Waugh S. M., DiBella E. E., Pilch P. F. Isolation of a proteolytically derived domain of the insulin receptor containing the major site of cross-linking/binding. Biochemistry. 1989 Apr 18;28(8):3448–3455. doi: 10.1021/bi00434a045. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Boyle T. R., Swanson M. L., Sweet L. J., Pessin J. E. Alteration of intramolecular disulfides in insulin receptor/kinase by insulin and dithiothreitol: insulin potentiates the apparent dithiothreitol-dependent subunit reduction of insulin receptor. Biochemistry. 1986 Jul 29;25(15):4381–4388. doi: 10.1021/bi00363a031. [DOI] [PubMed] [Google Scholar]
- Xu Q. Y., Paxton R. J., Fujita-Yamaguchi Y. Substructural analysis of the insulin receptor by microsequence analyses of limited tryptic fragments isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the absence or presence of dithiothreitol. J Biol Chem. 1990 Oct 25;265(30):18673–18681. [PubMed] [Google Scholar]