Abstract
Endometriosis is a heterogeneous disease where neurogenic sensitization can lead to chronic pain within and beyond the pelvis. Coincident pain and comorbidities merit specific attention. We discuss the causes, comorbidities, and management of endometriosis-associated chronic pelvic pain, advocating for a multidisciplinary approach to develop more effective treatments.
INTRODUCTION
Chronic pelvic pain (CPP) is a major cause of morbidity with an estimated prevalence of up to 25% in menstruating persons worldwide.1 Despite progress in recent years, CPP in these individuals remains an area of critical need for research into the underlying pathophysiology and factors that precipitate and sustain pain and for better evidence-based treatments. Endometriosis is present in about 10% of all reproductive-age biological females and 40%–87% of those with CPP, making it the most frequently identified cause.2
Endometriosis is defined by the presence of estrogen-dependent, progesterone-resistant lesions outside of the uterus that induce a chronic, inflammatory reaction, frequently resulting in chronic pain and infertility.2 Endometriosis lesions appear to arise from the common occurrence of retrograde menstruation in conjunction with neuroangiogenic factors that enable the endometrial cells to adhere to peritoneal surfaces, proliferate, and develop into endometriosis lesions. Lesion sub-types include superficial and/or deep lesions found on the pelvic peritoneum as well as bowel and bladder and cystic structures called “endometriomas” in the ovary.
The very heterogeneous presentation endometriosis encompasses visualized endometriosis lesions, multisystem symptomatology, and comorbid conditions. Although the endometriosis-associated pain often starts after menarche during adolescence, many of those affected wait years for the diagnosis to be made.2 Endometriosis-related pain impairs personal and work quality of life, severely impacting women over their life course.1 The constellation of pain symptoms include dysmenorrhea, non-menstrual CPP, and dyspareunia and frequently prompts the gynecologist to consider the diagnosis of endometriosis, but other clinicians do not consistently obtain a menstrual or sexual pain history as part of the routine medical history-taking and thus may overlook the symptoms suggesting this diagnosis (Figure 1). Once the diagnosis of endometriosis is considered, empiric treatment with hormonal agents like combined hormonal contraception or a progestin-releasing intrauterine system are frequently initiated to lessen and, at times, suppress menses. If the patient’s pain is successfully treated with these agents and over-the-counter pain medication, often no further evaluation proceeds.
Figure 1. Endometriosis phenome.
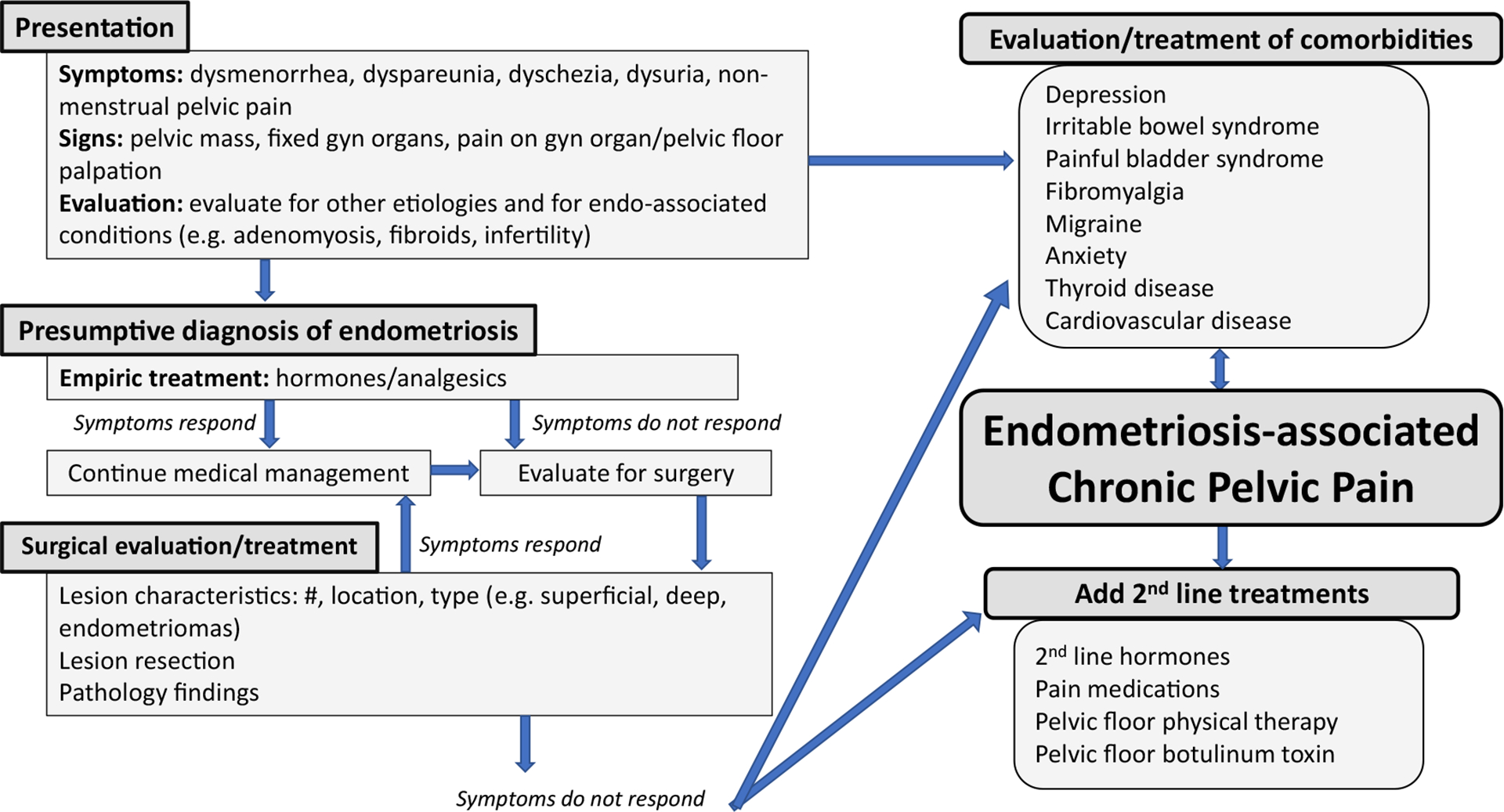
The pathway from presenting signs and symptoms to evaluation and treatment of endometriosis-associated chronic pelvic pain and comorbidities. Gyn, gynecologic; endo-associated, endometriosis associated.
Some consider the successful treatment of menstrual pain with hormonal agents and over-the-counter analgesics adequate to support a diagnosis of endometriosis; no non-invasive diagnostic standard exists. Diagnosis of superficial peritoneal endometriosis is made by a surgical evaluation, typically limited to those whose symptoms are resistant to empiric treatment and severe enough to justify a surgical procedure.2 Imaging can show deep lesions or ovarian endometriomas and is similarly often limited to those who are geographically, economically, and socially able to have such an evaluation by an experienced imaging specialist. For those who undergo laparoscopic surgery for clinical suspicion of endometriosis based on pain symptoms, the observation of a palpable mass on examination, suspicion of deep lesions, or endometriomas on imaging, the current approach is to excise any detected lesions. After surgery, hormones are continued to suppress further lesion growth and new lesion development.
Pain may not respond to hormones or lesion resection and may recur after lesion removal. In fact, despite surgical and/or hormonal treatment, about one-third of women with endometriosis develop CPP, defined as pain perceived in structures related to the pelvis and present for at least 3–6 months.3
Pain in endometriosis
The quality of endometriosis-related CPP (endo-CPP) varies widely. Affected menstruating persons experience cyclical and non-cyclical pain—dysmenorrhea and non-menstrual pain sometimes accompanied by dyschezia, dysuria, and, among those who are sexually active, dyspareunia. Pain may be felt throughout the pelvis and abdomen and can be referred to the back and legs.
Pain may arise from the lesions themselves. Endometriosis lesions are vascular and innervated by sensory and autonomic fibers, providing afferent access to peripheral and central pain and sensory pathways. However, the lesions cannot be the sole cause of pain. The intensity and duration of pain do not correlate with lesion number, location, or severity of endometriosis.
An abundance of proinflammatory cytokines and chemokines, angiogenic and nerve growth factors, neutrophils and pain mediators such as prostaglandin E2, tumor necrosis factor α (TNF-α), RANTES, interleukin-8 (IL-8), IL-1beta, calcitonin gene-related peptide (CGRP), substance P, and reactive oxygen species are present in endometriosis lesions and the surrounding peritoneal fluid, stimulated by the estrogen-dependent environment and resulting in a chronic local inflammation. These factors may contribute to lesion and neuronal growth, which may each serve to initiate and augment pain.4
Endo-CPP is thus multi-factorial and has nociceptive, neuropathic, and nociplastic features. Nociceptive pain arises from activation of nociceptors by actual or threatened damage to non-neural tissue from trauma, injury, or inflammatory processes. In endometriosis, nociceptive visceral pain may arise from endometriosis lesions and its associated inflammation and release of pain mediators. Neuropathic pain is pain caused by damage to the nerves or neural tissue or lesions directly affecting the somatosensory nervous system. Endometriosis may engender neuropathic pain elements through its own innervation when lesions impinge on pelvic nerves and via activation from the release of pain mediators.
Nociplastic pain is defined as pain arising from altered nociception without clear evidence of actual or threatened tissue damage, activation of peripheral nociceptors, or evidence for damage to neuronal somatosensory pathways or structures.5 Nociplastic pain appears to arise from peripheral, central, or cross-organ sensitization. Chronic pain persisting despite treatment of endometriosis is best classified as nociplastic.
Pain sensitization is defined by the International Association for the Study of Pain (IASP) as “increased responsiveness of nociceptive neurons to their normal input and/or recruitment of a response to normally subthreshold inputs.”5 This increased responsiveness is indicated by a decrease in pain threshold, an increase in response to stimuli above threshold, spontaneous neuronal discharges, and an increase in sensory receptive field size. In clinical research and in practice, sensitization is inferred from hyperalgesia (abnormally heightened sensitivity to noxious stimuli) and/or allodynia (pain in response to normally non-noxious stimuli).
The IASP further defines “central sensitization” as increased responsiveness of nociceptive neurons in the CNS to their normal or subthreshold afferent input. Central sensitization arises from hyper-function of endogenous pain control systems and can be inferred from pain and increased sensitivity in territories remote from a site of injury.
In cross-organ sensitization, pain in one visceral organ increases sensitivity to pain in another organ. It likely arises from convergence of innervation from multiple pelvic organs in the dorsal root ganglion and on the same spinal cord segments. Cross-organ sensitization has been demonstrated in animal models where acute cystitis lowers the colorectal pain threshold to balloon distension and, conversely, acute colitis causes irritative micturition patterns in an otherwise healthy bladder.6 Dyschezia and dysuria, when comorbid with endometriosis, may represent such cross-organ sensitization or may arise from deep endometriosis lesions within the respective viscera.
Sensitization is present in women with CPP, including endo-CPP. Phan et al. found myofascial dysfunction characterized by lowered pain-pressure thresholds, myofascial trigger points, and allodynia not only in the pelvis, but also paraspinally at thoracic levels in women with endo-CPP.7 As-Sanie et al. found a lowered pain threshold on the thumb nail bed in women with endometriosis compared with controls without endometriosis. CNS changes have been identified on brain voxel-based morphometry and fMRIs in women with endo-CPP including a decrease in gray matter volume and abnormal connectivity in pain-related structures and pathways.8,9
Peripheral and cross-organ sensitization may account for the frequent co-occurrence of comorbid pelvic pain conditions like irritable bowel syndrome, interstitial cystitis/painful bladder syndrome, and CPP in those with endometriosis. Central sensitization can also account for the association between CPP, including endo-CPP, and other non-pelvic pain syndromes, such as migraine and fibromyalgia, and for the persistence of pain after treatment in women with endometriosis.10,11
As a nociplastic pain condition, it may be that the initial pain due to endometriosis lesions leads to sensitization that then contributes to pain persistence in the pelvis and beyond. Further research is also needed into understanding whether and how dysmenor-rhea, common and often severe in those with endometriosis, as a pain condition in and of itself may contribute to sensitization and serve as a risk factor for pain chronification.12
Endo-CPP comorbidities extend beyond local and remote pain conditions. Along with other chronic pain conditions more common in women including fibromyalgia, migraine, irritable bowel syndrome, and painful bladder syndrome/interstitial cystitis, endo-CPP is frequently comorbid with non-pain conditions including depression, fatigue, sleep disturbances, and anxiety.
Management
The management of endometriosis initially falls to gynecologists, who provide evaluation and diagnosis resulting in surgery and medical hormonal management as needed. If pelvic pain persists in women with endometriosis, the clinician focus broadens hormonal therapy to accomplish menses suppression or initiates a hormonal therapy found to be effective in lessening endometriosis-associated pain (like gonadotropic-releasing agonists or antagonists rather than undertaking multiple surgeries) and engages a multidisciplinary team for pain management. Additional pain management approaches may include medications such as acetaminophen, muscle relaxants, non-steroidal anti-inflammatory drugs (NSAIDS), and medications for neuropathic pain including gabapentin, pregabalin, and duloxetine. The efficacy of cannabis, cannabis components cannabidiol (CBD) and tetrahydrocannabinol (THC), and smartphone self-help apps in endo-CPP remains uncertain. Pelvic floor physical therapy may be helpful, especially in those with pelvic floor muscle spasms or myofascial trigger points. An emerging treatment for endo-CPP is botulinum toxin injected into muscles of the pelvic floor.13,14 Intramuscular botulinum toxin injections may relieve pain by decreasing spasm in pelvic muscles and also by direct effects on the release of molecular pain mediators.14
Future directions
Research into endometriosis and the care of those with endometriosis should continue to investigate the disease pathophysiology, including the role of genetic risk, retrograde menstruation, the peri-lesional and peritoneal milieu, and factors associated with lesion implantation and growth so that symptoms can be identified and treated early and preventative measures can be developed. Fortunately, attention is moving beyond a focus solely on the lesions. Within the pelvis, better characterization of pelvic organ, overlapping pain phenotypes, and understanding of cross-organ sensitization may identify approaches that concurrently minimize bowel and bladder symptomatology.
Approaches solely focused on pelvic pain without considering the person as a whole are likely to be inadequate in those with further comorbid pain conditions and other symptomatology. The recent recognition that endo-CPP may portend central sensitization and neuroplastic pain and thus result in additional comorbid pain both inside and beyond the pelvis suggests that these coincident, co-occurring sites of pain merit assessment and treatment. Without a multidisciplinary team approach, healthcare can become fractionated, leaving patients with the need to navigate between primary care providers such as internists, family medicine practitioners, gynecologists, and specialists including other surgeons, urologists, gastroenterologists, psychiatrists, psychologists, and pain specialists. Optimally, the management of endo-CPP should encompass a comprehensive multi-modal approach. In research, more detailed characterization of the phenotypes of common chronic overlapping comorbid pain and non-pain conditions and of peripheral, central, and cross-organ sensitization will ultimately allow development of better approaches and precision medicine with effective treatments specific to the needs of individuals with endometriosis and endo-CPP.
ACKNOWLEDGMENTS
This work is funded by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institute of Health, United States.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Missmer SA, Tu FF, Agarwal SK, Chapron C, Soliman AM, Chiuve S, Eichner S, Flores-Caldera I, Horne AW, Kimball AB, et al. (2021). Impact of endometriosis on life-course Potential: a Narrative review. Int. J. Gen. Med 14, 9–25. 10.2147/IJGM.S261139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horne AW, and Missmer SA (2022). Pathophysiology, diagnosis, and management of endometriosis. BMJ 379, e070750. 10.1136/bmj-2022-070750. [DOI] [PubMed] [Google Scholar]
- 3.Engeler D, Baranowsky AP, Berghmans B, et al. EAU Guidelines on Chronic Pelvic Pain. Retrieved from https://uroweb.org/guidelines/chronic-pelvic-pain. (Accessed Feb 6, 2023).
- 4.McNamara HC, Frawley HC, Donoghue JF, Readman E, Healey M, Ellett L, Reddington C, Hicks LJ, Harlow K, Rogers PAW, and Cheng C (2021). Peripheral, central, and cross sensitization in endometriosis-associated pain and comorbid pain syndromes. Front Reprod Health 3, 729642. 10.3389/frph.2021.729642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Association for the Study of Pain (IASP). IASP Terminology. Retrieved from https://www.iasp-pain.org/resources/terminology/. (Accessed Feb 6, 2023).
- 6.Monten L, Forman A, and Andersson KE (2018). Pelvic organ cross-talk: a new paradigm for endometriosis-related pelvic pain? J. Endometr. Pelvic Pain Disord 10, 208–215. 10.1177/2284026518810573. [DOI] [Google Scholar]
- 7.Phan VT, Stratton P, Tandon HK, Sinaii N, Aredo JV, Karp BI, Merideth MA, and Shah JP (2021). Widespread myofascial dysfunction and sensitisation in women with endometriosis-associated chronic pelvic pain: a cross-sectional study. Eur. J. Pain 25, 831–840. 10.1002/ejp.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, and Schmidt-Wilcke T (2012). Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain 153, 1006–1014. 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V, and Harris RE (2016). Functional connectivity is associated with altered brain Chemistry in women with endometriosis-associated chronic pelvic pain. J. Pain 17, 1–13. 10.1016/j.jpain.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddern J, Grundy L, Castro J, and Brierley SM (2020). Pain in endometriosis. Front. Cell. Neurosci 14, 590823. 10.3389/fncel.2020.590823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng P, Zhang W, Leng J, and Lang J (2019). Research on central sensitization of endometriosis-associated pain: a systematic review of the literature. J. Pain Res 12, 1447–1456. 10.2147/JPR.S197667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R, Li B, Kreher DA, Benjamin AR, Gubbels A, and Smith SM (2020). Association between dysmenorrhea and chronic pain: a systematic review and meta-analysis of population-based studies. Am. J. Obstet. Gynecol 223, 350–371. 10.1016/j.ajog.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Karp BI, Tandon H, Vigil D, and Stratton P (2019). Methodological approaches to botulinum toxin for the treatment of chronic pelvic pain, vaginismus, and vulvar pain disorders. Int. Urogynecol. J 30, 1071–1081. 10.1007/s00192-018-3831-z. [DOI] [PubMed] [Google Scholar]
- 14.Tandon HK, Stratton P, Sinaii N, Shah J, and Karp BI (2019). Botulinum toxin for chronic pelvic pain in women with endometriosis: a cohort study of a pain-focused treatment. Reg. Anesth. Pain Med 10.1136/rapm-2019-100529. [DOI] [PMC free article] [PubMed] [Google Scholar]


