Abstract
BACKGROUND
Tonic and atonic “drop attack” seizures are a classic and morbid semiology in Lennox-Gastaut syndrome, resulting in frequent injuries and emergency room visits, in addition to neurocognitive sequelae. Recent years have seen a growing interest in less invasive techniques for performing the classic surgical treatment for drop attacks in Lennox-Gastaut syndrome, that is, corpus callosotomy.
OBSERVATIONS
A 5-year-old boy with Lennox-Gastaut syndrome presented for surgical evaluation. He experienced up to 20 daily tonic seizures despite multiple antiseizure medications. Preoperative imaging revealed highly abnormal anatomy with severe ventriculomegaly and thinning of the cortex and corpus callosum. Open microsurgery or an interhemispheric bimanual endoscopic approach to corpus callosotomy posed a risk for ventricular collapse and subdural hematoma, and the corpus callosum was too thin for laser ablation. A fully endoscopic transventricular “inside-out” complete corpus callosotomy was performed through a 7-mm burr hole via a single working channel without intraoperative complications. The patient continues to experience daily seizures but with a reduced frequency and intensity and a family-reported increased quality of life.
LESSONS
In cases of drug-resistant tonic and atonic seizures associated with ventriculomegaly, a fully endoscopic transventricular complete corpus callosotomy can be performed safely, potentially limiting the risk of ventricular collapse and subdural bleeding.
Keywords: epilepsy, corpus callosotomy, endoscopic, minimally invasive, Lennox-Gastaut syndrome
ABBREVIATIONS: CC = corpus callosotomy, CCC = complete CC, ETV = endoscopic third ventriculostomy, LITT = laser interstitial thermal therapy.
Corpus callosotomy (CC) represents a powerful palliative neurosurgical intervention for certain generalized and multifocal epilepsies.1 Most commonly, this surgery is used for the treatment of tonic or atonic “drop attack” seizures, and while generally considered a palliative intervention, CC is sometimes curative for this seizure semiology. Furthermore, this operation can be beneficial for the treatment of other seizure types and achieves comparable seizure control in pediatric and adult patients. In select cases, CC can serve as part of a multistage surgery plan: generalized seizures may show lateralization following callosal disconnection, thereby allowing for resection of a definitive seizure focus.2, 3
The goal of CC is to divide the fibers of the corpus callosum to prevent seizure propagation between hemispheres. While this is an old concept in neurosurgery, dating back to 1940, there has been a proliferation of surgical techniques by which this goal is achieved. In addition to open surgery, CC is now frequently performed in a minimally invasive fashion. Endoscopic CC generally refers to an open bimanual operation, wherein the endoscope is used in place of the surgical microscope, allowing a smaller incision and craniotomy to be used.4 Both open microsurgical and bimanual endoscopic CC are typically performed using an intrahemispheric approach, following the falx cerebri down to reach the commissural fibers.4 Minimally invasive CC can also be achieved via laser interstitial thermal therapy (LITT), wherein 2–3 fiberoptic laser catheters are passed along the length of the corpus callosum as the commissural fibers are thermally ablated.5
The corpus callosum is sometimes divided using a transventricular “inside-out” approach in the context of a lateral approach hemispherotomy.6 In this methodology, the corpus callosum is identified in the walls of the lateral ventricle, and dissection proceeds from the ependyma out to the pia mater of the interhemispheric fissure.
Despite this proliferation of techniques, patients with epilepsy syndromes sometimes have highly abnormal anatomy that precludes the use of some approaches. Here, we present the case of a child with Lennox-Gastaut syndrome, refractory tonic seizures, and abnormal anatomy, including marked ventriculomegaly and thinning of the cortex and corpus callosum, who was treated with a minimally invasive complete CC performed in a fully endoscopic, inside-out fashion via a single endoscopic working channel through a 7-mm burr hole.
Illustrative Case
A 5-year-old boy with a history of Lennox-Gastaut syndrome and ventriculomegaly presented for epilepsy surgery evaluation. He had initially developed seizures at 8 months of age and had been managed with multiple antiseizure medications. In the months preceding the presentation, his seizures had become more frequent, with as many as 20 daily tonic seizures. A primary evaluation noted generalized spike and slow wave activity followed by generalized paroxysmal fast activity associated with abrupt bilateral upper-extremity tonic extension, eyes rolling up, and behavioral arrest. Therefore, he was deemed a candidate for CC and/or vagus nerve stimulation. Given his frequent, debilitating tonic seizures and diagnosis of Lennox-Gastaut syndrome, a consensus was reached at an interdisciplinary epilepsy surgery conference that complete CC (CCC) offered the best chance of reducing the seizure burden and improving his quality of life.
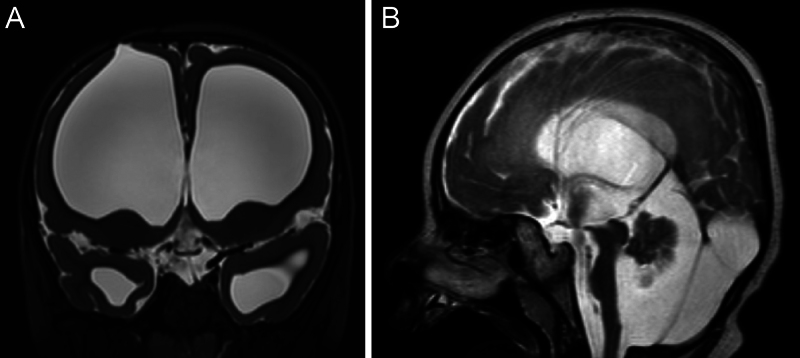
However, the patient’s anatomy presented obstacles to conventional CCC approaches: he had had severe ventriculomegaly in infancy, which was treated with a right-sided endoscopic third ventriculostomy (ETV) in the first year of life at another institution. The ETV had remained patent, and no shunt had been placed. His ventriculomegaly was associated with marked thinning of both the cortex and corpus callosum, all of which had remained stable (Fig. 1). An LITT-based callosotomy was ruled out because of the very thin corpus callosum, which would not accommodate a laser catheter. Interhemispheric techniques, either open microsurgical or bimanual endoscopic CCC, were considered, but because of his anatomy, such approaches posed a risk of ventricular collapse and subsequent subdural hemorrhage.
FIG. 1.
Preoperative coronal (A) and sagittal (B) T2-weighted magnetic resonance imaging of the brain. Coronal imaging demonstrates the bony and cortical entry point of the old ETV. Note the pronounced ventriculomegaly and thin cortex. The corpus callosum is so thin that it is not readily appreciable in the sagittal plane.
Therefore, a novel surgical procedure was devised, one relying on a fully endoscopic transventricular CCC conducted via a single working channel under continuous irrigation. Given the patient’s prior ETV, this operation could exploit the corridor created during the prior operation to minimize anatomical disruption. Furthermore, no arachnoid would be disrupted, preventing ventricular collapse during and after the surgery. Continuous irrigation would also assist in supporting the ventricular walls. Additional benefits of this approach include neither sacrificing nor stretching the bridging veins overlying the interhemispheric fissure, as well as using a small old incision, with no further scarring.
The procedure took place with the patient under general endotracheal anesthesia in a supine position with electromagnetic pinless navigation. Navigation was not relied on, as the procedure progressed based on patient-specific anatomical landmarks.
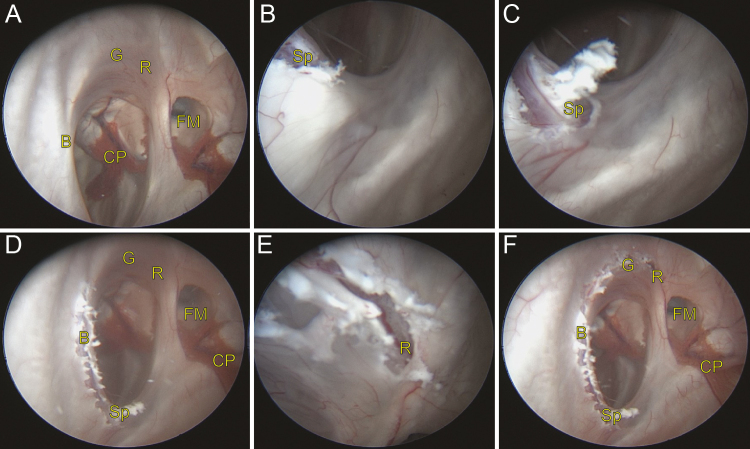
His old curvilinear incision in the right frontal area was opened, and the previous 7-mm burr hole was exposed (Fig. 2). A small rigid Lotta endoscope was inserted into the ventricle under continuous irrigation, and anatomical landmarks were identified, including the falcotentorial junction with overlying splenium, the falx with overlying thin corpus callosum, and the foramina of Monro. Meticulous hemostasis was maintained throughout the procedure. The callosotomy was performed in an inside-out fashion, as in a lateral approach hemispherotomy, working posterior to anterior (Fig. 3, Video 1). The splenium was divided with an endoscopic bipolar device, starting in the wall of the lateral ventricle and proceeding out to the pia along the falcotentorial junction. From this point, a view of the white matter–pia interface became the guide for complete dissection of the commissural fibers. Using the same technique, the body, genu, and rostrum of the corpus callosum were each sequentially divided. The end of the callosum could be appreciated when there was no longer white matter in the midline, and only pia and arachnoid. There was no appreciable bleeding during the procedure, and the endoscope was removed once the completeness of the callosotomy and hemostasis was confirmed.
FIG. 2.
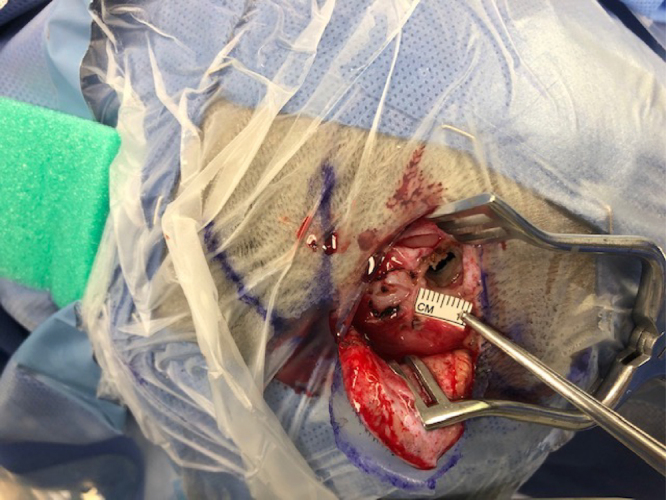
Intraoperative photograph of the 7-mm burr hole used to access the ventricle. This burr hole represents the entire surgical exposure for the case.
FIG. 3.
Serial intraoperative endoscopic photographs of the CC: identification of anatomical landmarks (A), disconnection of the splenium at the falcotentorial junction (BandC), disconnection of the posterior half of the body (D), disconnection of the genu and rostrum (E), and complete disconnection of the corpus callosum (F). B = body; CP = choroid plexus; FM = foramen of Monro; G = genu; R = rostrum; Sp = splenium.
VIDEO 1. Clip showing identification of the splenium and the start of the dissection of the splenium using an endoscopic bipolar device. An inside-out technique was used, starting at the splenium and working anteriorly. Click here to view.
In the immediate postoperative period, the patient’s home antiseizure medications were continued, and he was given a small dose of steroids along with standard perioperative prophylactic antibiotics. On his first postoperative day, he experienced transient status epilepticus requiring multiple antiseizure medication infusions. Continuous electroencephalographic monitoring revealed multifocal nongeneralized seizures. A lumbar puncture was performed, which revealed no evidence of infection or aseptic meningitis. He was successfully weaned from intravenous antiseizure medications by postoperative day 3. He was noted to be seizure free for the subsequent 4 days and was discharged to his home nursing facility.
He developed a pseudomeningocele after surgery that slowly increased in size over time, ultimately resulting in shunt placement at 4 months postoperatively. He was seizure free for 1 month following surgery, at which point seizures recurred. For the past 2.5 years of follow-up, he has been experiencing between 1 and 10 seizures per day, which are typically shorter in duration than his preoperative seizures, and he has shown improvements in cognition, mood, and head and hand control.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
This patient’s anatomy was not well suited for any of the established CC approaches. This case emphasizes the possibility of adapting operative approaches to patient-specific anatomy to achieve surgical goals through novel, minimally invasive means.
This report describes a CC performed in an entirely endoscopic fashion through a single working channel through the ventricle. This strategy allowed the complete length of the corpus callosum to be transected via a 7-mm burr hole. This minimally invasive approach could be achieved without brain retraction, without stretch or sacrifice of bridging veins, and with no more tissue disruption than an ETV or a shunt placement.
Patients without large ventricles are at risk for some degree of cerebral injury from endoscope movements, regardless of the angle of approach. Also, there is no concern for ventricular collapse in patients with normal-sized ventricles, so a transcortical endoscopic approach may not be justified in that population. However, in the setting of severe or potentially only moderate ventriculomegaly, this approach has unique advantages: the dilated ventricles create a natural surgical corridor with ample working space to ensure the transection of all callosal fibers. Furthermore, this strategy minimizes the risk of ventricular collapse by providing positive intraventricular pressure via continuous irrigation, avoids disrupting the arachnoid adhesions between the brain and dura, and requires no sacrifice or stretching of bridging veins.
With regard to this patient’s shunt placement, it is unclear whether he truly had hydrocephalus prior to undergoing CCC or if he developed hydrocephalus as a consequence of surgery. Hydrocephalus remains a risk for any surgery with entry into the ventricles, which can occur during interhemispheric approaches if the ventricular ependyma is violated. In this procedure, the ventricles are necessarily entered, which can increase the risk of subsequent hydrocephalus. Therefore, it is of paramount importance in this operation to limit bleeding into the ventricles, such as by maintaining meticulous hemostasis and using continuous irrigation.
Lessons
While novel methodologies have been developed by which a CC can be achieved, patients with seizures can present with complex surgical anatomy unsuited to these approaches, thereby warranting even newer surgical techniques. This case demonstrates the potential to tailor a novel, minimally invasive, transventricular, fully endoscopic approach through a 7-mm burr hole to achieve a complete callosotomy in the setting of large ventricles.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Atallah, Kennedy. Acquisition of data: Atallah, Galligan, Kennedy. Analysis and interpretation of data: Atallah, Galligan, Kennedy. Drafting the article: Baumgartner, Kennedy. Critically revising the article: all authors. Reviewed submitted version of manuscript: Baumgartner, Galligan, McDonnell, Kennedy. Approved the final version of the manuscript on behalf of all authors: Baumgartner. Administrative/technical/material support: Galligan, Kennedy. Study supervision: Kennedy.
Supplemental Information
Videos
Video 1. https://vimeo.com/960372325.
Correspondence
Michael E. Baumgartner: Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA. michael.baumgartner@pennmedicine.upenn.edu.
References
- 1.Wong TT, Kwan SY, Chang KP, et al. Corpus callosotomy in children. Childs Nerv Syst. 2006;22(8):999-1011. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner JE, Ajmal FQ, Baumgartner ME, et al. Palliation for catastrophic nonlocalizing epilepsy: a retrospective case series of complete corpus callosotomy at a single institution. J Neurosurg Pediatr. 2023;32(5):553-561. [DOI] [PubMed] [Google Scholar]
- 3.Chen PC, Baumgartner J, Seo JH, Korostenskaja M, Lee KH. Bilateral intracranial EEG with corpus callosotomy may uncover seizure focus in nonlocalizing focal epilepsy. Seizure. 2015;24:63-69. [DOI] [PubMed] [Google Scholar]
- 4.Smyth MD, Vellimana AK, Asano E, Sood S. Corpus callosotomy–open and endoscopic surgical techniques. Epilepsia. 2017;58(suppl 1):73-79. [DOI] [PubMed] [Google Scholar]
- 5.Badger CA, Lopez AJ, Heuer G, Kennedy BC. Systematic review of corpus callosotomy utilizing MRI guided laser interstitial thermal therapy. J Clin Neurosci. 2020;76:67-73. [DOI] [PubMed] [Google Scholar]
- 6.Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49(4):891-901. [DOI] [PubMed] [Google Scholar]