Abstract
BACKGROUND
A syringosubarachnoid (SS) shunt combined with keyhole hemilaminectomy is a beneficial procedure that can reduce the size of the skin incision and the risk of complications. However, ingenuity is needed to confirm the position of the syrinx during surgery. The authors present a case in which they treated syringomyelia in the upper thoracic spine using augmented reality (AR) to confirm syrinx formation, bone resection, and skin incision.
OBSERVATIONS
Microscope-based AR was an appropriate and practical choice in this case. By placing the reference array at the Mayfield clamp, it was possible to use AR from the point of skin incision. Under AR navigation, an SS shunt tube can be placed in the short syrinx.
LESSONS
AR navigation enables pinpoint SS shunt tube insertion with minimal skin incision and bone resection. It is particularly useful for upper thoracic and small syrinx lesions.
Keywords: syringomyelia, augmented reality, surgical navigation systems
ABBREVIATIONS: 3D = three-dimensional, AR = augmented reality, CBCT = cone beam CT, CT = computed tomography, DREZ = dorsal root entry zone, MRI = magnetic resonance imaging, NRS = numeric rating scale, SS = syringosubarachnoid.
Syringomyelia is a chronically progressive illness characterized by the presence of a syrinx in the spinal cord.1 It can occur due to several etiologies, including Chiari malformation, spinal cord trauma, adhesive arachnoiditis, and spina bifida, and it can also be idiopathic. Shunting of the syrinx is widely used for treating syringomyelia of various etiologies.2 Different surgical methods performed to place a shunt tube in the syrinx include a syringosubarachnoid (SS) shunting, syringoperitoneal shunting, and syringopleural shunting; however, SS shunting is the most commonly performed procedure.3
Laminectomy and dural incision are necessary to expose the spinal cord while placing the SS shunt. Multilevel hemilaminectomy or total laminectomy can enable wide exposure of the spinal cord; however, each is frequently associated with several complications such as extensive epidural fibrosis, spinal instability and deformity, nerve root compression, and infection.4 Few previous studies have reported SS shunt placement with keyhole hemilaminectomy and dorsal root entry zone (DREZ) myelotomy as a method that could reduce the size of the incision required and the risk of complications.2, 4
However, during the surgery, confirming the location of the syrinx when placing the SS shunt tube is very important. Ultrasonography effectively enables confirmation of the position of the syrinx in real time during surgery.5, 6 However, ultrasonography is associated with the disadvantage that it cannot be used from the bone surface and requires a bone window into which the probe can be inserted. Therefore, fluoroscopy, which requires an enlarged bone window, is used to check the bone resection range. However, confirmation using fluoroscopy with a lateral view is difficult in the upper thoracic spine.
We herein report our experience of treating syringomyelia in the upper thoracic spine while using augmented reality (AR) to confirm syrinx formation, bone resection, and skin incision.
Illustrative Case
History, Physical Examination, and Preoperative Imaging Findings
A 72-year-old woman had a past medical history of diabetes and an allergy to iodinated contrast agents. She visited her previous doctor with the complaint of pain in the right side of her chest for 6 months. She was started on medication for suspected herpes zoster, and follow-up was performed. She was prescribed mirogabalin 20 mg a day for pain control, but her symptoms failed to improve. Because of the persisting symptoms, magnetic resonance imaging (MRI) was performed, which revealed a cystic lesion in the spinal cord at the upper thoracic spine. She was then referred to our hospital for further investigations and treatment.
On examination, a tingling pain equivalent to a numeric rating scale (NRS) score of 4 was noted from the right armpit to the anterior chest. The patient experienced no motor weakness or bladder or bowel disturbance. Deep tendon reflexes in the extremities were normal, and no pathologic reflexes were observed.
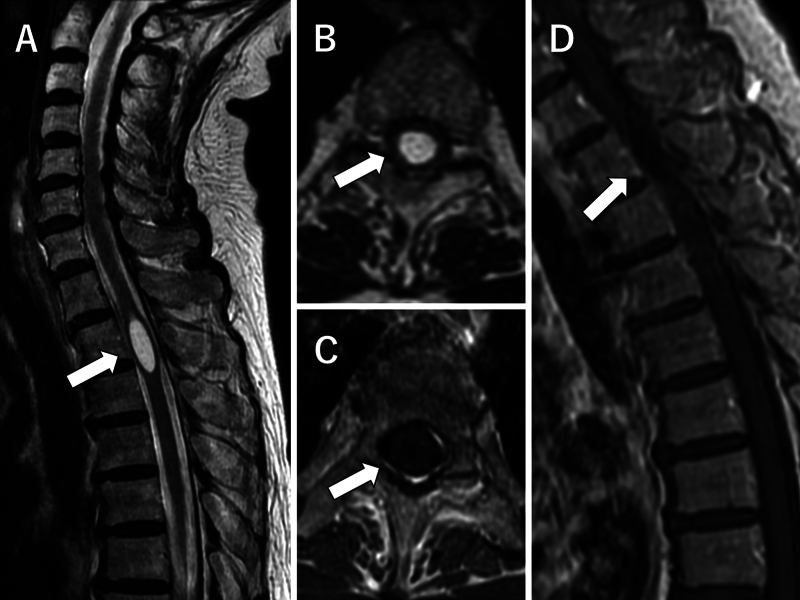
MRI revealed an intramedullary cystic lesion with a major diameter of 25 mm at the level of the T2 vertebral body. The lesion was observed as low intensity on the T1-weighted image and high intensity on the T2-weighted image (Fig. 1A and B). Gadolinium contrast was not used for any portion (Fig. 1C and D). No arachnoid adhesion was observed around the lesion. MRI excluded the presence of Chiari malformation and tethered cord syndrome. Given the above findings, the patient was diagnosed with idiopathic syringomyelia.
FIG. 1.
Preoperative sagittal T2-weighted MR image reveals a high-intensity cystic lesion (arrow) with a major diameter of 25 mm at the level of the T2 vertebral body (A). Axial T2-weighted MR image reveals an intramedullary lesion (arrow) and mild spinal cord swelling (B). Axial (C) and sagittal (D) contrast-enhanced T1-weighted images reveal a low-intensity lesion (arrows) resembling cerebrospinal fluid. No contrast effect was observed.
Operation
As sensory impairment gradually progressed with conservative therapy alone, it was decided to perform surgical treatment. Although syringoperitoneal and syringopleural shunting were feasible, we opted for the SS shunting due to because the syrinx was localized and had no adhesion to the surrounding arachnoid mater. This procedure involves positioning the tube exclusively within the dura mater.
The surgery was performed in a hybrid operating room equipped with an Artis Zeego robotic C-arm (Siemens and a Curve navigation system (Brainlab). The patient was positioned prone on a Maquet Magnus operating table (Getinge). Her head was fixed using a Mayfield skull clamp. The reference array for the patient was also affixed to a Mayfield clamp. Cone beam computed tomography (CBCT; Artis Zeego) was used to obtain three-dimensional (3D) computed tomography (CT) images, which were then transferred to the Curve navigation system. Following the immediate execution of the automatic registration, CT data were integrated with preoperatively registered 3D simulation data. Before initiating surgery, a pointer was used to confirm accuracy. A Kinevo 900 microscope (Carl Zeiss) was used, with the reference array for the microscope recognized by the Curve navigation system to confirm AR navigation (Fig. 2).
FIG. 2.
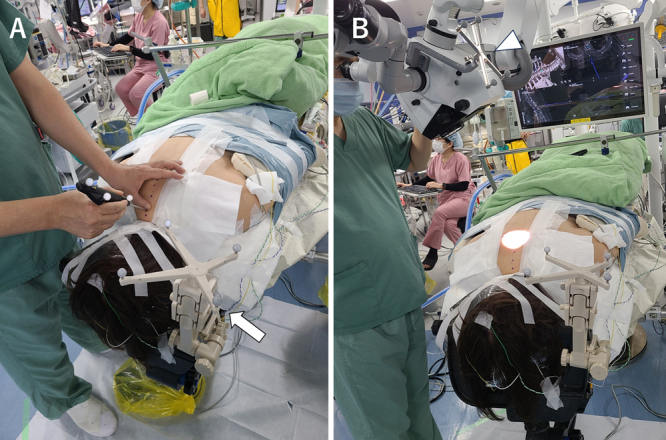
Photographs of the surgical setting. A: Placement of the reference array for the patient (arrow) at the Mayfield clamp. The surgeon uses a pointer to confirm the accuracy of navigation. B: The reference array for the microscope (arrowhead) is placed at the microscope. The surgeon confirms the AR navigation.
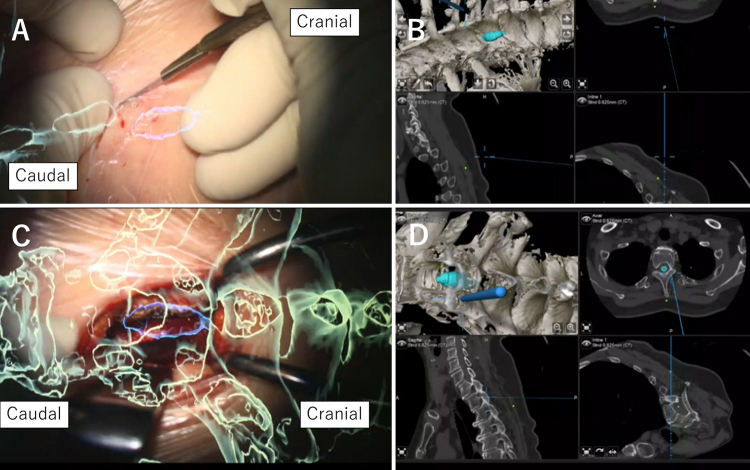
Referring to the navigation, a slightly curved skin incision was made from the midline to the right caudal side (Fig. 3A and B). The paravertebral muscles on the right side were dissected to expose the caudal and cranial sides of the T2 and T3 laminae. Keyhole hemilaminectomy was performed between the right laminae at T2–3, and the dura was exposed, while appropriately confirming the shunt insertion site of the syrinx using AR navigation (Fig. 3C and D).
FIG. 3.
Intraoperative microscopic views with AR objects and navigation maps during the approach. The green object represents the bone, and the blue object represents the syrinx. A: Skin incision. B: Navigation map during skin incision. C: Exposure of the lamina. D: Navigation map during exposure of the lamina.
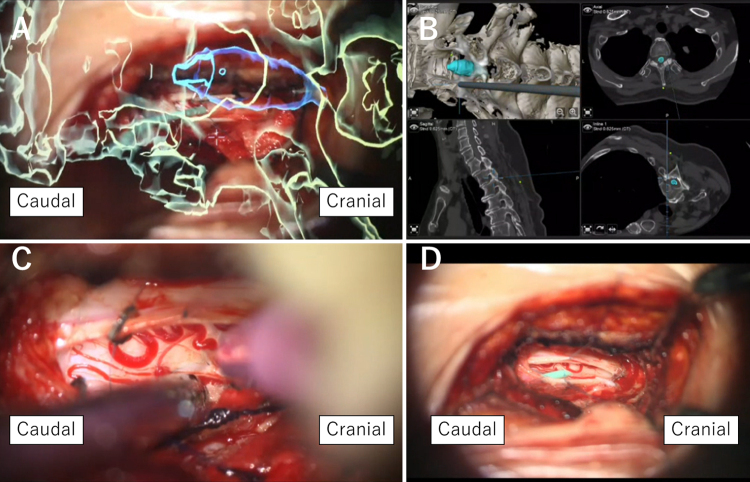
The SS shunt tube (Create Medic), with a diameter of 1 mm and a length of 40 mm, was used in this patient. Side holes on the proximal side existed up to 20 mm from the tip of the SS shunt tube. Therefore, it was necessary to insert the tube from the bottom of the syrinx to fit all the side holes into the 25-mm syrinx. DREZ myelotomy was performed at the level of the bottom of the syrinx using AR navigation. A 2-mm incision in the pia mater was made with a micro-knife at the DREZ. Utilizing a micro-forceps and guided by AR navigation, we delved deep into the spinal cord to access the syrinx. The tube was inserted into the syrinx (Fig. 4). The SS shunt tube was sutured to the pia. Myelotomy, tube insertion, and suturing of the tube to the pia mater were meticulously performed under microscopy. Because of the limited size of the keyhole laminectomy bone window, an AnastoClip AC (LeMaitre) was utilized to close the dura, which was subsequently covered with fibrin glue and a collagen-based dural graft.
FIG. 4.
Intraoperative microscopic views with AR objects and navigation map during tube insertion. A: The surgeon applies the tube to confirm the location of the myelotomy. The green object represents the bone, and the blue object represents the syrinx. B: Navigation map during confirmation of the location of the myelotomy. C: DREZ myelotomy. D: The tube is placed into the syrinx.
Postoperative Course
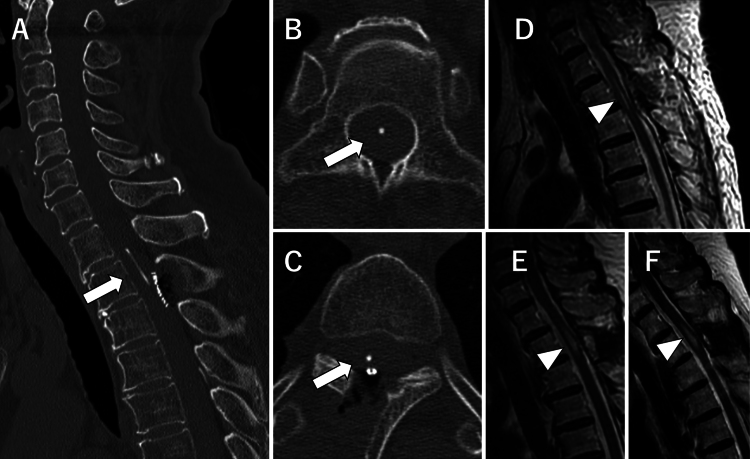
Postoperative CT revealed that the SS shunt tube was inserted into the central canal of the spinal cord at the T2 level (Fig. 5A–C). There was no new neurological deficit. The patient’s pain improved from an NRS score of 4 to 1, and oral medication for pain relief was no longer required. MRI performed 4 days following surgery indicated that the syrinx had shrunk, and follow-up MRI performed 3 months after surgery showed no recurrence of syringomyelia (Fig. 5D–F).
FIG. 5.
Postoperative CT and MR images. CT studies from the day after surgery (A–C). Sagittal view demonstrates that the tube (arrow, A) fits perfectly into the syrinx. Axial views demonstrate that the tip of the tube (arrow, B) is located in the central canal, and the tube (arrow, C) is inserted into the spinal cord from the right DREZ. Sagittal T2-weighted MR image obtained 4 days after surgery, indicating a shrunken syrinx (arrowhead, D). Sagittal T2-weighted MR image obtained 3 months after surgery, showing a slightly larger syrinx (arrowhead, E) than on the immediate postoperative image, but there is no obvious recurrence. Sagittal T2-weighted MR image obtained 9 months after surgery, showing that the size of the syrinx (arrowhead, F) is similar to that after 3 months.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
This case, which had an excellent outcome, demonstrated that AR is very useful in SS shunting. This is the first case report that includes the use of AR navigation for placing an SS shunt.
AR is fast gaining popularity as a valuable tool in spine surgery and7 has been applied to various types of surgeries such as pedicle screw placement, targeted cervical foraminotomy, bone biopsy, osteotomy planning, and percutaneous intervention.8 Optical see-through AR, which often requires the use of goggles, allows AR objects to be displayed alongside real structures in the field of view.9 A microscope can also serve as an optical see-through AR display, similar to goggles. Compared to goggles, a microscope has several advantages as an AR display; it does not need to be worn on the surgeon’s head, thus avoiding the weight and movement issues associated with goggles. A previously conducted study has reported the use of microscope-based AR in intradural spinal tumor surgery.10 The use of a microscope has long been recommended for SS shunt surgery, and microscope-based AR was practical in the current case.5
Conventional navigation, which displays the map on a separate monitor, can also be used instead of AR navigation. However, in conventional navigation, the surgeon needs to look away from the surgical field into a dedicated navigation monitor to verify anatomical positions.11 Optical see-through AR navigation enables the surgeon to navigate without looking at another monitor, and if AR objects obstruct the surgeon’s view, turning it off within the display is easy. Surgeries such as myelotomies involve delicate techniques; therefore, the ability to concentrate on the surgical field without looking away is advantageous for the surgeon.
By placing the reference array at the Mayfield clamp, we were able to use AR navigation from the point of skin incision. There are reports indicating that placing the reference array at a Mayfield clamp during endoscopic cervical spine surgery maintains minimal invasiveness.12, 13 However, if the lesion and reference array are at a greater distance, the positional relationship between the vertebral bodies can shift, resulting in reduced accuracy. This issue can be effectively addressed by using optical see-through AR, since deviation in navigation can be noticed due to the visibility of actual structures such as bones. Moreover, in the event of a deviation, it is possible to retake CBCT or move the reference array to the spinous process.
If the syrinx occupies an extensive area, myelotomy should simply be performed at the area where the syrinx is large.6 However, for syringes occupying a limited area, as in the present case, care must be taken regarding the point of myelotomy. A previous report indicated that inserting the shunt 20–30 mm into the spinal cord ensures that all side holes in the tube lie within the syrinx, thereby helping to prevent dislodgement.14 Therefore, the position of the myelotomy must be such that the SS shunt tube fits within the syrinx. Although the syrinx is not visible from the spinal cord surface, AR navigation allows for pinpoint tube insertion.
The SS shunt can be inserted via either DREZ myelotomy or midline myelotomy. We opted for the DREZ myelotomy due to the feasibility of shunt insertion solely through a hemilaminectomy while conserving the spinous process. Given that the sole presenting symptom in our patient was pain, the objective was to conserve the posterior element to the greatest extent possible and mitigate postoperative pain. The keyhole hemilaminectomy conserves the nuchal and interspinous ligaments, paraspinous muscles, and bilateral facet joints, thereby diminishing the risk of spinal instability.2, 4 The disadvantage of DREZ myelotomy with keyhole hemilaminectomy is that the bone window is small, making it difficult to confirm with ultrasonography. AR navigation is considered effective in compensating for this disadvantage.
Lessons
AR navigation enables pinpointing SS shunt tube insertion with minimal skin incision and bone resection. It is particularly useful for upper thoracic and small syrinx lesions.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Matsuoka, Shin, Shiraishi, Hayami, Wada. Acquisition of data: Matsuoka, Shin, Fukumori, Shiraishi, Aketa, Wada. Analysis and interpretation of data: Matsuoka, Shiraishi, Wada. Drafting the article: Matsuoka, Shiraishi, Wada. Critically revising the article: Matsuoka, Shin, Shiraishi, Wada. Reviewed submitted version of manuscript: Shiraishi, Wada. Approved the final version of the manuscript on behalf of all authors: Matsuoka. Statistical analysis: Shiraishi. Administrative/technical/material support: Mitsui, Shiraishi. Study supervision: Shin, Shiraishi, Fukutome, Tei, Motoyama.
Correspondence
Ryuta Matsuoka: Osaka Police Hospital, Osaka, Japan. koumei108@gmail.com.
References
- 1.Donauer E, Rascher K. Syringomyelia: a brief review of ontogenetic, experimental and clinical aspects. Neurosurg Rev. 1993;16(1):7-13. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki Y, Koyanagi I, Hida K, Abe H. Syringo-subarachnoid shunt for syringomyelia using partial hemilaminectomy. Br J Neurosurg. 1999;13(1):41-45. [DOI] [PubMed] [Google Scholar]
- 3.Rothrock RJ, Lu VM, Levi AD. Syrinx shunts for syringomyelia: a systematic review and meta-analysis of syringosubarachnoid, syringoperitoneal, and syringopleural shunting. J Neurosurg Spine. 2021;35(4):535-545. [DOI] [PubMed] [Google Scholar]
- 4.Gezen F, Kahraman S, Ziyal IM, Canakçi Z, Bakir A. Application of syringosubarachnoid shunt through key-hole laminectomy. Technical note. Neurosurg Focus. 2000;8(3):E10. [DOI] [PubMed] [Google Scholar]
- 5.Tator CH, Briceno C. Treatment of syringomyelia with a syringosubarachnoid shunt. Can J Neurol Sci. 1988;15(1):48-57. [DOI] [PubMed] [Google Scholar]
- 6.Hussain I, Greenfield JP. Ultrasound-guided syringosubarachnoid shunt insertion for cervicothoracic syringomyelia. Clin Spine Surg. 2020;33(5):185-191. [DOI] [PubMed] [Google Scholar]
- 7.Azad TD, Warman A, Tracz JA, Hughes LP, Judy BF, Witham TF. Augmented reality in spine surgery—past, present, and future. Spine J. 2024;24(1):1-13. [DOI] [PubMed] [Google Scholar]
- 8.Ghaednia H, Fourman MS, Lans A, et al. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617-1625. [DOI] [PubMed] [Google Scholar]
- 9.Gibby JT, Swenson SA, Cvetko S, Rao R, Javan R. Head-mounted display augmented reality to guide pedicle screw placement utilizing computed tomography. Int J Comput Assist Radiol Surg. 2019;14(3):525-535. [DOI] [PubMed] [Google Scholar]
- 10.Carl B, Bopp M, Saß B, Pojskic M, Nimsky C. Augmented reality in intradural spinal tumor surgery. Acta Neurochir (Wien). 2019;161(10):2181-2193. [DOI] [PubMed] [Google Scholar]
- 11.Burström G, Persson O, Edström E, Elmi-Terander A. Augmented reality navigation in spine surgery: a systematic review. Acta Neurochir (Wien). 2021;163(3):843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin Y, Sunada H, Shiraishi Y, et al. Navigation-assisted full-endoscopic spine surgery: a technical note. J Spine Surg. 2020;6(2):513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Wu J, Xu C, et al. Minimally invasive full-endoscopic posterior cervical foraminotomy assisted by O-arm-based navigation. Pain Phys. 2018;21(3):E215-E223. [PubMed] [Google Scholar]
- 14.Tator CH, Meguro K, Rowed DW. Favorable results with syringosubarachnoid shunts for treatment of syringomyelia. J Neurosurg. 1982;56(4):517-523. [DOI] [PubMed] [Google Scholar]