Abstract
Although maternal stress during pregnancy and even before conception shapes offspring risk for mental health problems, relatively little is known about the mechanisms through which these associations operate. In theory, preconception and prenatal stress may affect offspring mental health by influencing child responses to postnatal caregiving. To address this knowledge gap, this study had two aims. First, we examined associations between preconception and prenatal stress with child temperament profiles at age four using multilevel assessment of maternal perceived stress and stress physiology. Second, we tested child temperament profiles as moderators of associations between observed parenting behaviors during a parent–child free-play interaction when children were 4 years old and child behavior problems 1 year later. Latent profile analyses yielded four distinct child temperament profiles: inhibited, exuberant, regulated low reactive, and regulated high reactive. Consistent with hypotheses, preconception, and prenatal stress each independently predicted the likelihood of children having temperament profiles characterized by higher negative emotionality and lower regulation. Specifically, preconception perceived stress and prenatal cortisol predicted likelihood of children having an exuberant temperament, whereas prenatal perceived stress predicted likelihood of children having an inhibited temperament. Contrary to hypotheses, temperament profiles did not moderate predictions of child behavior problems from observed parenting behaviors; however, responsive parenting behaviors inversely predicted child behavior problems independently of child temperament. These findings add to growing evidence regarding effects of preconception factors on child outcomes and underscore a central role for responsive parenting behaviors in predicting more favorable child mental health independent of child temperament.
Keywords: preconception stress, prenatal stress, parenting, temperament, mental health
Maternal stress during pregnancy shapes offspring risk for mental health problems across development (Barker, 2007; Bateson et al., 2014; Gluckman et al., 2005; Monk et al., 2019; O’Donnell & Meaney, 2017; Sandman et al., 2012). More recently, emerging evidence suggests that maternal stress even before conception uniquely predicts offspring risk (Hipwell et al., 2019; Keenan et al., 2018; Olsson et al., 2021; Spry et al., 2020, 2022). Critically, however, putative effects of maternal stress in both of these periods are probabilistic, not deterministic. According to developmental cascades (Masten & Cicchetti, 2010), influences of preconception and prenatal stress on child outcomes are sensitive to other biological, psychological, and social factors, including aspects of the postnatal environment (Hentges et al., 2019; Huizink & De Rooij, 2018). That is, although maternal inputs prior to conception and during pregnancy may guide offspring developmental trajectories, postnatal environmental factors may alter these outcomes (Barker, 2007; Bateson et al., 2014; Gluckman et al., 2005; Sandman et al., 2012). For example, individual differences in child temperament are sensitive to preconception and prenatal maternal stress and may in turn interact with postnatal caregiving to shape adaptive or maladaptive outcomes (Hartman & Belsky, 2018; Hartman et al., 2023; Pluess & Belsky, 2011). The current study examined how preconception, prenatal, and postnatal factors independently and interactively shape developmental cascades. Specifically, this study (a) evaluated associations of multilevel measures of preconception and prenatal stress with child temperament profiles and (b) examined whether child temperament profiles in turn moderated predictions of child behavior problems from maternal parenting behaviors.
Preconception and Prenatal Developmental Origins
Decades of prenatal programming evidence suggests persuasively that fetal adaptations to prenatal stress are associated with long-term offspring health (Entringer et al., 2012; Glover et al., 2010; Lautarescu et al., 2020; Rogers et al., 2020; Van den Bergh et al., 2020). More recently, growing evidence indicates that maternal stress even before pregnancy shapes birth outcomes and offspring health (Atrash et al., 2006; Keenan et al., 2018; Lu & Halfon, 2003). From a life course perspective, maternal experiences prior to conception “set the stage” for pregnancy, potentially through processes such as uterine priming, zygote implantation, and early placental development (Chan et al., 2018; Kee et al., 2021; Keenan et al., 2018; Stephenson et al., 2018). Indeed, in recent work maternal preconception stress was associated with birth and developmental outcomes, including shorter length of gestation (Mahrer et al., 2021), lower birth weight (Guardino et al., 2016), increased behavioral reactivity in infancy (Spry et al., 2020), and attention problems in early childhood (Class et al., 2013). Despite this growing evidence, it remains unclear if putative effects of preconception stress on child outcomes operate mainly through prenatal influences (i.e., indirect effects) or if they contribute uniquely to offspring outcomes above and beyond prenatal exposures (i.e., direct effects; Bowers & Yehuda, 2016; Keenan et al., 2018; Yehuda & Lehrner, 2018).
Preconception stress may indirectly predict outcomes through prenatal factors such as maternal stress or physiological functioning during pregnancy. For example, consistent with prenatal programming, elevated stress prior to conception may continue through the prenatal period and in turn shape fetal development (Davis & Narayan, 2020; Lu & Halfon, 2003; Keenan et al., 2018). Because of their sensitivity to stress and role in fetal development, dysregulated maternal cortisol levels and diurnal patterns during pregnancy may be a central prenatal biological pathway linking preconception stress to child outcomes (Harris & Seckl, 2011; McGowan & Matthews, 2018). Maternal preconception stress is associated with differences in maternal hypothalamic-pituitary-adrenal (HPA) axis regulation during pregnancy (Epstein et al., 2021; Rinne, Hartstein, et al., 2023) and various measures of maternal prenatal cortisol predict infant and child outcomes (Bolten et al., 2011; Howland et al., 2017; Zijlmans et al., 2015). HPA axis activity is multidimensional, however, with different cortisol measurement strategies yielding separable indicators (e.g., total cortisol output, diurnal cortisol slope, and cortisol awakening response) with distinct neurobiological underpinnings (Adam & Kumari, 2009; Adam et al., 2017; Howland et al., 2017). Furthermore, different cortisol indices show meaningfully distinct patterns of associations with both maternal stress and child outcomes (Duthie & Reynolds, 2013; Entringer et al., 2010; for reviews, see Rinne, Hartstein, et al., 2023; Zijlmans et al., 2015). Thus, although use of multiple measures of maternal cortisol during pregnancy may usefully elucidate separable prenatal biological pathways linking maternal preconception stress to child outcomes, they are infrequently tested in the same study (Zijlmans et al., 2015).
Spanning human and nonhuman animal models, other empirical work has found that preconception influences are independent of prenatal factors, suggesting direct effects of preconception factors on offspring outcomes (Class et al., 2013; Guardino et al., 2022; Keenan et al., 2018; Rinne, Carroll, et al., 2023; Swales et al., 2023; Yehuda et al., 2000). For example, experimental manipulation of parental preconception stress among rodents produced altered offspring behavioral and physiological differences characteristic of stress-related disorders (e.g., epigenetic changes; altered stress coping behaviors), independent of pre- and postnatal factors (for reviews, see Chan et al., 2018; Keenan et al., 2018). In humans, even with control of prenatal factors, parental preconception stress and parental emotional distress were associated with greater negative emotionality, HPA axis dysregulation, altered neurodevelopment, and shorter telomere length during infancy and childhood (e.g., Guardino et al., 2022; Keenan et al., 2018; Rinne, Carroll, et al., 2023; Swales et al., 2023; Yehuda et al., 2000). Collectively, this multimethod evidence converges around the centrality of preconception influences on offspring outcomes that are distinct from prenatal influences, potentially operating through germline epigenetic mechanisms (Chan et al., 2018; Yehuda & Lehrner, 2018). However, further research is necessary to determine whether preconception factors independently predict child outcomes or indirectly through prenatal factors. Repeated measures designs should be prioritized given that they are particularly well-positioned to rigorously test direct effects and indirect, mediating processes.
Preconception and Prenatal Influences on Child Responses to the Postnatal Environment
Reflecting developmental cascades, preconception, and prenatal factors may affect offspring developmental outcomes by influencing offspring responses to the postnatal environment (Hartman & Belsky, 2018; Hartman et al., 2023; Pluess & Belsky, 2011). In particular, individual differences in temperament, defined as an early-emerging and biologically based set of dispositional characteristics, may constitute a pathway through which preconception and prenatal stress shape offspring responses to postnatal environmental factors (Hartman et al., 2023; Rothbart et al., 2000; Rothbart & Posner, 2006; Rothbart & Putnam, 2002). Theoretically and empirically, temperament dimensions such as emotionality, impulsivity, and regulation significantly affect child sensitivity to the caregiving environment (Belsky, 1997, 2005; Chess & Thomas, 1977; Lerner & Lerner, 1994). According to diathesis-stress models (e.g., Zuckerman, 1999), temperamental vulnerabilities such as high negative emotionality and low regulation confer risk to behavior problems, especially when accompanied by negative parenting (e.g., overprotective, intrusive; Gilliom & Shaw, 2004; Hastings et al., 2008; Leerkes et al., 2009; Maziade et al., 1985, 1990; Stoltz et al., 2017). Conversely, differential susceptibility proposes that particular child traits, including stress reactivity and negative emotionality, simultaneously confer enhanced sensitivity to both risk-promoting and development-enhancing environments (Boyce & Ellis, 2005; Ellis et al., 2011). Consistent with differential susceptibility, evidence from recent reviews indicates that children with temperaments characterized by high negative emotionality and intensity as well as low regulation and low adaptability were simultaneously more strongly influenced by both positive and negative maternal affect (Belsky, 2013; Kiff et al., 2011; Slagt et al., 2016; Stoltz et al., 2017). Taken together, there is persuasive evidence that dimensions of temperament critically affect how children respond to postnatal caregiving and subsequent developmental outcomes.
Individual differences in temperament are sensitive to preconception and prenatal factors (Blair et al., 2011; Davis et al., 2018; Spry et al., 2020; Swales et al., 2023). Although maternal preconception mental health was associated with higher infant and early childhood negative emotionality in several recent studies (Spry et al., 2020; Swales et al., 2023), studies have yet to test if maternal preconception stress relates to offspring temperament. Regarding prenatal factors, a large body of evidence demonstrates that prenatal stress is associated with lower infant and child regulatory abilities as well as higher infant and child negative emotionality (e.g., Bush et al., 2017; Huizink et al., 2002; Lin et al., 2014; Van den Bergh et al., 2020). Some studies also suggest that prenatal stress is associated with offspring extraversion and surgency; however, the direction of effects was contingent on how prenatal stress was measured (Bush et al., 2017; Lin et al., 2014). Moreover, elevated maternal plasma and salivary cortisol during pregnancy were positively associated with infant negative reactivity at 2 months among 247 full-term infants (Davis et al., 2007) and with greater observer-reported behavioral reactivity to novelty among 103 4-month-old infants (Werner et al., 2013), respectively. Cumulatively, these findings suggest that preconception and prenatal factors, including maternal stress and cortisol, prospectively predict individual differences in dimensions of offspring temperament, which may in turn influence responses to postnatal environmental influences.
Person-Centered Approaches to Temperament
Evidence on preconception and prenatal contributors to child temperament to date has largely focused on specific individual dimensions of temperament (e.g., effortful control, negative affect; Van den Bergh et al., 2020). Similarly, interaction models with parenting often focus on specific temperament dimensions, although theoretically derived behavioral profiles (e.g., “behaviorally inhibited”; “difficult temperament”) are also periodically employed and have been found to moderate predictions of behavior problems from parenting behaviors (see Slagt et al., 2016 for a review). However, these approaches have historically been agnostic about covariation among temperament dimensions and delimit understanding how temperament traits cluster together (see Scott et al., 2016; Shiner et al., 2012; and Lin et al., 2018 for further discussion). More recent person-centered approaches leverage the full spectrum of individual differences in multiple dimensions of temperament to identify latent profiles of phenotypically similar infants and children (e.g., Gartstein et al., 2017; Lin et al., 2018; Ostlund et al., 2021; Prokasky et al., 2017). Indeed, there is growing empirical evidence supporting the utility of person-centered approaches in characterizing temperament profiles across dimensions of emotionality, regulation, and impulsivity (e.g., Beekman et al., 2015; Brown et al., 2022; Gartstein et al., 2017; Lin et al., 2018; Ostlund et al., 2021; Prokasky et al., 2017; Scott et al., 2016).
Recent studies also found that temperament profiles derived from latent profile analysis (LPA) are sensitive to prenatal factors and moderate effects of parenting on child outcomes (Brown et al., 2022; Gartstein et al., 2017; Lin et al., 2018; Ostlund et al., 2021; Prokasky et al., 2017). For example, greater prenatal substance use was associated with infant temperament profiles characterized by high behavioral and emotional reactivity in a large sample of infants from two longitudinal multisite studies (Lin et al., 2018). Among 247 toddlers, maternal anger and hostility during pregnancy predicted a greater likelihood of toddlers exhibiting behaviorally dysregulated temperament profiles resembling irritability or inhibition (Ostlund et al., 2021). With respect to person-centered approaches of temperament and their interactions with parenting behavior, among 150 mother–child pairs, profiles characterized by high impulsivity, high emotionality, and low effortful control modified predictions of externalizing problems from parenting behaviors. Specifically, self-reported negative parenting (composite of hostile, overreactive, and lax parenting) predicted more externalizing problems among children with exuberant temperament compared to regulated temperament profiles (Brown et al., 2022). Thus, testing preconception and prenatal stress as unique predictors of empirically derived child temperament profiles as well as the role of child temperament profiles in modifying links between parenting and subsequent child behavior problems may elucidate developmental cascades following preconception and prenatal stress.
The Current Study
The present study aimed to disentangle the putative effects of preconception and prenatal stress on child temperament, and to test effects of child temperament, parenting behaviors, and their interaction in prospective prediction of child behavior problems. In a predominantly low socioeconomic status and racially and ethnically diverse sample of mother–child pairs (n = 127), we tested two key aims:
Aim 1 tested independent associations of preconception perceived stress, prenatal perceived stress, and prenatal cortisol with child temperament profiles at age four derived from LPA. Specifically, using serial mediation, we tested whether preconception perceived stress was directly associated with child temperament profiles independently of prenatal perceived stress and cortisol (i.e., direct effect) or whether preconception stress operates indirectly through prenatal perceived stress and cortisol to influence child temperament profiles (i.e., indirect effect). We assessed perceived stress in women before conception and during pregnancy as a measure of maternal stress appraisals and maternal cortisol in pregnancy as a physiological measure of maternal stress to test distinct influences of different types of stress on child outcomes (Bush et al., 2017). Motivated by previous evidence that greater maternal prenatal cortisol output was associated with offspring temperament (Davis et al., 2007; Werner et al., 2013), our primary analysis focused on total cortisol output as a measure of maternal stress physiology in pregnancy. Secondary analyses evaluated the cortisol slope and cortisol awakening response as measures of maternal prenatal stress physiology to test whether links with temperament profiles differed as a function of the cortisol measure. Next, Aim 2 tested LPA-derived profiles as moderators of observed parenting behaviors as prospective predictors of child behavior problems 1 year later. Figure 1 presents a conceptual overview of study aims.
Figure 1. Conceptual Overview of the Current Study.
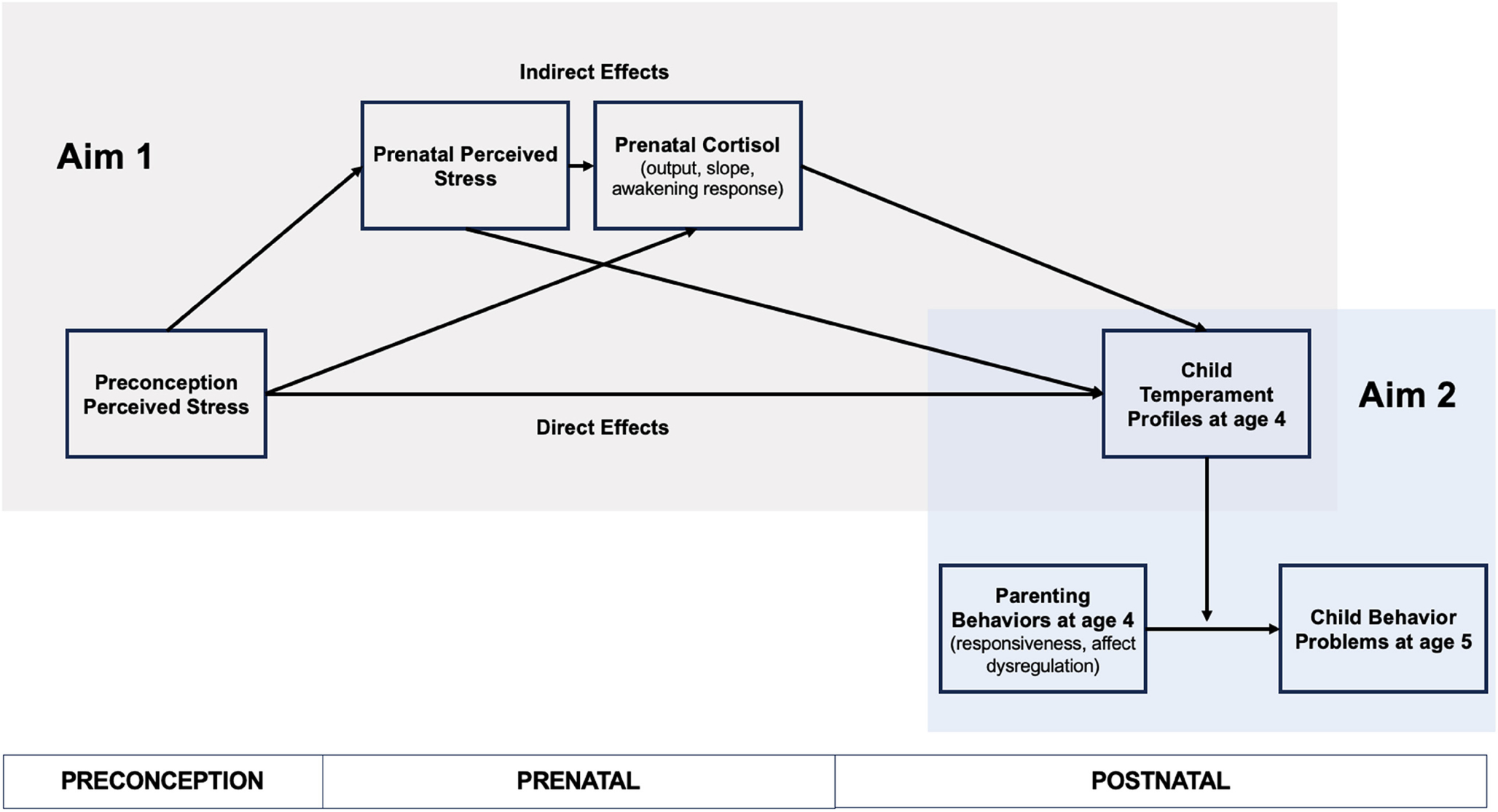
Note. Guided by the theoretical framework developmental cascades, this study tests the direct effects of preconception perceived stress on child temperament profiles at age four and indirect effects through perceived stress and cortisol during pregnancy (Aim 1). Aim 2 then tests whether child temperament profiles modify associations of parenting behaviors measured during a parent–child free-play interaction at age four and child behavior problems at age 5. See the online article for the color version of this figure.
We hypothesized that preconception perceived stress would predict temperament profiles characterized by high emotionality, high impulsivity, and low regulation indirectly through prenatal perceived stress and cortisol. Specifically, we predicted that higher preconception perceived stress would be associated with higher perceived stress and dysregulated cortisol indices during pregnancy (i.e., greater cortisol output in primary analyses and flatter cortisol slope and a greater cortisol awakening response in secondary analyses) that would then predict temperament profiles. In turn, consistent with differential susceptibility, we predicted that temperament profiles characterized by high emotionality, high impulsivity, and low regulation would be more sensitive to both positive and negative parenting behaviors. That is, children with this temperament profile would show fewer behavior problems in the context of responsive maternal parenting behaviors compared to children with other temperament profiles but more behavior problems in the context of dysregulated maternal affect (i.e., high negative and flat affect, low positive affect).
Method
Transparency and Openness
We report how we determined our sample size, all data exclusions (if any), all manipulations, and all measures in the study, and we follow JARS (Kazak, 2018). All data, analysis code, and research materials are available from the first author upon request. Data were analyzed using R Version 4.0.0 (R Core Team, 2020) and Mplus Version 8 (Muthén & Muthén, 2017). This study’s design and its analyses were not preregistered.
Participants and Procedure
The current study includes women enrolled in the multisite Community Child Health Network (CCHN; Guardino et al., 2016; Ramey et al., 2015). Participants were recruited following a birth (index birth) in five study sites and completed visits at regular intervals for 2 years after that birth (n = 2,510). Women who became pregnant again within the 2-year study period completed visits through a subsequent pregnancy and postpartum period (n = 362). Of the participants in CCHN who reported a subsequent pregnancy, those enrolled at three eligible CCHN study sites (Washington, District of Columbia; Lake County, Illinois; and North Carolina) were invited to enroll in a follow-up study of the subsequent child’s (study child) development (n = 287) and to complete additional visits when the study child was between 3 and 5 years of age. Of those invited, 127 participants enrolled in the follow-up study. The present study includes all participants enrolled in the follow-up study (n = 127) who completed early childhood follow-up visits when the study child was approximately 4 years old (M = 3.85 years, SD = 0.52, range = 3.35–5.48) and 1 year later (M = 5.07, SD = 0.46, range = 4.31–6.11). Trained research staff conducted structured interviews during in-home visits. The study was approved by the Institutional Review Boards at each site. Mothers provided written and informed consent for themselves and their children and were compensated for study visits.
The current study includes data from visits following the index birth and prior to conception of the subsequent pregnancy, during the subsequent pregnancy, and the early childhood visits. Here, preconception is defined as prior to conception of the study child, which for all participants in the sample was also an interconception period between consecutive births (i.e., between the birth of the index child and study child). An overview of study visits in the current study is presented in Figure 2. Regarding sample characteristics, mean maternal age was 34 years (M = 33.98, SD = 5.48) at the first early childhood visit. Mothers identified as Hispanic/Latina (48.8%), non-Hispanic White (31.5%), and Black/African American (21.3%). Mean per capita income adjusted for cost of living at each study site was $12,604 (SD = $12,725, range = $22.08–$70,000) and the modal level of educational attainment was high school diploma or GED (40.2%; M years of education = 12.72, SD = 3.57, range = 6–21). Most of the mothers were married to the study child’s father (46.4%) or cohabiting with but not married to the study child’s father (30.7%) at the early childhood study visits. Just over half of the study children were girls (53.5%). About half of the study children were the second-born child (51.2%). All study children were singleton births.
Figure 2.
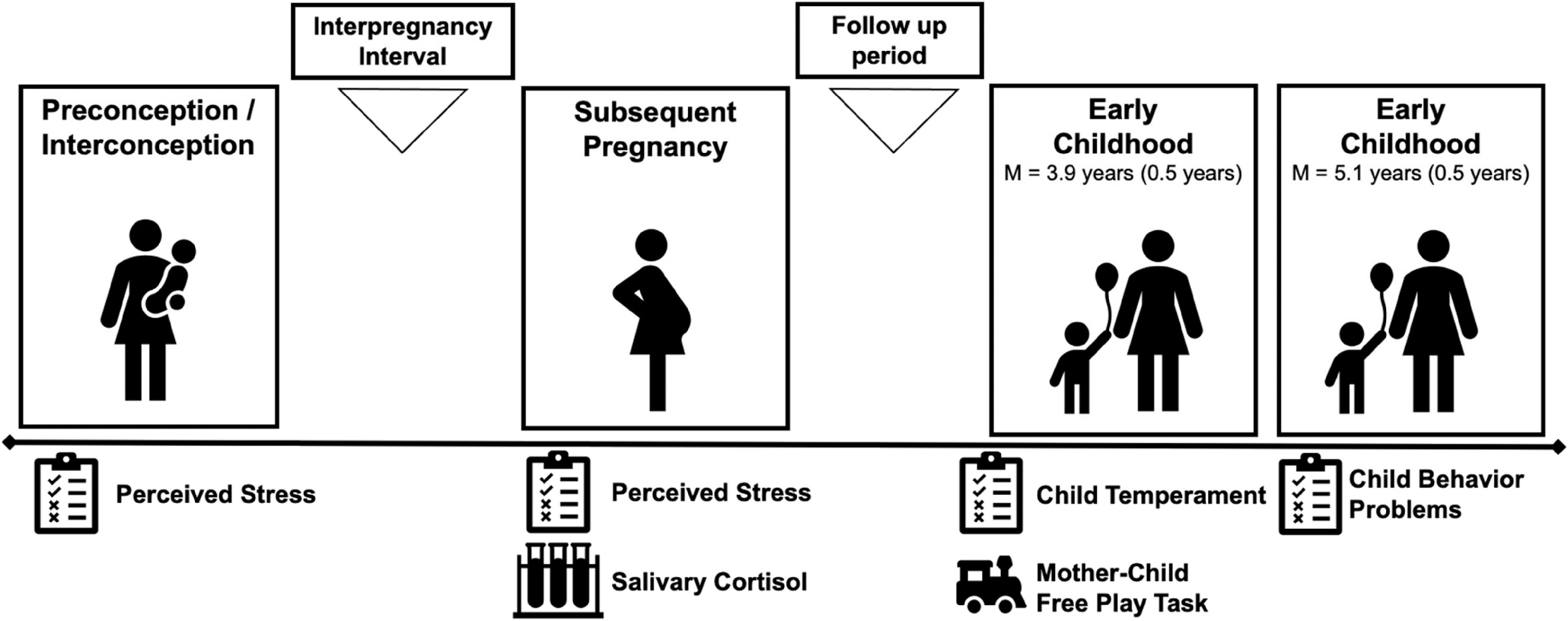
Study Overview and Measures
Measures
Maternal Preconception and Prenatal Perceived Stress
The 10-item version of the Perceived Stress Scale was used to measure maternal perceived stress prior to conception and during pregnancy (Cohen et al., 1983). Participants reported on the degree to which they found their lives to be unpredictable, uncontrollable, and overwhelming in the past month on a 5-point Likert scale from 0 (never) to 4 (almost always). The Perceived Stress Scale is an established index of general stress appraisal that is commonly used and validated for use during pregnancy (Karam et al., 2012). Mothers reported on perceived stress at each study visit after the index birth and prior to conception of the study child and during the second- and third-trimester of the study child pregnancy. Cronbach’s alpha indicated good reliability at each study visit (range = .77–.87). Scores from the study visit most proximal to conception of the study child were used in primary analyses to measure preconception stress (M = 27.13 weeks before conception, SD = 28.72 weeks). During pregnancy, mothers’ perceived stress scores at each pregnancy visit were positively correlated and were averaged for primary analyses (r = .56).
Maternal Prenatal Salivary Cortisol
Saliva samples were collected by mothers after each prenatal study visit which were assayed for cortisol. During study visits, research staff provided saliva sampling kits and instructions on procedures for collecting saliva, completed a practice sample with the participant, and answered questions. The kits included sterile cryogenic vials, labels, straws for passive drool collection of saliva, and morning and bedtime diaries for sample collection. Participants collected saliva at three times over the course of each sampling day (upon waking, 30 min after waking, and bedtime). Participants reported on time of sampling using morning and bedtime diaries. Participants mailed back samples in preaddressed, prepaid envelopes to each study office. Saliva samples were stored at −80 °C. Saliva samples were shipped to ZRT Laboratories (Beaverton, Oregon) on dry ice and assayed for cortisol using competitive luminescence immunoassay (IBL-America, Minneapolis, Minnesota) with a lower limit of detection of 0.015 μg/dL. Intra- and interassay coefficients of variance were 5.5% and 7.6%, respectively.
Repeated assessments of cortisol over the course of a day can provide information about different aspects of HPA axis activity. In the current study, we calculated measures of the cortisol awakening response, cortisol slope, and total cortisol output, calculated as per standard practice (Adam & Kumari, 2009; Guardino et al., 2016). The cortisol awakening response is defined as the increase in cortisol within the first 30 min after waking from waking cortisol values and was calculated as the difference between cortisol levels 30 min after waking and waking cortisol levels. The diurnal cortisol slope reflects circadian changes in cortisol, specifically declines in cortisol from morning to evening, and was calculated by subtracting morning cortisol levels from bedtime cortisol levels divided by the number of hours between the morning and bedtime cortisol measurements. Whereas the cortisol awakening response and diurnal cortisol slope capture dynamic changes in HPA axis activity over the course of the day, total cortisol output measures cumulative cortisol levels over the course of the day. Total cortisol output was calculated using area under the curve with respect to ground (Pruessner et al., 2003).
Cortisol measures were calculated for each prenatal study visit in the second and third trimester. For parsimony and to estimate HPA axis regulation over the second half of pregnancy, cortisol indices for each prenatal visit were averaged in primary analyses. Mean values of each cortisol measure were largely similar in the second (Mgestational age = 20.24 weeks, SD = 5.00) and third-trimester (Mgestational age = 32.70 weeks, SD = 3.79; see Table S1 in the online supplemental materials) and positively intercorrelated. In the current sample, the strength of the association between each cortisol measure varied from a small to medium correlation (|r| = .18–.72).
Child Temperament
Maternal ratings of child temperament were obtained via the Children’s Behavior Questionnaire Very Brief (CBQ-VB) version at the first early childhood visit when children were approximately 4 years old. The CBQ-VB is widely used and has demonstrated validity and reliability for children in this study’s age range (Putnam & Rothbart, 2006). Consistent with prior research identifying temperament profiles (e.g., Brown et al., 2022), subscales of temperament dimensions from the CBQ-VB were included in the LPA rather than higher-order scales of effortful control, negative affect, and surgency. Simulation studies suggest that including at least five indicators in LPA, as done here, improves acquisition of stable profiles and statistical power, including in sample sizes similar to the current study (Tein et al., 2013; Wurpts & Geiser, 2014). Subscales in the current analysis were previously identified in the original creation of the CBQ-VB and have demonstrated reliability and validity (Putnam & Rothbart, 2006). In total, there are 13 subscales from the CBQ-VB and each subscale includes two or three items. Subscales consisted of anger, discomfort, sadness, soothability, fear, impulsivity, shyness, activity level, high-intensity pleasure, inhibitory control, attention focusing, low-intensity pleasure, and perceptual sensitivity. Descriptive statistics and intercorrelations of temperament subscales appear in Table S2 in the online supplemental materials; temperament subscales were correlated in expected ways.
Parenting Behaviors
Observed maternal parenting behaviors were scored during a semistructured play task at the first early childhood visit. During the task, mothers and children were offered toy boxes and instructed to play with the toys for 15 min in mothers’ language of preference (69% English, 31% Spanish).1 Three trained coders scored parenting behaviors from 1 (not at all characteristic) to 4 (highly characteristic) along seven dimensions using the validated 36-month mother–child interaction coding system from the National Institute of Child Development Study of Early Childcare and Youth Development (1993). The seven dimensions of parenting behaviors were sensitivity to nondistress (attuned to and centered on child; letting the child guide play), positive regard (warmth, praise, and affection), stimulation of cognitive development (efforts to facilitate learning; promote more mature play), intrusiveness (being adult-centered; controlling the interaction), negative regard (anger, frustration, impatience, and/or other indicators of negative regard), detachment (disengaged from child or emotionally uninvolved with the child), and flatness of affect (not being animated in play session; displaying flat affect in facial expressions and vocal tone). All coders were blind to other data gathered on study participants. The coders achieved reliability with an expert coder before coding independently. To assess interrater reliability on independently coded videos, 24% of the videos were randomly selected and coded independently by an expert coder (mean percent agreement across subscales = 94%; range = 81%–100%).
Prior to analysis, we conducted exploratory and confirmatory factor analyses to discern the underlying factor structure of the seven dimensions of maternal parenting behaviors.2 We employed exploratory factor analysis with oblique goemin rotation and allowed for correlated factors. The two-factor solution fit the data better than the one-factor solution, χ2(14) = 54.71, p < .001. The two-factor solution was subsequently confirmed in confirmatory factor analysis with good fit to the data, χ2(10) = 12.87, p = .23; comparative fit index (CFI) = .99; Tucker–Lewis index (TLI) = .98; root-mean-square error of approximation (RMSEA) = .05, 90% CI [0.00, 0.12]; standardized root-mean-square residual (SRMR) = 0.05. Maternal sensitivity (λ = .97), stimulation of cognitive development (λ = .72), detachment (λ = −.54), and intrusiveness (λ = −.33) significantly loaded onto a maternal responsive parenting factor (all ps < .001) and maternal flat affect (λ = .75), negative regard (λ = .51), and positive regard (λ = −.83) significantly loaded onto a maternal affect dysregulation factor (all ps < .001). Saved factor scores for maternal responsive parenting and maternal affect dysregulation were used in primary analyses. Factor loadings appear in Figure S2 in the online supplemental materials.
Child Behavior Problems
Child behavior problems were measured with the Child Behavior Checklist (CBCL; age 1.5–5-year version) at the second early childhood visit. The CBCL is a well-validated 100-item parent-report checklist that assesses emotional and behavioral problems in children. Parents were asked to rate how true each item is for their child in the past 2 months on a scale of 0 (not true) to 2 (very true), which yielded estimates of total behavior problems and separate internalizing and externalizing problems broadband subscales. Given their high intercorrelation in the current sample (r = .62) and to maximize parsimony, we examined total behavioral problems (T-scores) as an outcome in primary analyses. Scores between 65 and 69 indicate clinical elevations whereas score above 70 indicate clinical significance (Achenbach & Rescorla, 2000).
Sociodemographic and Medical Variables
Women reported their racial/ethnic identity, age, and education level at the time of CCHN enrollment and reported updates to household income and relationship with their partner at each study visit. Maternal prepregnancy body mass index (BMI) was calculated based on maternal height and weight measured at the study visit most proximal to the conception of the subsequent pregnancy. Data on maternal medical conditions (preeclampsia, gestational diabetes, pregnancy infection, and anemia) during the subsequent pregnancy, study child birth weight, and study child length of gestation were extracted from medical records. Women in the follow-up study reported on study child age and child biological sex at the first early childhood visit.
Data Analytic Plan
Prior to analysis, primary study variables were examined for normality (skewness > 2 and kurtosis > 7) and outliers (>3 standard deviations from sample mean). All primary study variables were normally distributed. One observation was an outlier on cortisol slope (>3 SD above mean) during pregnancy and was winsorized. Missing data were handled using full information maximum likelihood (FIML), which is recommended when missing data exceeds 10%. FIML reduces bias of estimates, increases power, and improves generalizability of results relative to other missing data procedures (e.g., complete case analysis; listwise deletion; Dong & Peng, 2013; Enders, 2001, 2010). FIML is also appropriate in the context of quantitative moderators. Missing data on primary study variables ranged from 9% (perceived stress duringpregnancy) to 52% (cortisol output during pregnancy).
LPA of Child Temperament
First, we used LPA in MPlus to identify profiles of child temperament. LPA is a person-centered approach that aims to identify subgroups within a population based on certain sets of continuous variables (S. L. Ferguson et al., 2020). We compared models fitting one to five classes successively given that prior research has most often identified between three and five temperament profiles in similar age ranges. The best-fitting model was selected based on model fit statistics including Bayesian information criteria (BIC; lower values preferred), bootstrapped log-ratio test (BLTR; compares whether the model with k classes is a significant improvement from the model with k−1 classes), and entropy (higher values preferred; Jung & Wickrama, 2008; S. L. Ferguson et al., 2020). The best-fitting model was also selected based on theoretical parsimony and consistency with infant and child temperament profiles identified in prior research which commonly include profiles characterized by: (a) high negative affect, high activity level and impulsivity, and low regulatory abilities (i.e., exuberant or impulsive); (b) high negative affect, low positive affect and activity, and low regulatory abilities (i.e., inhibited); (c) high positive affect and regulatory abilities (i.e., regulated); and (d) below average levels on all temperament dimensions (i.e., average; Beekman et al., 2015; Brown et al., 2022; Gartstein et al., 2017; Lin et al., 2018; Ostlund et al., 2021; Prokasky et al., 2017; Scott et al., 2016). For the best-fitting model, we also evaluated differences between classes on each indicator included in the LPA to assess statistical power to detect the correct number of classes. Prior simulation work has found that while the effect of sample size on power to detect the correct number of classes is minimal, the mean difference between classes affects statistical power (Tein et al., 2013).
Aim 1: Preconception Stress, Prenatal Stress, and Child Temperament Profiles
In Aim 1 primary analyses, we conducted a serial mediation model using structural equation modeling to test (a) the direct effect of maternal preconception perceived stress on child temperament profiles and (b) the indirect effect of maternal preconception perceived stress on child temperament profiles through perceived stress and total cortisol output during pregnancy. Each serial mediation model estimated the following: (a) the effect of preconception perceived stress on child temperament (direct effect); (b) the effect of preconception perceived stress on prenatal perceived stress and prenatal cortisol (a1 path and a2 path, respectively); (c) the effect of prenatal perceived stress on prenatal cortisol (d21); (d) the effect of prenatal perceived stress and prenatal cortisol on child temperament profiles (b1 path and b2 path, respectively); and (e) the effect of preconception perceived stress on child temperament profiles through prenatal perceived stress and cortisol. In each serial mediation model, we used multinominal logistic regression to evaluate the effect of each predictor on LPA-derived temperament profiles. One temperament profile was set to the reference category and the others were dummy coded and entered simultaneously into the serial mediation model. As indicated in Figure 1, direct effects of preconception perceived stress on child temperament were interpreted as the effect of preconception stress on temperament profiles independently of each prenatal stress mediator whereas indirect effects were interpreted as the effects of maternal preconception perceived stress through each prenatal stress mediator.
For secondary analyses in Aim 1, additional serial mediation models evaluated the same effects with cortisol slope and the cortisol awakening response as cortisol measures. Each cortisol measure was tested in a separate model (two total models).
Aim 2: Temperament Profiles as Moderators of Parenting and Child Behavior Problems
In Aim 2, we used multiple linear regression with a Parenting Behavior × Temperament Profile interaction term predicting child behavior problems. For these analyses, temperament was included as a multicategorical moderator, which compares the effect of parenting behaviors on subsequent child behavior problems as a function of temperament profile; as noted above, one profile was the reference group whereas other profiles were dummy coded. Primary analyses for Aim 2 initially tested two linear regression models, with each Parenting Behavior × Temperament interaction term tested in separate models. If the interaction term was not significant, it was dropped from the model and only main effects were interpreted.
Covariates
Primary analyses for Aims 1 and 2 adjusted for maternal education, birth order, and length of gestation. These covariates were included in primary models based on associations with primary study variables in prior work and the current sample (p < .10). More specifically, maternal education was associated with child temperament and child behavior problems, and birth order was associated with child behavior problems. Additionally, maternal cortisol output and slope during pregnancy were associated with length of gestation. Maternal age, maternal medical conditions during the subsequent pregnancy, maternal prepregnancy BMI, relationship status, birth order, child age, and child biological sex were evaluated as potential covariates based on prior work but were not meaningfully associated with primary variables in this sample thus were not retained as covariates.
Sensitivity Analyses
We conducted three sensitivity analyses to evaluate robustness of results. First, we examined whether the Parenting Behaviors × Temperament interaction predicted child behavior problems differentially for internalizing or externalizing problems. Second, we tested whether the results persisted when adjusting for maternal perceived stress at each early childhood study visit. Third, we tested second- and third-trimester cortisol measures as mediators to evaluate whether there were timing differences in associations of maternal stress with prenatal cortisol or associations of prenatal cortisol with child temperament profiles.
Results
Descriptive Statistics
Descriptive statistics and bivariate correlations of primary study variables are presented in Table 1. Mean child behavior problems were low to moderate but there was a range across the sample. About 10% of the sample had scores indicative of risk for clinically significant behavior problems and less than 1% of the sample had scores indicative of clinically significant behavior problems (Achenbach & Rescorla, 2000). Maternal preconception and prenatal perceived stress were modestly correlated. Maternal preconception and prenatal stress were associated with higher child behavior problems. Maternal responsive parenting behaviors were associated with lower child behavior problems whereas maternal affect dysregulation was associated with higher child behavior problems.
Table 1.
Descriptive Statistics and Bivariate Correlations of Primary Study Variables
| Variable | M | SD | Range | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1. Preconception perceived stress | 12.74 | 5.04 | 3 to 26 | — | ||||||
| 2. Prenatal perceived stress | 16.74 | 6.00 | 0 to 32 | .40** | — | |||||
| 3. Prenatal cortisol slope | −0.03 | 0.02 | −0.09 to 0.02 | .16 | .28* | — | ||||
| 4. Prenatal cortisol awakening response | 0.10 | 0.34 | −0.78 to 1.20 | −.18 | .09 | .18 | — | |||
| 5. Prenatal total cortisol output | 6.95 | 3.19 | 0.64 to 15.76 | −.14 | .06 | −.24 | 72** | — | ||
| 6. Responsive parenting behaviors | 0.00 | 0.99 | −0.61 to 2.06 | −.12 | −.29** | .03 | .13 | .14 | — | |
| 7. Maternal affect dysregulation | 0.00 | 0.90 | −1.64 to 2.88 | .07 | .11 | −.09 | −.06 | −.14 | −.79*** | — |
| 8. Child total behavior problems | 50.75 | 10.10 | 28 to 79 | .21* | .23* | .04 | −.16 | −.00 | −.45** | −.10 |
Note. All cortisol units are nmol/L.
p < .05.
p < .01.
p < .001.
LPA of Temperament
We compared models fitting one to five classes successively using LPA. The four-class model best fit the data based on the sample-size adjusted BIC, the BLRT, and good entropy; this was strong evidence delineating the number of classes (see Table S3 in the online supplemental materials for fit indices). The number and nature of these four temperament profiles were consistent with theoretical models of temperament and with prior research on temperament profiles in this age range (e.g., Brown et al., 2022). Mean scores for each of the latent profiles on temperament subscales are visually displayed in Figure 3.
Figure 3. Predicted Means on CBQ-VB Subscales by Latent Profile.
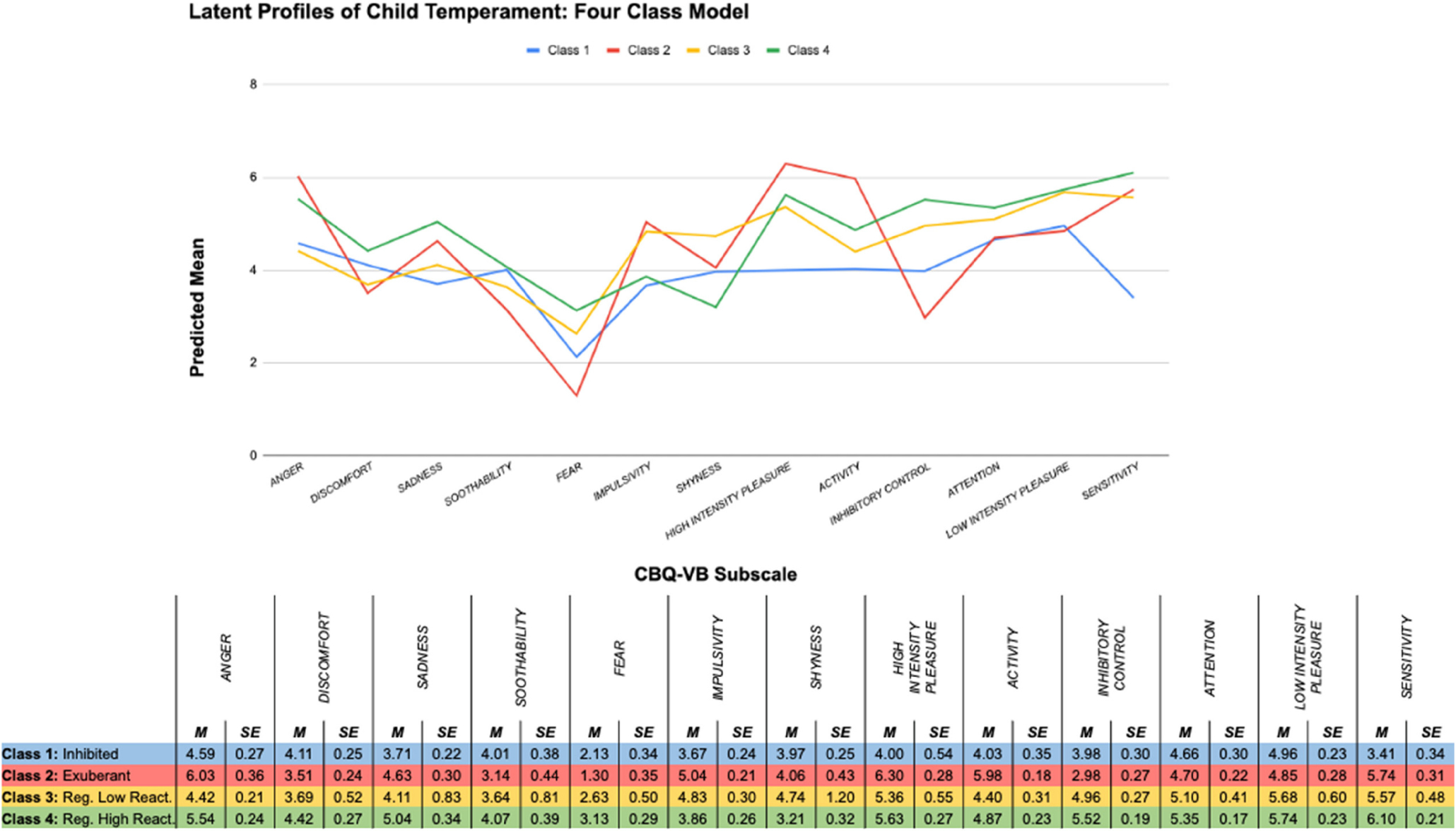
Note. CBQ-VB = Children’s Behavior Questionnaire Very Brief; Reg. Low React. = regulated low reactive; Reg. High React. = regulated high reactive. See the online article for the color version of this figure.
Class 1 included children with moderate levels of anger discomfort, sadness, soothability, and fear; low to moderate levels of regulation; and low levels of impulsivity, extraversion, and activity (16.5%; “inhibited”). Class 2 included children with high levels of impulsivity, extraversion, and activity; moderate levels of discomfort, sadness, soothability, and fear; and low to moderate levels of regulation (7.9%; “exuberant”). Class 3 was the largest profile and included children with moderate to high levels of regulation; moderate impulsivity, extraversion, and activity; and low levels of discomfort, sadness, soothability, and fear (44.1%; “regulated low reactive”). Class 4 included children with high levels of regulation; high levels of discomfort, sadness, soothability, and fear; and moderate levels of impulsivity, extraversion, and activity (31.5%; “regulated high reactive”). As the temperament profile with the largest proportion of children, the regulated low reactive temperament profile was included as the reference group in primary analyses. The four classes significantly differed on all temperament dimensions except soothability and the mean difference between classes on each indicator included in the LPA ranged from moderate to large on average (Cohen’s d; range = 0.50–1.00).
Primary Results
Aim 1: Associations of Preconception and Prenatal Stress With Child Temperament Profiles
We tested the direct effects of preconception perceived stress on child temperament and indirect effects of preconception stress on child temperament via perceived stress and cortisol during pregnancy using serial mediation models, which adjusted for maternal education, birth order, and length of gestation.
Primary Analyses: Total Cortisol Output.
Primary analyses included cortisol output as the measure of prenatal cortisol. Preconception perceived stress predicted greater likelihood of children being in the exuberant temperament profile compared to the regulated low reactive temperament profile (OR = 1.24, 95% CI [1.02, 1.52], p = .04). Consistent with hypotheses, preconception perceived stress was also positively associated with prenatal perceived stress (β= .36, SE = 0.09, p < .001) but prenatal perceived stress was unrelated to child temperament profiles. Contrary to hypotheses, neither preconception nor prenatal perceived stress was associated with prenatal cortisol output. However, children of mothers with greater prenatal cortisol output were significantly more likely to be in the exuberant temperament profile compared to the regulated low reactive temperament profile (OR = 1.55, 95% CI [1.04, 2.31], p = .03). Prenatal perceived stress and cortisol output did not mediate the effect of preconception perceived stress on child temperament profiles (all ps > .10). Complete regression coefficients are presented in Table 2.
Table 2.
Primary Aim 1 Results With Prenatal Cortisol Output
| Predictor | Outcome | OR or β | SE | 95% CI | B (SE) | p |
|---|---|---|---|---|---|---|
|
| ||||||
| Preconception perceived stress | Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. | Ref. |
| Temperament profile: regulated high reactive | 1.00 | 0.05 | [0.90, 1.11] | −0.04 (0.11) | .68 | |
| Temperament profile: inhibited | 0.92 | 0.06 | [0.80, 1.05] | −0.08 (0.07) | .22 | |
| Temperament profile: exuberant | 1.24 | 0.13 | [1.02, 1.52] | 0.22(0.10) | .04** | |
| Preconception perceived stress | Prenatal perceived stress | 0.36 | 0.09 | [0.18,0.53] | 0.43 (0.12) | <.001*** |
| Prenatal cortisol output | −0.14 | 0.15 | [−0.42, 0.15] | −0.09 (0.09) | .36 | |
| Prenatal perceived stress | Prenatal cortisol output | 0.20 | 0.13 | [−0.05, 0.45] | 0.11 (0.07) | .11 |
| Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Temperament profile: regulated high reactive | 1.04 | 0.05 | [0.95, 1.15] | 0.04 (0.06) | .41 | |
| Temperament profile: inhibited | 1.09 | 0.06 | [0.98, 1.21] | 0.08 (0.05) | .14 | |
| Temperament profile: exuberant | 0.82 | 0.09 | [0.67, 1.01] | −0.20(0.10) | .06** | |
| Prenatal cortisol output | Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. | Ref. |
| Temperament profile: regulated high reactive | 0.96 | 0.10 | [0.77, 1.18] | −0.04 (0.11) | .68 | |
| Temperament profile: inhibited | 1.07 | 0.13 | [0.84, 1.36] | 0.07 (0.12) | .58 | |
| Temperament profile: exuberant | 1.55 | 0.32 | [1.04, 2.31] | 0.44 (0.20) | .03* | |
|
| ||||||
| Indirect effects | B | SE | p | |||
|
| ||||||
| Preconception perceived stress → prenatal perceived stress → regulated high reactive | 0.02 | 0.02 | .42 | |||
| Preconception perceived stress → prenatal perceived stress → inhibited | 0.04 | 0.03 | .18 | |||
| Preconception perceived stress → prenatal perceived stress → exuberant | −0.09 | 0.05 | .10 | |||
| Preconception perceived stress → prenatal cortisol output → regulated high reactive | 0.00 | 0.01 | .69 | |||
| Preconception perceived stress → prenatal cortisol output → inhibited | −0.01 | 0.01 | .67 | |||
| Preconception perceived stress → prenatal cortisol output → exuberant | −0.04 | 0.04 | .39 | |||
| Preconception perceived stress → prenatal perceived stress → prenatal cortisol output → regulated high reactive | 0.00 | 0.01 | .70 | |||
| Preconception perceived stress → prenatal perceived stress → prenatal cortisol output → inhibited | 0.00 | 0.01 | .59 | |||
| Preconception perceived stress → prenatal perceived stress → prenatal cortisol output → exuberant | 0.02 | 0.02 | .29 | |||
Note. All models adjusted for length of gestation, maternal education, and birth order. CI = confidence interval.
p < .05.
p < .10.
p < .001.
Secondary Analyses: Diurnal Cortisol Slope.
The first secondary analysis included diurnal cortisol slope as the measure of prenatal cortisol. Preconception perceived stress was again positively associated with prenatal perceived stress. In turn, prenatal perceived stress predicted a greater likelihood of children being in the inhibited profile versus the regulated high reactive profile (OR = 1.11, 95% CI [1.001, 1.23], p = .047), consistent with hypotheses. There was a marginal indirect effect of preconception perceived stress on the likelihood of being in the inhibited temperament profile via prenatal perceived stress (B = 0.05, SE = 0.03, p = .09). Greater perceived stress during pregnancy was also associated with a flatter cortisol slope during pregnancy (β= .25, SE = 0.13, p = .047). Moreover, prenatal diurnal cortisol slope predicted likelihood of children being in the exuberant temperament profile. Contrary to hypotheses, however, children of mothers with a flatter cortisol slope during pregnancy were significantly less likely to be in the exuberant temperament profile compared to the regulated low reactive temperament profile (OR = 0.61, 95% CI [0.39, 0.98], p = .03). Complete regression coefficients are presented in Table 3.
Table 3.
Secondary Aim 1 Results With Prenatal Cortisol Slope
| Predictor | Outcome | OR or β | SE | 95% CI | B (SE) | p |
|---|---|---|---|---|---|---|
|
| ||||||
| Preconception perceived stress | Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. | Ref. |
| Temperament profile: regulated high reactive | 1.01 | 0.06 | [0.91, 1.12] | 0.01 (0.05) | .91 | |
| Temperament profile: inhibited | 0.91 | 0.06 | [0.80, 1.04] | −0.09 (0.07) | .16 | |
| Temperament profile: exuberant | 1.14 | 0.10 | [0.95, 1.36] | 0.13 (0.09) | .16 | |
| Preconception perceived stress | Prenatal perceived stress | 0.36 | 0.09 | [0.19,0.53] | 0.43 (0.12) | <.001*** |
| Prenatal cortisol slope | −0.06 | 0.13 | [−0.31,0.19] | −0.02 (0.05) | .64 | |
| Prenatal perceived stress | Prenatal cortisol slope | 0.25 | 0.13 | [0.004, 0.50] | 0.09 (0.05) | .046* |
| Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Temperament profile: regulated high reactive | 1.02 | 0.05 | [0.93, 1.13] | 0.02 (0.05) | .64 | |
| Temperament profile: inhibited | 1.11 | 0.06 | [1.001, 1.23] | 0.10 (0.05) | .047* | |
| Temperament profile: exuberant | 0.93 | 0.08 | [0.79, 1.11] | −0.07 (0.09) | .42 | |
| Prenatal cortisol slope | Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. | Ref. |
| Temperament profile: regulated high reactive | 1.17 | 0.27 | [0.75, 1.82] | 0.15 (0.23) | .51 | |
| Temperament profile: inhibited | 0.80 | 0.17 | [0.53, 1.21] | −0.23 (0.21) | .28 | |
| Temperament profile: exuberant | 0.61 | 0.15 | [0.39, 0.98] | −0.49 (0.24) | .03* | |
|
| ||||||
| Indirect effects | B | SE | p | |||
|
| ||||||
| Preconception perceived stress → prenatal perceived stress → regulated high reactive | 0.01 | 0.02 | .64 | |||
| Preconception perceived stress → prenatal perceived stress → inhibited | 0.05 | 0.03 | .09** | |||
| Preconception perceived stress → prenatal perceived stress → exuberant | −0.03 | 0.04 | .44 | |||
| Preconception perceived stress → prenatal cortisol slope → regulated high reactive | 0.00 | 0.01 | .74 | |||
| Preconception perceived stress → prenatal cortisol slope → inhibited | 0.01 | 0.01 | .66 | |||
| Preconception perceived stress → prenatal cortisol slope → exuberant | 0.01 | 0.03 | .65 | |||
| Preconception perceived stress → prenatal perceived stress → prenatal cortisol slope → regulated high reactive | 0.01 | 0.01 | .56 | |||
| Preconception perceived stress → prenatal perceived stress → prenatal cortisol slope → inhibited | −0.01 | 0.01 | .33 | |||
| Preconception perceived stress → prenatal perceived stress → prenatal cortisol slope → exuberant | −0.02 | 0.01 | .12 | |||
Note. All models adjusted for length of gestation, maternal education, and birth order. CI = confidence interval.
p < .05.
p < .10.
p < .001.
Secondary Analyses: Cortisol Awakening Response.
The next secondary analysis included the cortisol awakening response as a measure of prenatal cortisol. Again, preconception perceived stress positively predicted children being in the exuberant versus regulated low reactive temperament profile in this model. Prenatal perceived stress positively predicted cortisol awakening response during pregnancy (β= .28, SE = 0.11, p = .03); however, cortisol awakening response during pregnancy was unrelated to temperament profiles. Complete regression coefficients are presented in Table S4 in the online supplemental materials.
Aim 2: Temperament Profiles as Moderators of Parenting and Child Behavior Problems
Next, we tested whether age 4 temperament profiles moderated predictions of age five child behavior problems from age four maternal responsive behaviors and affect dysregulation during the parent–child interaction using multiple linear regression. Complete regression coefficients for Aim 2 appear in Table 4. In the first model, the Maternal Responsive Behaviors × Child Temperament Profile interaction was unrelated to child behavior problems; therefore, we interpreted main effects with the interaction term dropped from the model. Responsive parenting behaviors inversely predicted child behavior problems 1 year later (β=−.33, SE = 0.10, p = .001). In the next model, once again, the Maternal Affect Dysregulation × Child Temperament Profiles interaction was unrelated to child behavior problems. With those interactions dropped, maternal affect dysregulation was not significantly associated with child behavior problems (β= .15, SE = 0.10, p = .15).
Table 4.
Primary Aim 2 Results
| Predictor | β | SE | 95% CI | p |
|---|---|---|---|---|
|
| ||||
| Model 1: main effects of responsive parenting behaviors on child behavior problems | ||||
| Responsive parenting behaviors | −0.33 | 0.10 | [−0.52, −0.14] | .001** |
| Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. |
| Temperament profile: regulated high reactive | 0.17 | 0.10 | [−0.03, 0.36] | .09* |
| Temperament profile: inhibited | 0.17 | 0.10 | [−0.02, 0.35] | .08* |
| Temperament profile: exuberant | 0.15 | 0.11 | [−0.07, 0.36] | .19 |
| Model 2: main effects of maternal affect dysregulation on child behavior problems | ||||
| Maternal affect dysregulation | 0.15 | 0.10 | [−0.05, 0.35] | .15 |
| Temperament profile: regulated low reactive | Ref. | Ref. | Ref. | Ref. |
| Temperament profile: regulated high reactive | 0.17 | 0.10 | [−0.04, 0.37] | .11 |
| Temperament profile: inhibited | 0.20 | 0.10 | [0.00, 0.39] | .05* |
| Temperament profile: exuberant | 0.17 | 0.10 | [−0.05, 0.40] | .12 |
Note. All models adjusted for length of gestation, maternal education, and birth order. Interaction effects of maternal parenting behaviors. CI = confidence interval.
p < .10.
p < .01.
Sensitivity Analyses
The pattern of results remained the same in each sensitivity analysis. First, the results were robust to separate internalizing and externalizing problem outcomes, suggesting their shared sensitivity to the Parenting × Temperament interactions. Second, results did not change with statistical control of maternal perceived stress at each early childhood study visit; this indicates that preconception and prenatal influences were independent of concurrent maternal stress. Third, there were no differences in patterns of (a) associations of maternal stress with prenatal cortisol measures or (b) associations of prenatal cortisol with child temperament profiles. Thus, there was no evidence of timing effects.
Discussion
According to developmental cascades, predictions of offspring development from preconception and prenatal factors depends on the postnatal caregiving environment. To date, however, methodological challenges in prospective assessment of preconception, prenatal, and postnatal factors have limited understanding of developmental cascades following preconception and prenatal stress. The current study aimed to address this research gap by testing the effects of preconception and prenatal stress on child temperament profiles at age four and examining whether child temperament profiles, in turn, modified associations of parenting behaviors with child behavior problems. We tested these questions in a racially and ethnically diverse and predominantly low socioeconomic status sample of mother–child pairs. Notably, this study is the first to our knowledge to prospectively assess preconception, prenatal, and postnatal factors that shape child outcomes based on multimodal assessment of maternal stress, stress physiology, and observed parenting.
We identified four distinct child temperament profiles using LPA: regulated low reactive, regulated high reactive, inhibited, and exuberant. These temperament profiles align with prior empirical work using LPA in similarly aged children as well as theoretical conceptualizations of temperament (Brown et al., 2022; Dollar et al., 2017; Putnam & Rothbart, 2006). Consistent with study hypotheses, multilevel measures of maternal preconception and prenatal stress based on perceived stress and stress physiology each predicted child temperament profiles. Specifically, greater preconception perceived stress and higher prenatal cortisol output were each independently associated with children being more likely to have an exuberant temperament in primary analyses whereas a flatter cortisol slope was associated with children being less likely to have an exuberant temperament in secondary analyses. Furthermore, prenatal perceived stress was associated with a greater likelihood of children having an inhibited temperament. However, contrary to hypotheses, neither prenatal stress nor cortisol were indirect pathways through which preconception perceived stress predicted child temperament. Also contrary to hypotheses, child temperament profiles did not modify associations of parenting behaviors with child behavior problems, although responsive parenting behaviors were associated with lower child behavior problems independently of child temperament. The conceptual overview summarizing results is presented in Figures S1–S3 in the online supplemental materials.
Preconception and Prenatal Influences on Child Temperament Profiles
Preconception and prenatal stress prospectively predicted child temperament profiles in the current study. Specifically, preconception maternal perceived stress positively predicted a greater likelihood of children being in the exuberant temperament profile characterized by high negative emotionality, impulsivity, and low regulation, independently of maternal perceived stress and cortisol during pregnancy. This finding aligns with prior work that preconception maternal posttraumatic stress and depressive symptoms were associated with greater child negative emotionality, above and beyond the effects of prenatal factors (Spry et al., 2020; Swales et al., 2023). Of note, the present study innovatively employed prospective assessment of preconception and prenatal factors, thereby allowing for temporally ordered, rigorous tests of mechanistic pathways linking preconception factors to child outcomes. Although prenatal stress and cortisol did not mediate predictions of child temperament profiles from preconception stress, it is plausible that preconception stress operates through other unmeasured prenatal pathways (e.g., inflammatory processes; Hantsoo et al., 2019; Nazzari & Frigerio, 2020) or pathways that are independent of prenatal factors (e.g., germline epigenetic mechanisms; Bowers & Yehuda, 2016; Chan et al., 2018; Yehuda et al., 2000, 2014; Yehuda & Lehrner, 2018). Ultimately, these findings underscore the importance of applying life course perspectives to better understand how preconception factors shape offspring development (Keenan et al., 2018).
Although these results did not provide support for an indirect pathway whereby preconception stress predicted child temperament profiles indirectly through prenatal factors, we did find that prenatal stress, based on maternal stress appraisals and stress physiology, were associated with differences in child temperament. These findings align with a considerable body of evidence within the prenatal programming model where prenatal stress–conceptualized and measured in multiple ways–shapes fetal development and offspring outcomes (Barker, 2007; Bateson et al., 2014; Gluckman et al., 2005; Olsson et al., 2021; Sandman et al., 2012). For example, measures of maternal stress and stress physiology during pregnancy have been associated with offspring mental and physical health extending through adulthood (Glover et al., 2010; Rogers et al., 2020; Van den Bergh et al., 2020). Overall, in the present study, prenatal stress and stress physiology were associated with the likelihood of children being in the exuberant and inhibited temperament profiles. Children with exuberant and inhibited temperaments were similarly high on dimensions related to negative emotionality (discomfort, sadness, soothability, and fear) and low on dimensions related to regulation. This finding is consistent with prior work reporting that prenatal stress is associated with higher negative emotionality and lower regulatory abilities in infants and children (Davis et al., 2007, 2011; Irwin et al., 2021; Lin et al., 2018; Ostlund et al., 2021; Van den Bergh et al., 2020). Notably, this study extends prior work by examining associations of preconception and prenatal stress with child temperament profiles across dimensions rather than focusing on specific temperament dimensions (Shiner et al., 2012).
Several key findings emerged from the multilevel assessment of maternal prenatal stress in the current study that may advance understanding of how different forms of prenatal stress influence child temperament, particularly the clustering of temperament traits across dimensions. First, two of three indices of prenatal cortisol tested in this study predicted the likelihood of children being in the exuberant temperament profile. In primary analyses, greater prenatal cortisol output was associated with a greater likelihood of children having exuberant temperaments. The maternal HPA axis regulation undergoes substantial changes during pregnancy that leads to changes in both maternal cortisol levels and diurnal cortisol rhythms, including an increase in circulating cortisol levels (Duthie & Reynolds, 2013; Entringer et al., 2010). These changes support fetal development as well as labor and delivery processes, but variations in fetal exposure to cortisol levels and dysregulated cortisol patterns can adversely affect subsequent offspring functioning (Howland et al., 2017; Zijlmans et al., 2015). For example, approximately 15% of maternal cortisol crosses the placenta to the fetal compartment such that greater cortisol output during pregnancy may excessively expose the fetus to glucocorticoids and alter fetal development (Davis et al., 2007; Howland et al., 2017). Consistent with the present findings, prior studies have also reported that excessive fetal exposure to glucocorticoids is associated with infant and child temperament (Davis et al., 2007; Werner et al., 2013; Zijlmans et al., 2015).
Importantly, HPA axis regulation is multidimensional and different cortisol measures, such as cortisol output, awakening response, and slope, yield distinct information about neuroendocrine functioning (Adam & Kumari, 2009). Although primary analyses focused on maternal cortisol output as a measure of prenatal stress physiology, maternal prenatal diurnal cortisol patterns may also influence fetal development and offspring outcomes (Rinne, Hartstein, et al., 2023; Zijlmans et al., 2015). Thus, secondary analyses in the present study included maternal diurnal cortisol slope and cortisol awakening response as additional cortisol measures to elucidate separable prenatal biological pathways that may influence child temperament. In these analyses, a flatter cortisol slope was associated with a lower likelihood of children having exuberant temperaments whereas the cortisol awakening response was not associated with child temperament profiles. The finding that a flatter cortisol slope was associated with a lower likelihood of children having exuberant temperament is contrary to study hypotheses but could be due in part to changes in diurnal cortisol regulation from a nonpregnant to a pregnant state. Specifically, regulation of diurnal cortisol patterns changes over the course of pregnancy such that the cortisol slope gradually flattens from early to late pregnancy (Duthie & Reynolds, 2013; Entringer et al., 2010). Therefore, whereas flatter cortisol slopes are associated with less favorable health in nonpregnant samples (Adam et al., 2017), a flatter cortisol slope in the current sample may reflect normative flattening of the diurnal slope that occurs during pregnancy and may protect the fetus from excessive exposure to glucocorticoids (Duthie & Reynolds, 2013; Entringer et al., 2010). Indeed, previously, flattened diurnal slopes during pregnancy were associated with optimal fetal growth (Kivlighan et al., 2008). These findings warrant replication in future studies that also employ multiple indices of prenatal cortisol but suggest that further investigation of how different measures of maternal prenatal HPA axis activity relate to child outcomes may advance understanding of prenatal biological mechanisms that shape child developmental trajectories.
Second, whereas measures of prenatal cortisol were associated with offspring being more likely to have an exuberant temperament profile, prenatal perceived stress predicted a greater likelihood of children having an inhibited temperament. These findings provide evidence of distinct associations of prenatal stress physiology and stress appraisals with child temperament profiles. The inhibited and exuberant temperament profiles diverged on dimensions related to surgency such that children with exuberant temperaments were higher in impulsivity, extraversion, and activity than children with inhibited temperaments. Therefore, prenatal stress appraisals and stress physiology may differentially relate to temperament dimensions pertaining to child surgency specifically (Bush et al., 2017; Lin et al., 2014; Van den Bergh et al., 2020). Studies testing associations of multiple types of prenatal stress with child temperament are rare, but consistent with the current study, there is preliminary evidence that different forms of prenatal stress show specific associations with child surgency (Bush et al., 2017; Lin et al., 2014). Future studies could further test associations of multiple forms of prenatal stress with child outcomes (e.g., acute stress, chronic stress, stress physiology) to advance understanding of the mechanistic pathways through which different forms of prenatal stress influence developmental cascades (Bush et al., 2017).
Parenting, Child Temperament, and Child Behavior Problems
Informed by the theoretical framework of developmental cascades, our second study aim tested whether child temperament profiles moderated the associations of parenting behaviors with child behavior problems one year later. Contrary to study hypotheses, however, child temperament profiles did not moderate the effects of parenting on subsequent child behavior problems. This finding differs from theory and empirical evidence that child characteristics like temperament interact with parenting behavior to differentially predict child outcomes (e.g., Belsky, 2013; Brown et al., 2022; Pitzer et al., 2011; Rubin et al., 2003; Slagt et al., 2016). There may be several reasons for null interaction effects. It is plausible that the small size of some temperament profile groups may have limited our ability to detect interaction effects; however, recent work testing Parenting × Temperament Profile interactions with similar sample and class sizes have detected significant interaction effects (e.g., Brown et al., 2022; McClelland & Judd, 1993). These null effects may also be partly attributable to the nature of the mother–child interaction task. Whereas free-play tasks typically capture maternal behaviors in response to child nondistress, other tasks may yield more variability in parenting behaviors and could strengthen inferences about interactions between parenting behaviors and child temperament (e.g., frustration, dyadic problem-solving, or attachment-relevant tasks; Cassidy et al., 1992; Goldsmith, 1996; Lunkenheimer et al., 2020). Finally, developmental influences are also likely salient, as estimated from the age of children: in one meta-analysis, parenting by temperament interactions occurred more consistently in studies during infancy than early childhood (Slagt et al., 2016).
Although temperament profiles in the current study did not significantly moderate predictions of child behavior problems from parenting behaviors, maternal responsiveness predicted fewer child behavior problems independently of child temperament. This finding is notable given that inhibited and exuberant temperament profiles have been previously associated with more child behavior problems (Putnam & Rothbart, 2006). In the current study, the inhibited and exuberant temperament profiles were also positively associated with child behavior problems one year later, with small effect sizes, but effects were not statistically significant when controlling for maternal parenting behaviors. Collectively, these results suggest that parent-based interventions that serve to increase parental responsiveness could facilitate improved child outcomes even beyond the effects of individual differences in temperament secondary to preconception and prenatal stress.
Strengths and Limitations
Given its prospective design spanning preconception, prenatal, and postnatal periods, the current study was well-positioned to isolate independent and interactive preconception, prenatal, and postnatal influences on child outcomes. Additionally, the current sample was diverse with regard to race/ethnicity, predominantly low income, and included representation from rural, urban, and suburban study sites. Another notable strength of the current study was its use of person-centered LPA to innovatively characterize and discern empirically distinct profiles of child temperament. Person-centered approaches elucidate covariation across temperament dimensions to identify phenotypically similar profiles of children and can help facilitate more targeted assessment of child risk factors for psychopathology to support the optimization of prevention and intervention efforts (Brown et al., 2022; Gartstein et al., 2017; Lin et al., 2018; Ostlund et al., 2021; Prokasky et al., 2017). This method also highlighted distinct associations of multilevel measures of prenatal stress with configurations of temperament dimensions. Such differences may not have been detected in variable-centered approaches (Shiner et al., 2012). Moreover, the multimethod approach to understanding maternal influences represents a critical strength of the study. Integrating multimodal assessments of maternal perceived stress and stress physiology innovatively assays preconception and prenatal stress, thus elucidating their putative independent effects on offspring outcomes. Moreover, the use of objective behavioral observation of maternal parenting behaviors during a parent–child free-play interaction rigorously assessed parenting behaviors and reduced the potential bias associated with self-report measures. Finally, the use of modern missing data handling techniques (i.e., FIML), as employed in the current study, is an effective strategy to decrease Type-II error rates, and increase precision of estimates (Enders, 2001, 2010).
Despite the noted strengths, the current study also has limitations. First, mothers reported on both child temperament and behavior problems, resulting in the possibility of shared method variance. Next, there was also variability across participants in the amount of time between the preconception and prenatal assessment which may contribute to variation in the strength of associations between preconception and prenatal factors. Nonetheless, the prospective assessment of preconception and prenatal factors is a particularly notable strength of this study that extends prior work on preconception influences that largely rely on retrospective report. It is also plausible that observer characteristics may influence ratings of parent–child interactions and a few of the video-recorded interactions were translated prior to coding. This should be kept in mind when interpreting results, particularly given the diverse demographic composition of the current sample. Although parenting codes did not differ as a function of participant language or translation in this sample, prior work has indicated that these factors are critical to consider in the context of observer ratings of parent–child interactions (e.g., Gonzales et al., 1996; Harvey et al., 2009; Yasui & Dishion, 2008). Lastly, the modest sample size may have limited statistical power to detect interactions between parenting and temperament in predicting child behavior problems as there were small class sizes for certain temperament profiles. However, the present sample size and class sizes align with prior work as well as recommendations for LPA and structural equation modeling (e.g., Brown et al., 2022; S. L. Ferguson et al., 2020; Wolf et al., 2013). Specifically, all profiles included more than 5% of the sample and there were moderate to large effect size differences between classes, suggesting clear delineation of the temperament profiles (C. J. Ferguson, 2016; Tein et al., 2013). Post hoc power analyses for primary analyses also indicated they achieved sufficient power (.0.80) and effect sizes from primary analyses were small to medium (Faul et al., 2009; C. J. Ferguson, 2016). Despite the present limitations, the overall design features of this study could be prioritized in future work in larger samples, including the longitudinal design spanning several developmental periods, multimodal assessments, diverse sample composition, and use of latent variables approaches, to advance understanding of preconception, prenatal, and postnatal influences on developmental trajectories.
Conclusions
Guided by the theoretical framework of developmental cascades and a life course perspective, the current longitudinal study used data from the preconception, prenatal, and postnatal period to elucidate developmental cascades following preconception and prenatal stress. Maternal preconception and prenatal stress, using multilevel assessment of maternal perceived stress and cortisol, each independently predicted the likelihood of children exhibiting inhibited and exuberant temperament profiles. Although temperament profiles did not modify associations of maternal parenting behaviors with child behavior problems, observed maternal responsiveness inversely predicted subsequent child behavior problems when controlling for child temperament, highlighting a critical protective role of postnatal caregiving inputs in shaping developmental trajectories over and above child effects. Further research that attends to multilevel, independent and interactive influences on child outcomes across the preconception, prenatal, and postnatal period is warranted to elucidate developmental cascades following preconception and prenatal stress.
Supplementary Material
Public Significance Statement.
While prior work has demonstrated prenatal and even preconception developmental origins of psychopathology risk, elucidating the independent and joint effects of preconception, prenatal, and postnatal factors on child mental health is critical to innovate and maximize intervention and prevention efforts. In the present study, preconception and prenatal stress each independently predicted child temperament; however, postnatal responsive parenting behaviors were associated with lower child behavior problems regardless of child temperament. These findings inform understanding of distinct preconception, prenatal, and postnatal influences on child outcomes.
Acknowledgments
This work was supported through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U HD44207, U HD44219, U HD44226, U HD44245, U HD44253, U HD54791, U HD54019, U HD44226–05S1, U HD44245–06S1, R03 HD59584, and R01HD072021–05) and the National Institute of Nursing Research (U NR008929). Gabrielle R. Rinne was supported by T32-MH015750. The authors report no conflicts of interest. The authors report how they determined their sample size, all data exclusions (if any), all manipulations, and all measures in the study, and they followed JARS (Kazak, 2018). All data, analysis code, and research materials are available from Gabrielle R. Rinne upon request. Data were analyzed using R Version 4.0.0 (R Core Team, 2020) and Mplus Version 8 (Muthén & Muthén, 2017). This study’s design and its analyses were not preregistered.
Footnotes
Supplemental materials: https://doi.org/10.1037/dev0001728.supp
Two coders were monolingual English-speaking, and one coder was bilingual English and Spanish speaking. Videos in Spanish were coded by the Spanish-speaking bilingual coder or translated for coding when that was not possible for a small proportion of the sample (n = 4). Mean levels of the dimensions of parenting behaviors did not significantly differ for mothers coded in English vs. Spanish (ps > .06). Additionally, the pattern of primary results remained the same in a post hoc sensitivity analysis excluding videos that were translated prior to coding.
Maternal negative affect and detachment were log-transformed prior to factor analysis to account for nonnormality (skewness >2; kurtosis >7).
References
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA pre-school forms & profiles. University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Adam EK, & Kumari M (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34(10), 1423–1436. 10.1016/j.psyneuen.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, & Gilbert KE (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrash HK, Johnson K, Adams M, Cordero JF, & Howse J (2006). Preconception care for improving perinatal outcomes: The time to act. Maternal and Child Health Journal, 10(S1), 3–11. 10.1007/s10995-006-0100-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP (2007). The origins of the developmental origins theory. Journal of Internal Medicine, 261(5), 412–417. 10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- Bateson P, Gluckman P, & Hanson M (2014). The biology of developmental plasticity and the predictive adaptive response hypothesis. The Journal of Physiology, 592(11), 2357–2368. 10.1113/jphysiol.2014.271460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman C, Neiderhiser JM, Buss KA, Loken E, Moore GA, Leve LD, Ganiban JM, Shaw DS, & Reiss D (2015). The development of early profiles of temperament: Characterization, continuity, and etiology. Child Development, 86(6), 1794–1811. 10.1111/cdev.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J (1997). Variation in susceptibility to environmental influence: An evolutionary argument. Psychological Inquiry, 8(3), 182–186. 10.1207/s15327965pli0803_3 [DOI] [Google Scholar]
- Belsky J (2005). The developmental and evolutionary psychology of intergenerational transmission of attachment. In Carter CS, Ahnert L, Grossmann KE, Hrdy SB, Lamb ME, Porges SW, & Sachser N (Eds.), Attachment and bonding: A new synthesis (pp. 169–198). Boston Review. [Google Scholar]
- Belsky J (2013). Differential susceptibility to environmental influences. International Journal of Child Care and Education Policy, 7(2), 15–31. 10.1007/2288-6729-7-2-15 [DOI] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, & Davis EP (2011). Prenatal maternal anxiety and early childhood temperament. Stress, 14(6), 644–651. 10.3109/10253890.2011.594121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolten MI, Wurmser H, Buske-Kirschbaum A, Papoušek M, Pirke KM, & Hellhammer D (2011). Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Archives of Women’s Mental Health, 14(1), 33–41. 10.1007/s00737-010-0183-1 [DOI] [PubMed] [Google Scholar]
- Bowers ME, & Yehuda R (2016). Intergenerational transmission of stress in humans. Neuropsychopharmacology, 41(1), 232–244. 10.1038/npp.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KM, Pérez-Edgar K, & Lunkenheimer E (2022). Understanding how child temperament, negative parenting, and dyadic parent–child behavioral variability interact to influence externalizing problems. Social Development, 31(4), 1020–1041. 10.1111/sode.12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, Coleman-Phox K, Wadhwa PD, Laraia BA, Adler NE, & Epel ES (2017). Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Development and Psychopathology, 29(5), 1553–1571. 10.1017/S0954579417001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy J, Marvin RS, & with the MacArthur Working Group. (1992). Attachment organization in preschool children: Procedures and coding manual [Unpublished manuscript] ( pp. 125–131). University of Virginia. [Google Scholar]
- Chan JC, Nugent BM, & Bale TL (2018). Parental advisory: Maternal and paternal stress can impact offspring neurodevelopment. Biological Psychiatry, 83(10), 886–894. 10.1016/j.biopsych.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S, & Thomas A (1977). Temperamental individuality from childhood to adolescence. Journal of the American Academy of Child Psychiatry, 16(2), 218–226. 10.1016/S0002-7138(09)60038-8 [DOI] [PubMed] [Google Scholar]
- Class QA, Khashan AS, Lichtenstein P, Långström N, & D’Onofrio BM (2013). Maternal stress and infant mortality: the importance of the preconception period. Psychological Science, 24(7), 1309–1316. 10.1177/0956797612468010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46(6), 737–746. 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry, 52(2), 119–129. 10.1111/j.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Hankin BL, Swales DA, & Hoffman AMC (2018). An experimental test of the fetal programming hypothesis: Can we reduce child ontogenetic vulnerability to psychopathology by decreasing maternal depression? Development and Psychopathology, 30(3), 787–806. 10.1017/S0954579418000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Narayan AJ (2020). Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Development and Psychopathology, 32(5), 1625–1639. 10.1017/S0954579420001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollar JM, Stifter CA, & Buss KA (2017). Exuberant and inhibited children: Person-centered profiles and links to social adjustment. Developmental Psychology, 53(7), 1222–1229. 10.1037/dev0000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, & Peng CYJ (2013). Principled missing data methods for researchers. SpringerPlus, 2(1), Article 222. 10.1186/2193-1801-2-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie L, & Reynolds RM (2013). Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology, 98(2), 106–115. 10.1159/000354702 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2011). Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology, 23(1), 7–28. 10.1017/S0954579410000611 [DOI] [PubMed] [Google Scholar]
- Enders CK (2001). The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educational and Psychological Measurement, 61(5), 713–740. 10.1177/0013164401615001 [DOI] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. Guilford Press. [Google Scholar]
- Entringer S., Buss C., Shirtcliff EA., Cammack AL., Yim IS., Chicz-DeMet A., Sandman CA., & Wadhwa PD. (2010). Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress, 13(3), 258–268. 10.3109/10253890903349501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, & Wadhwa PD (2012). Prenatal stress, telomere biology, and fetal programming of health and disease risk. Science Signaling, 5(248), Article pt12. 10.1126/scisignal.2003580 [DOI] [PubMed] [Google Scholar]
- Epstein CM, Houfek J, Rice MJ, & Weiss SJ (2021). Integrative review of early life adversity and cortisol regulation in pregnancy. Journal of Obstetric, Gynecologic & Neonatal Nursing, 50(3), 242–255. 10.1016/j.jogn.2020.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang AG (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Ferguson CJ (2016). An effect size primer: A guide for clinicians and researchers. Professional Psychology: Research and Practice, 40(5), 532–538. 10.1037/a0015808 [DOI] [Google Scholar]
- Ferguson SL, Moore EWG, & Hull DM (2020). Finding latent groups in observed data: A primer on latent profile analysis in Mplus for applied researchers. International Journal of Behavioral Development, 44(5), 458–468. 10.1177/0165025419881721 [DOI] [Google Scholar]
- Gartstein MA, Prokasky A, Bell MA, Calkins S, Bridgett DJ, Braungart-Rieker J, Leerkes E, Cheatham CL, Eiden RD, Mize KD, Jones NA, Mireault G, & Seamon E (2017). Latent profile and cluster analysis of infant temperament: Comparisons across person-centered approaches. Developmental Psychology, 53(10), 1811–1825. 10.1037/dev0000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliom M, & Shaw DS (2004). Codevelopment of externalizing and internalizing problems in early childhood. Development and Psychopathology, 16(2), 313–333. 10.1017/S0954579404044530 [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, & O’Donnell K (2010). Prenatal stress and the programming of the HPA axis. Neuroscience & Biobehavioral Reviews, 35(1), 17–22. 10.1016/j.neubiorev.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, & Spencer HG (2005). Predictive adaptive responses and human evolution. Trends in Ecology & Evolution, 20(10), 527–533. 10.1016/j.tree.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH (1996). Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development, 67(1), 218–235. 10.2307/1131697 [DOI] [PubMed] [Google Scholar]
- Gonzales NA, Cauce AM, & Mason CA (1996). Interobserver agreement in the assessment of parental behavior and parent–adolescent conflict: African American mothers, daughters, and independent observers. Child Development, 67(4), 1483–1498. 10.2307/1131713 [DOI] [PubMed] [Google Scholar]
- Guardino CM, Rahal D, Rinne GR, Mahrer NE, Davis EP, Adam EK, Shalowitz MU, Ramey SL, & Schetter CD (2022). Maternal stress and mental health before pregnancy and offspring diurnal cortisol in early childhood. Developmental Psychobiology, 64(7), Article e22314. 10.1002/dev.22314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardino CM, Schetter CD, Saxbe DE, Adam EK, Ramey SL, & Shalowitz MU (2016). Diurnal salivary cortisol patterns prior to pregnancy predict infant birth weight. Health Psychology, 35(6), 625–633. 10.1037/hea0000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantsoo L, Kornfield S, Anguera MC, & Epperson CN (2019). Inflammation: A proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biological Psychiatry, 85(2), 97–106. 10.1016/j.biopsych.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, & Seckl J (2011). Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior, 59(3), 279–289. 10.1016/j.yhbeh.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Hartman S, & Belsky J (2018). Prenatal stress and enhanced developmental plasticity. Journal of Neural Transmission, 125(12), 1759–1779. 10.1007/s00702-018-1926-9 [DOI] [PubMed] [Google Scholar]
- Hartman S, Belsky J, & Pluess M (2023). Prenatal programming of environmental sensitivity. Translational Psychiatry, 13(1), Article 161. 10.1038/s41398-023-02461-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EA, Friedman-Weieneth JL, Miner AL, Bartolomei RJ, Youngwirth SD, Hashim RL, & Arnold DH (2009). The role of ethnicity in observers’ ratings of mother–child behavior. Developmental Psychology, 45(6), 1497–1508. 10.1037/a0017200 [DOI] [PubMed] [Google Scholar]
- Hastings PD, Sullivan C, McShane KE, Coplan RJ, Utendale WT, & Vyncke JD (2008). Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: Direct mothers and moderated fathers. Child Development, 79(1), 45–64. 10.1111/j.1467-8624.2007.01110.x [DOI] [PubMed] [Google Scholar]
- Hentges RF, Graham SA, Plamondon A, Tough S, & Madigan S (2019). A developmental cascade from prenatal stress to child internalizing and externalizing problems. Journal of Pediatric Psychology, 44(9), 1057–1067. 10.1093/jpepsy/jsz044 [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Tung I, Northrup J, & Keenan K (2019). Transgenerational associations between maternal childhood stress exposure and profiles of infant emotional reactivity. Development and Psychopathology, 31(3), 887–898. 10.1017/S0954579419000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland MA, Sandman CA, & Glynn LM (2017). Developmental origins of the human hypothalamic–pituitary–adrenal axis. Expert Review of Endocrinology & Metabolism, 12(5), 321–339. 10.1080/17446651.2017.1356222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, De Medina PGR, Mulder EJH, Visser GHA, & Buitelaar JK (2002). Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 41(9), 1078–1085. 10.1097/00004583-200209000-00008 [DOI] [PubMed] [Google Scholar]
- Huizink AC, & De Rooij SR (2018). Prenatal stress and models explaining risk for psychopathology revisited: Generic vulnerability and divergent pathways. Development and Psychopathology, 30(3), 1041–1062. 10.1017/S0954579418000354 [DOI] [PubMed] [Google Scholar]
- Irwin JL, Meyering AL, Peterson G, Glynn LM, Sandman CA, Hicks LM, & Davis EP (2021). Maternal prenatal cortisol programs the infant hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology, 125, Article 105106. 10.1016/j.psyneuen.2020.105106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, & Wickrama KAS (2008). An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass, 2(1), 302–317. 10.1111/j.1751-9004.2007.00054.x [DOI] [Google Scholar]
- Karam F, Bérard A, Sheehy O, Huneau MC, Briggs G, Chambers C, Einarson A, Johnson D, Kao K, Koren G, Martin B, Polifka JE, Riordan SH, Roth M, Lavigne SV, Wolfe L, Braddock S, Quinn D, Jones KL, … Wisner K (2012). Reliability and validity of the 4-item perceived stress scale among pregnant women: Results from the OTIS antidepressants study. Research in Nursing & Health, 35(4), 363–375. 10.1002/NUR.21482 [DOI] [PubMed] [Google Scholar]
- Kazak AE (2018). Editorial: Journal article reporting standards. American Psychologist, 73(1), 1–2. 10.1037/amp0000263 [DOI] [PubMed] [Google Scholar]
- Kee MZL, Ponmudi S, Phua DY, Rifkin-Graboi A, Chong YS, Tan KH, Chan JKY, Broekman BFP, Chen H, & Meaney MJ (2021). Preconception origins of perinatal maternal mental health. Archives of Women’s Mental Health, 24(4), 605–618. 10.1007/s00737-020-01096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE, Class QA, & Mbayiwa K (2018). Extending the developmental origins of disease model: Impact of preconception stress exposure on offspring neurodevelopment. Developmental Psychobiology, 60(7), 753–764. 10.1002/dev.21773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, & Zalewski M (2011). Nature and nurturing: Parenting in the context of child temperament. Clinical Child and Family Psychology Review, 14(3), 251–301. 10.1007/s10567-011-0093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivlighan KT, DiPietro JA, Costigan KA, & Laudenslager ML (2008). Diurnal rhythm of cortisol during late pregnancy: Associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology, 33(9), 1225–1235. 10.1016/j.psyneuen.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautarescu A, Craig MC, & Glover V (2020). Prenatal stress: Effects on fetal and child brain development. International Review of Neurobiology 150,17–40. 10.1016/bs.irn.2019.11.002 [DOI] [PubMed] [Google Scholar]
- Leerkes EM, Blankson AN, & O’Brien M (2009). Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Development, 80(3), 762–775. 10.1111/j.1467-8624.2009.01296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner JV, & Lerner RM (1994). Explorations of the goodness-of-fit model in early adolescence. In Carey WB & McDevitt SC (Eds.), Prevention and early intervention: Individual differences as risk factors for the mental health of children: A festschrift for Stella Chess and Alexander Thomas (pp. 161–169). Brunner/Mazel. [Google Scholar]
- Lin B, Crnic KA, Luecken LJ, & Gonzales NA (2014). Maternal prenatal stress and infant regulatory capacity in Mexican Americans. Infant Behavior and Development, 37(4), 571–582. 10.1016/j.infbeh.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Ostlund BD, Conradt E, Lagasse LL, & Lester BM (2018). Testing the programming of temperament and psychopathology in two independent samples of children with prenatal substance exposure. Development and Psychopathology, 30(3), 1023–1040. 10.1017/S0954579418000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, & Halfon N (2003). Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal, 7(1), 13–30. 10.1023/A:1022537516969 [DOI] [PubMed] [Google Scholar]
- Lunkenheimer E, Hamby CM, Lobo FM, Cole PM, & Olson SL (2020). The role of dynamic, dyadic parent–child processes in parental socialization of emotion. Developmental Psychology, 56(3), 566–577. 10.1037/dev0000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrer NE, Guardino CM, Hobel C, & Dunkel Schetter C (2021). Maternal stress before conception is associated with shorter gestation. Annals of Behavioral Medicine, 55(3), 242–252. 10.1093/abm/kaaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Development and Psychopathology, 22(3), 491–495. 10.1017/S0954579410000222 [DOI] [PubMed] [Google Scholar]
- Maziade M, Capéraà P, Laplante B, Boudreault M, Thivierge J, Côté R, & Boutin P (1985). Value of difficult temperament among 7-year-olds in the general population for predicting psychiatric diagnosis at age 12. The American Journal of Psychiatry, 142(8), 943–946. 10.1176/ajp.142.8.943 [DOI] [PubMed] [Google Scholar]
- Maziade M, Caron C, Côté R, Mérette C, Bernier H, Laplante B, Boutin P, & Thivierge J (1990). Psychiatric status of adolescents who had extreme temperaments at age 7. The American Journal of Psychiatry, 147(11), 1531–1536. 10.1176/ajp.147.11.1531 [DOI] [PubMed] [Google Scholar]
- McClelland GH, & Judd CM (1993). Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin, 114(2), 376–390. 10.1037/0033-2909.114.2.376 [DOI] [PubMed] [Google Scholar]
- McGowan PO, & Matthews SG (2018). Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology, 159(1), 69–82. 10.1210/en.2017-00896 [DOI] [PubMed] [Google Scholar]
- Monk C, Lugo-Candelas C, & Trumpff C (2019). Prenatal developmental origins of future psychopathology: Mechanisms and pathways. Annual Review of Clinical Psychology, 15(1), 317–344. 10.1146/annurev-clinpsy-050718-095539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus user’s guide (version 8.0). Mplus user’s guide ( pp. 1–950). [Google Scholar]
- National Institute of Child Development. (1993). NICHD study of early child care manual, chapter 29.12. [Google Scholar]
- Nazzari S, & Frigerio A (2020). The programming role of maternal antenatal inflammation on infants’ early neurodevelopment: A review of human studies. Journal of Affective Disorders, 263, 739–746. 10.1016/j.jad.2019.10.010 [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, & Meaney MJ (2017). Fetal origins of mental health: The developmental origins of health and disease hypothesis. American Journal of Psychiatry, 174(4), 319–328. 10.1176/appi.ajp.2016.16020138 [DOI] [PubMed] [Google Scholar]
- Olsson CA, Spry EA, Yvette A, Moreno-Betancur M, Youssef G, Greenwood C, Letcher P, Macdonald JA, McIntosh J, Hutchinson D, & Patton GC (2021). Preconception depression and anxiety symptoms and maternal–infant bonding: A 20-year intergenerational cohort study. Archives of Women’s Mental Health, 24(3), 513–523. 10.1007/s00737-020-01081-5 [DOI] [PubMed] [Google Scholar]
- Ostlund BD, Pérez-Edgar KE, Shisler S, Terrell S, Godleski S, Schuetze P, & Eiden RD (2021). Prenatal substance exposure and maternal hostility from pregnancy to toddlerhood: Associations with temperament profiles at 16 months of age. Development and Psychopathology, 33(5), 1566–1583. 10.1017/S0954579421001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer M, Jennen-Steinmetz C, Esser G, Schmidt MH, & Laucht M (2011). Differential susceptibility to environmental influences: The role of early temperament and parenting in the development of externalizing problems. Comprehensive Psychiatry, 52(6), 650–658. 10.1016/j.comppsych.2010.10.017 [DOI] [PubMed] [Google Scholar]
- Pluess M, & Belsky J (2011). Prenatal programming of postnatal plasticity? Development and Psychopathology, 23(1), 29–38. 10.1017/S0954579410000623 [DOI] [PubMed] [Google Scholar]
- Prokasky A, Rudasill K, Molfese VJ, Putnam S, Gartstein M, & Rothbart M (2017). Identifying child temperament types using cluster analysis in three samples. Journal of Research in Personality, 67, 190–201. 10.1016/j.jrp.2016.10.008 [DOI] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. 10.1016/S0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- Putnam SP, & Rothbart MK (2006). Development of short and very short forms of the Children’s Behavior Questionnaire. Journal of Personality Assessment, 87(1), 102–112. 10.1207/s15327752jpa8701_09 [DOI] [PubMed] [Google Scholar]
- Ramey SL, Schafer P, DeClerque JL, Lanzi RG, Hobel C, Shalowitz M, Chinchilli V, & Raju TNK (2015). The preconception stress and resiliency pathways model: A multi-level framework on maternal, paternal, and child health disparities derived by community-based participatory research. Maternal and Child Health Journal, 19(4), 707–719. 10.1007/s10995-014-1581-1 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. Foundation for Statistical Computing. [Google Scholar]
- Rinne GR, Carroll JE, Guardino CM, Shalowitz MU, Ramey SL, & Schetter CD (2023). Parental preconception posttraumatic stress symptoms and maternal prenatal inflammation prospectively predict shorter telomere length in children. Psychosomatic Medicine. Advance online publication. 10.1097/PSY.0000000000001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne GR, Hartstein J, Guardino CM, & Dunkel Schetter C (2023). Stress before conception and during pregnancy and maternal cortisol during pregnancy: A scoping review. Psychoneuroendocrinology, 153, Article 106115. 10.1016/j.psyneuen.2023.106115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Obst S, Teague SJ, Rossen L, Spry EA, Macdonald JA, Sunderland M, Olsson CA, Youssef G, & Hutchinson D (2020). Association between maternal perinatal depression and anxiety and child and adolescent development: A meta-analysis. JAMA Pediatrics, 174(11), 1082–1092. 10.1001/jamapediatrics.2020.2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, & Evans DE (2000). Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology, 78(1), 122–135. 10.1037/0022-3514.78.1.122 [DOI] [PubMed] [Google Scholar]
- Rothbart MK, & Posner MI (2006). Temperament, attention, and developmental psychopathology. In Cicchetti D & Cohen DJ (Eds.), Developmental psychopathology: Developmental neuroscience (pp. 465–501). John Wiley & Sons. https://psycnet.apa.org/record/2006-03610-011 [Google Scholar]
- Rothbart MK, & Putnam SP (2002). Temperament and socialization. In Pulkkinen L & Caspi A (Eds.), Paths to successful development: Personality in the life course (pp. 19–45). Cambridge University Press. https://books.google.com/books?hl=en&lr=&id=IcxFeiSD8xMC&oi=fnd&pg=PR9&ots=q3Be53-snF&sig=DufljqmoPEdzpY_lrNZcoGnMLrY [Google Scholar]
- Rubin KH., Burgess KB., Dwyer KM., & Hastings PD. (2003). Predicting preschoolers’ externalizing behaviors from toddler temperament, conflict, and maternal negativity. Developmental Psychology, 39(1), 164–176. 10.1037/0012-1649.39.1.164 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, & Glynn LM (2012). Prescient human fetuses thrive. Psychological Science, 23(1), 93–100. 10.1177/0956797611422073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BG, Lemery-Chalfant K, Clifford S, Tein JY, Stoll R, & Goldsmith HH (2016). A twin factor mixture modeling approach to childhood temperament: Differential heritability. Child Development, 87(6), 1940–1955. 10.1111/cdev.12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner RL, Buss KA, McClowry SG, Putnam SP, Saudino KJ, & Zentner M (2012). What is temperament now? Assessing progress in temperament research on the twenty-fifth anniversary of Goldsmith et al. (). Child Development Perspectives, 6(4), 436–444. 10.1111/j.1750-8606.2012.00254.x [DOI] [Google Scholar]
- Slagt M, Dubas JS, Deković M., & van Aken MAG. (2016). Differences in sensitivity to parenting depending on child temperament: A meta-analysis. Psychological Bulletin, 142(10), 1068–1110. 10.1037/bul0000061 [DOI] [PubMed] [Google Scholar]
- Spry E, Moreno-Betancur M, Becker D, Romaniuk H, Carlin JB, Molyneaux E, Howard LM, Ryan J, Letcher P, McIntosh J, Macdonald JA, Greenwood CJ, Thomson KC, McAnally H, Hancox R, Hutchinson DM, Youssef GJ, Olsson CA, & Patton GC (2020). Maternal mental health and infant emotional reactivity: A 20-year two-cohort study of preconception and perinatal exposures. Psychological Medicine, 50(5), 827–837. 10.1017/S0033291719000709 [DOI] [PubMed] [Google Scholar]
- Spry EA, Letcher P, Patton GC, Sanson AV, & Olsson CA (2022). The developmental origins of stress reactivity: An intergenerational life-course perspective. Current Opinion in Behavioral Sciences, 43, 187–192. 10.1016/j.cobeha.2021.10.005 [DOI] [Google Scholar]
- Stephenson J, Heslehurst N, Hall J, Schoenaker DAJM, Hutchinson J, Cade JE, Poston L, Barrett G, Crozier SR, Barker M, Kumaran K, Yajnik CS, Baird J, & Mishra GD (2018). Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. The Lancet, 391(10132), 1830–1841. 10.1016/S0140-6736(18)30311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz S, Beijers R, Smeekens S, & Deković M. (2017). Diathesis stress or differential susceptibility? Testing longitudinal associations between parenting, temperament, and children’s problem behavior. Social Development, 26(4), 783–796. 10.1111/sode.12237 [DOI] [Google Scholar]
- Swales DA, Davis EP, Mahrer NE, Guardino CM, Shalowitz MU, Ramey SL, & Schetter CD (2023). Preconception maternal posttraumatic stress and child negative affectivity: Prospectively evaluating the intergenerational impact of trauma. Development and Psychopathology, 35(2), 619–629. 10.1017/S0954579421001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tein JY, Coxe S, & Cham H (2013). Statistical power to detect the correct number of classes in latent profile analysis. Structural Equation Modeling: a Multidisciplinary Journal, 20(4), 640–657. 10.1080/10705511.2013.824781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, & Schwab M (2020). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience & Biobehavioral Reviews, 117, 26–64. 10.1016/J.NEUBIOREV.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Werner E, Zhao Y, Evans L, Kinsella M, Kurzius L, Altincatal A, Mcdonough L, & Monk C (2013). Higher maternal prenatal cortisol and younger age predict greater infant reactivity to novelty at 4 months: An observation based study. Developmental Psychobiology, 55(7), 707–718. 10.1002/dev.21066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Harrington KM, Clark SL, & Miller MW (2013). Sample size requirements for structural equation models: An evaluation of power, bias, and solution propriety. Educational and Psychological Measurement, 73(6), 913–934. 10.1177/0013164413495237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurpts IC, & Geiser C (2014). Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a monte-carlo study. Frontiers in Psychology, 5, Article 920. 10.3389/fpsyg.2014.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui M, & Dishion TJ (2008). Direct observation of family management: Validity and reliability as a function of coder ethnicity and training. Behavior Therapy, 39(4), 336–347. 10.1016/j.beth.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau I, & Dolan S (2000). Low cortisol and risk for PTSD in adult offspring of holocaust survivors. American Journal of Psychiatry, 157(8), 1252–1259. 10.1176/appi.ajp.157.8.1252 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, Flory JD, Bierer LM, & Meaney MJ (2014). Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. American Journal of Psychiatry, 171(8), 872–880. 10.1176/appi.ajp.2014.13121571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehud R., & Lehrne A. (2018). Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry, 17(3), 243–257. 10.1002/wps.20568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans MA, Riksen-Walraven JM, & de Weerth C (2015). Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neuroscience & Biobehavioral Reviews, 53, 1–24. 10.1016/J.NEUBIOREV.2015.02.015 [DOI] [PubMed] [Google Scholar]
- Zuckerman M (1999). Diathesis-stress models. In Zuckerman M (Eds.), Vulnerability to psychopathology: A biosocial model (pp. 3–23). American Psychological Association. 10.1037/10316-001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


