Abstract
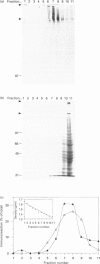
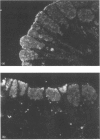
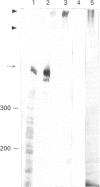
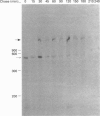
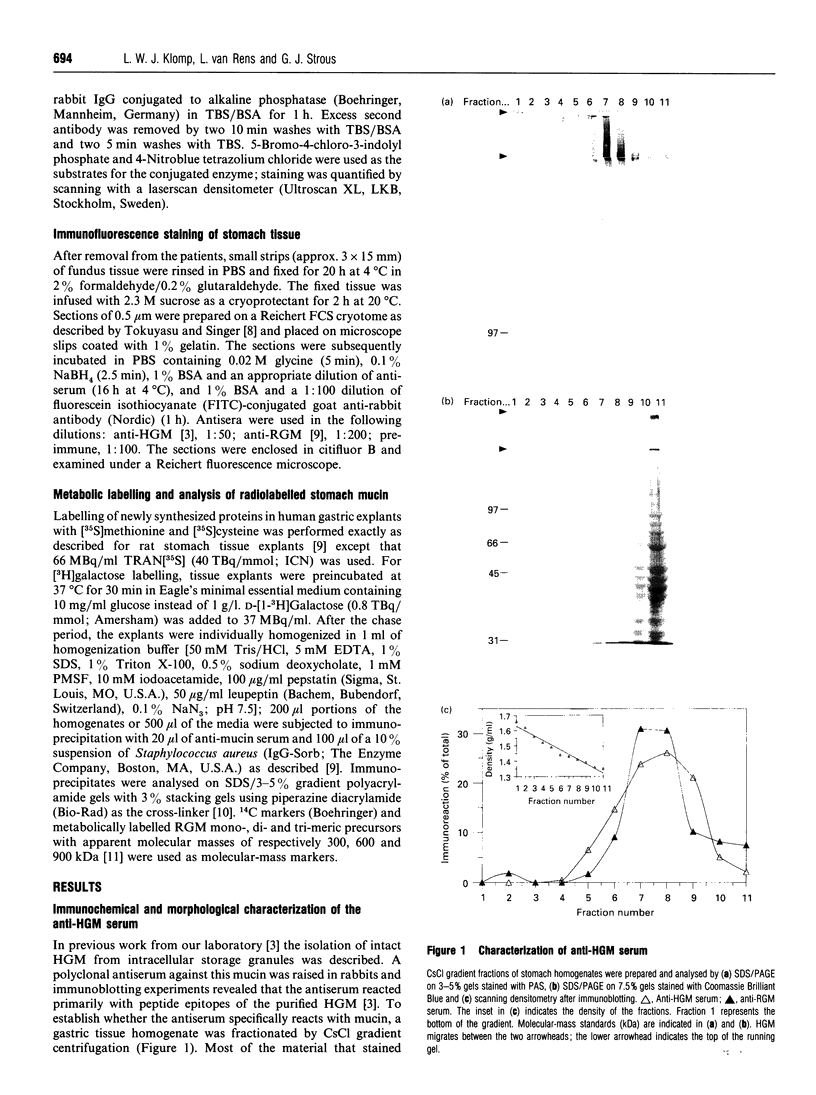
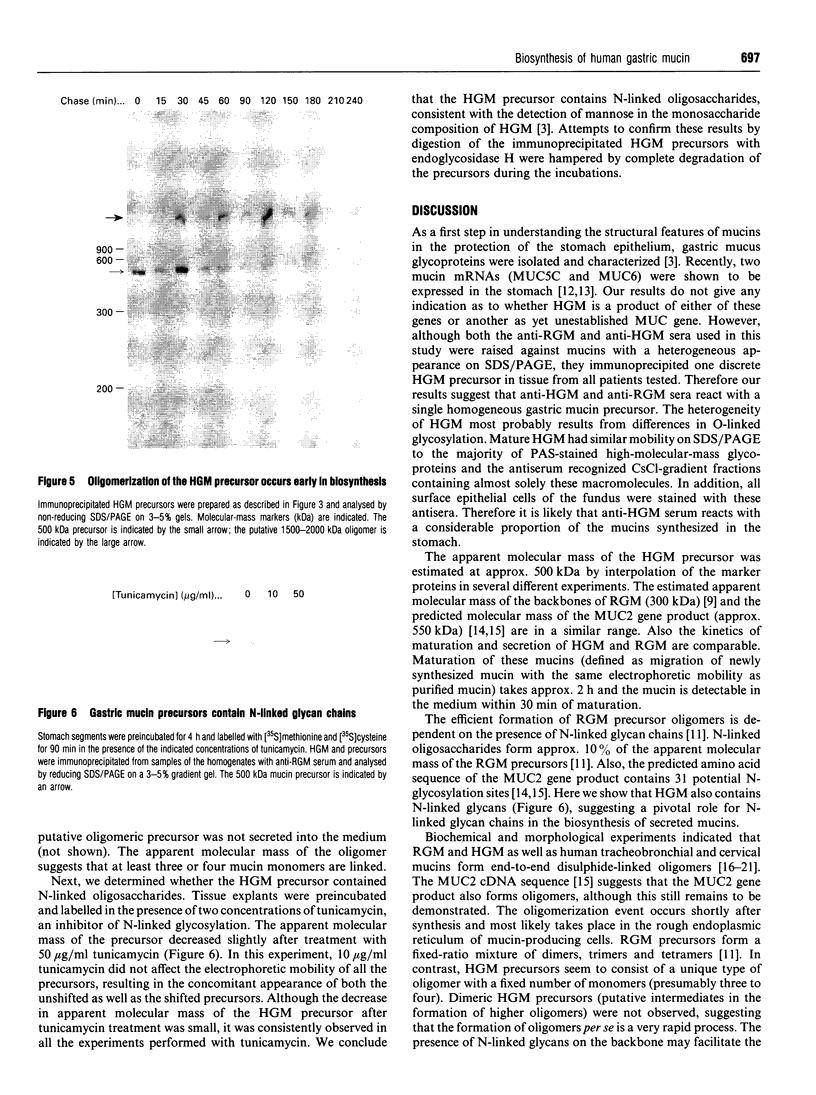
Gastric mucin plays an important role in the protection of the stomach wall from chemical, microbiological and mechanical damage. We have previously isolated human gastric mucus glycoproteins and raised a polyclonal antiserum against these macromolecules. This antiserum specifically reacted with gastric mucins in immunoblotting experiments and stained mucous granules at the apical side of gastric surface epithelial cells. A similar staining pattern was obtained after incubation with an antiserum against rat gastric mucin. Next we used the antiserum in pulse-chase experiments of human stomach tissue explants. After short labelling periods with [35S]methionine and [35S]cysteine, the antiserum reacted with a polypeptide with an apparent molecular mass of approx. 500 kDa as determined by SDS/PAGE, which was converted after 90 min into a heterogeneous high-molecular-mass glycoprotein. This high-molecular-mass form, but not the 500 kDa polypeptide, was detectable in the culture medium after 2 h. This strongly suggests that the 500 kDa polypeptide is the precursor of the purified gastric mucin. Analysis of pulse-chase experiments by non-reducing SDS/PAGE revealed that the precursors form disulphide-linked oligomers early in biosynthesis, before the addition of O-linked sugars. After preincubation with the N-glycosylation inhibitor, tunicamycin, the apparent molecular mass of the precursor decreased marginally but consistently, indicating that N-linked glycan chains are present on the mucin precursor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978 Jan;34(1):28–33. [PubMed] [Google Scholar]
- Audie J. P., Janin A., Porchet N., Copin M. C., Gosselin B., Aubert J. P. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found Symp. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Dekker J., Aelmans P. H., Strous G. J. The oligomeric structure of rat and human gastric mucins. Biochem J. 1991 Jul 15;277(Pt 2):423–427. doi: 10.1042/bj2770423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Strous G. J. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent, and precedes initial O-glycosylation. J Biol Chem. 1990 Oct 25;265(30):18116–18122. [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Strous G. J. Biosynthesis of gastric mucus glycoprotein of the rat. J Biol Chem. 1989 Jun 25;264(18):10431–10437. [PubMed] [Google Scholar]
- Dekker J., van der Ende A., Aelmans P. H., Strous G. J. Rat gastric mucin is synthesized and secreted exclusively as filamentous oligomers. Biochem J. 1991 Oct 1;279(Pt 1):251–256. doi: 10.1042/bj2790251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether J. K., Harris J. A., Neutra R., Kizer K. W. Exposure to aerial malathion application and the occurrence of congenital anomalies and low birthweight. Am J Public Health. 1987 Aug;77(8):1009–1010. doi: 10.2105/ajph.77.8.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Rothe E. M., Lagace R. E., Kim Y. S. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992 Oct 25;267(30):21375–21383. [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Siddiki B., Kim Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994 Jan 28;269(4):2440–2446. [PubMed] [Google Scholar]
- Harding S. E., Rowe A. J., Creeth J. M. Further evidence for a flexible and highly expanded spheroidal model for mucus glycoproteins in solution. Biochem J. 1983 Mar 1;209(3):893–896. doi: 10.1042/bj2090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I. Electron microscopy of cervical-mucus glycoproteins and fragments therefrom. The use of colloidal gold to make visible 'naked' protein regions. Biochem J. 1990 Jan 1;265(1):169–177. doi: 10.1042/bj2650169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Oates K., Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem J. 1986 Oct 1;239(1):147–153. doi: 10.1042/bj2390147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Davies J. R., Kraayenbrink M., Richardson P. S., Sheehan J. K., Carlstedt I. Mucus glycoproteins from 'normal' human tracheobronchial secretion. Biochem J. 1990 Jan 1;265(1):179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribara N. W., Roberton A. M., Ho S. B., Kuo W. L., Gum E., Hicks J. W., Gum J. R., Jr, Byrd J. C., Siddiki B., Kim Y. S. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993 Mar 15;268(8):5879–5885. [PubMed] [Google Scholar]