Abstract
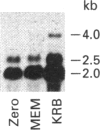
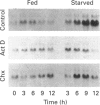
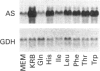
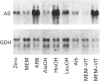
A full-length cDNA clone for rat asparagine synthetase (AS) was isolated from a cDNA library enriched for amino acid-regulated sequences. The AS cDNA was used to investigate the amino acid-dependent repression of AS mRNA content in rat Fao hepatoma cells. In response to complete amino acid starvation, there was an approximately 10-fold increase in the level of AS mRNA. Three species of mRNA, of approx. sizes 2.0, 2.5 and 4.0 kb, were detected and each was simultaneously regulated to the same degree. The expression of AS mRNA increased by 6 h after removal of amino acids, reached a plateau after 9 h, and was blocked by either actinomycin D or cycloheximide. Partial repression of the AS mRNA content was maintained by the presence of a single amino acid in the culture medium, but the degree of effectiveness for each one varied widely. Glutamine showed the greatest ability to repress the AS mRNA content, even at an extracellular concentration 10 times below its plasma level. Other effective repressors included the amino acids asparagine, histidine and leucine, as well as ammonia. Depletion of selected single amino acids from an otherwise complete culture medium also caused up-regulation. In particular, removal of histidine, threonine or tryptophan from the medium, or the addition of histidinol to inhibit histidinyl-tRNA synthetase, resulted in a significant increase in AS mRNA content. The data indicate that nutrient regulation of AS mRNA occurs by a general control mechanism that is responsive to a spectrum of amino acids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Miller P. F., Jackson B. M., Hinnebusch A. G. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis for GCN4 translational control. Mol Cell Biol. 1991 Jan;11(1):486–496. doi: 10.1128/mcb.11.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrulis I. L., Chen J., Ray P. N. Isolation of human cDNAs for asparagine synthetase and expression in Jensen rat sarcoma cells. Mol Cell Biol. 1987 Jul;7(7):2435–2443. doi: 10.1128/mcb.7.7.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrulis I. L., Hatfield G. W., Arfin S. M. Asparaginyl-tRNA aminoacylation levels and asparagine synthetase expression in cultured Chinese hamster ovary cells. J Biol Chem. 1979 Nov 10;254(21):10629–10633. [PMC free article] [PubMed] [Google Scholar]
- Bracy D. S., Handlogten M. E., Barber E. F., Han H. P., Kilberg M. S. Cis-inhibition, trans-inhibition, and repression of hepatic amino acid transport mediated by System A. Substrate specificity and other properties. J Biol Chem. 1986 Feb 5;261(4):1514–1520. [PubMed] [Google Scholar]
- Bremner I., Beattie J. H. Metallothionein and the trace minerals. Annu Rev Nutr. 1990;10:63–83. doi: 10.1146/annurev.nu.10.070190.000431. [DOI] [PubMed] [Google Scholar]
- Cassio D., Lemoine F., Waller J. P., Sandrin E., Boissonnas R. A. Selective inhibition of aminoacyl ribonucleic acid synthetases by aminoalkyl adenylates. Biochemistry. 1967 Mar;6(3):827–836. doi: 10.1021/bi00855a024. [DOI] [PubMed] [Google Scholar]
- Chen Z. P., Chen K. Y. Mechanism of regulation of ornithine decarboxylase gene expression by asparagine in a variant mouse neuroblastoma cell line. J Biol Chem. 1992 Apr 5;267(10):6946–6951. [PubMed] [Google Scholar]
- Chiles T. C., Laine R. O., Shay N. F., Handlogten M. E., Nick H. S., Kilberg M. S. Enhanced mRNA content in response to amino acid starvation for a 73 kDa protein of the inner mitochondrial membrane. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1068–1075. doi: 10.1006/bbrc.1993.1734. [DOI] [PubMed] [Google Scholar]
- Chiles T. C., O'Brien T. W., Kilberg M. S. Production of monospecific antibodies to a low-abundance hepatic membrane protein using nitrocellulose immobilized protein as antigen. Anal Biochem. 1987 May 15;163(1):136–142. doi: 10.1016/0003-2697(87)90103-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. D., Abraham S. Gene expression: nutrient control of pre- and posttranscriptional events. FASEB J. 1992 Oct;6(13):3146–3152. doi: 10.1096/fasebj.6.13.1397836. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Metherall J. E., Ridgway N. D., Brown M. S., Goldstein J. L. Genetic distinction between sterol-mediated transcriptional and posttranscriptional control of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1991 May 15;266(14):9128–9134. [PubMed] [Google Scholar]
- Dever T. E., Feng L., Wek R. C., Cigan A. M., Donahue T. F., Hinnebusch A. G. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992 Feb 7;68(3):585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Gong S. S., Basilico C. A mammalian temperature-sensitive mutation affecting G1 progression results from a single amino acid substitution in asparagine synthetase. Nucleic Acids Res. 1990 Jun 25;18(12):3509–3513. doi: 10.1093/nar/18.12.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S. S., Guerrini L., Basilico C. Regulation of asparagine synthetase gene expression by amino acid starvation. Mol Cell Biol. 1991 Dec;11(12):6059–6066. doi: 10.1128/mcb.11.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco A., Gong S. S., Ittmann M., Basilico C. Organization and expression of the cell cycle gene, ts11, that encodes asparagine synthetase. Mol Cell Biol. 1989 Jun;9(6):2350–2359. doi: 10.1128/mcb.9.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco A., Ittmann M., Basilico C. Molecular cloning of a gene that is necessary for G1 progression in mammalian cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1565–1569. doi: 10.1073/pnas.84.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini L., Gong S. S., Mangasarian K., Basilico C. Cis- and trans-acting elements involved in amino acid regulation of asparagine synthetase gene expression. Mol Cell Biol. 1993 Jun;13(6):3202–3212. doi: 10.1128/mcb.13.6.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B. S., Vaughan M. H., Wang L. Reversible inhibition by histidinol of protein synthesis in human cells at the activation of histidine. J Biol Chem. 1972 Jun 25;247(12):3854–3857. [PubMed] [Google Scholar]
- Hinnebusch A. G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988 Jun;52(2):248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo S., Fujimori M., Shioda S., Nakai Y., Takeda M., Sato T. Immunochemical characterization of rat testicular asparagine synthetase. Arch Biochem Biophys. 1992 May 15;295(1):120–125. doi: 10.1016/0003-9861(92)90496-j. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell. 1985 Nov;43(1):177–188. doi: 10.1016/0092-8674(85)90022-4. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Hutson R. G., Laine R. O. Amino acid-regulated gene expression in eukaryotic cells. FASEB J. 1994 Jan;8(1):13–19. doi: 10.1096/fasebj.8.1.8299885. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Lee A. S. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992 Apr;4(2):267–273. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- Marten N. W., Burke E. J., Hayden J. M., Straus D. S. Effect of amino acid limitation on the expression of 19 genes in rat hepatoma cells. FASEB J. 1994 May;8(8):538–544. doi: 10.1096/fasebj.8.8.8181673. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Fujioka M., Su Y., Kanamoto R., Pitot H. C. Nutritional regulation and tissue-specific expression of the serine dehydratase gene in rat. J Biol Chem. 1991 Oct 25;266(30):20412–20417. [PubMed] [Google Scholar]
- Paluh J. L., Orbach M. J., Legerton T. L., Yanofsky C. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3728–3732. doi: 10.1073/pnas.85.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay N. F., Nick H. S., Kilberg M. S. Molecular cloning of an amino acid-regulated mRNA (amino acid starvation-induced) in rat hepatoma cells. J Biol Chem. 1990 Oct 15;265(29):17844–17848. [PubMed] [Google Scholar]
- Sheng S., Moraga D. A., Van Heeke G., Schuster S. M. High-level expression of human asparagine synthetase and production of monoclonal antibodies for enzyme purification. Protein Expr Purif. 1992 Aug;3(4):337–346. doi: 10.1016/1046-5928(92)90010-t. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Mattes P. M., Jayme D. W., Oxender D. L. Regulation of amino acid transport system L in Chinese hamster ovary cells. J Biol Chem. 1982 Mar 25;257(6):2974–2980. [PubMed] [Google Scholar]