Abstract
Technical innovation in neuroscience introduced powerful tools for measuring and manipulating neuronal activity via optical, chemogenetic, and calcium imaging tools. In this review we consider how these tools may work differently in males and females. We review sex differences in the metabolism of chemogenetic ligands and their downstream signaling effects. Optical tools more directly alter depolarization or hyperpolarization of neurons, but biological sex and gonadal hormones modulate synaptic inputs and intrinsic excitability. Optogenetic manipulations can be consistent across the rodent estrous cycle but within certain circuits, manipulations can vary across the cycle. Finally, calcium imaging methods could be affected by testosterone and estradiol, which can directly modulate calcium influx. Together, our findings suggest that these neuroscientific tools can work differently in males and females and that users should be aware of these differences when applying these methods in males and females.
1. Introduction
The introduction of in vivo calcium imaging, optogenetics, and chemogenetics [1–3] brought circuit- and cell type-specific approaches to behavioral neuroscience [4,5]. These tools were primarily developed in male rodents. With increasing awareness of the importance of including both males and females in neuroscience studies [6], these tools are increasingly used to study neural circuits in both sexes. These tools tap into the fundamental properties of neural function, so it is usually assumed they will operate similarly in both sexes. However, this may not always be a valid assumption. Sex differences in pharmacokinetics and signal transduction can impact the performance of chemogenetic tools. Steroid hormones such as estradiol, progesterone, and testosterone can modulate synaptic inputs and neuronal excitability that could affect optogenetic tools. Finally, steroid hormones can alter the function of calcium channels that could influence the performance of genetically encoded calcium indicators. We review the extent to which these methods are impacted by gonadal hormones and discuss recommendations for assessing the significance of these effects.
2. Chemogenetics
Chemogenetic methods modulate neural activity using genetically modified G-protein receptors and a ligand to activate them [7]. Engineered human muscarinic receptors hM3Dq (excitatory) and hM4Di (inhibitory) are activated by clozapine N-oxide (CNO) or compound 21. A second class of receptors was constructed based on kappa opioid receptors (KORD) that are activated by salvinorin A [8]. Combining hM3Dq and KORD allows for activating and inhibiting different populations of cells in the same animal. Both systems rely on a ligand and G-protein signal transduction to alter neuronal activity. Sex differences in pharmacokinetics and G-protein signal transduction could impact the performance of these tools.
2.1. Sex differences in pharmacokinetics
It is now more widely appreciated that CNO is metabolized to clozapine, which has psychoactive properties. There is strong evidence for sex differences in clozapine pharmacokinetics [9]. Women generally have higher blood levels of clozapine than men when given the same dose, and women require lower doses for therapeutic effects. These differences may be driven by CYP1A2, an enzyme with higher activity in men versus women [10] and female rodents [11]. In both rats and mice, CNO is rapidly converted clozapine [12] which is further back metabolized to CNO or the pharmacologically active metabolite N-desmethylclozapine (NDMC). Some evidence suggests that clozapine is the key ligand for hM3Dq or hM3Di activation [7]. Together, these results suggest that the same dose of CNO could have different efficacies in males and females, and that investigators should consider using lower doses of CNO for females than males (Fig. 1).
Figure 1: Sex difference in pharmacokinetics and signal transduction: implications for chemogenetics.
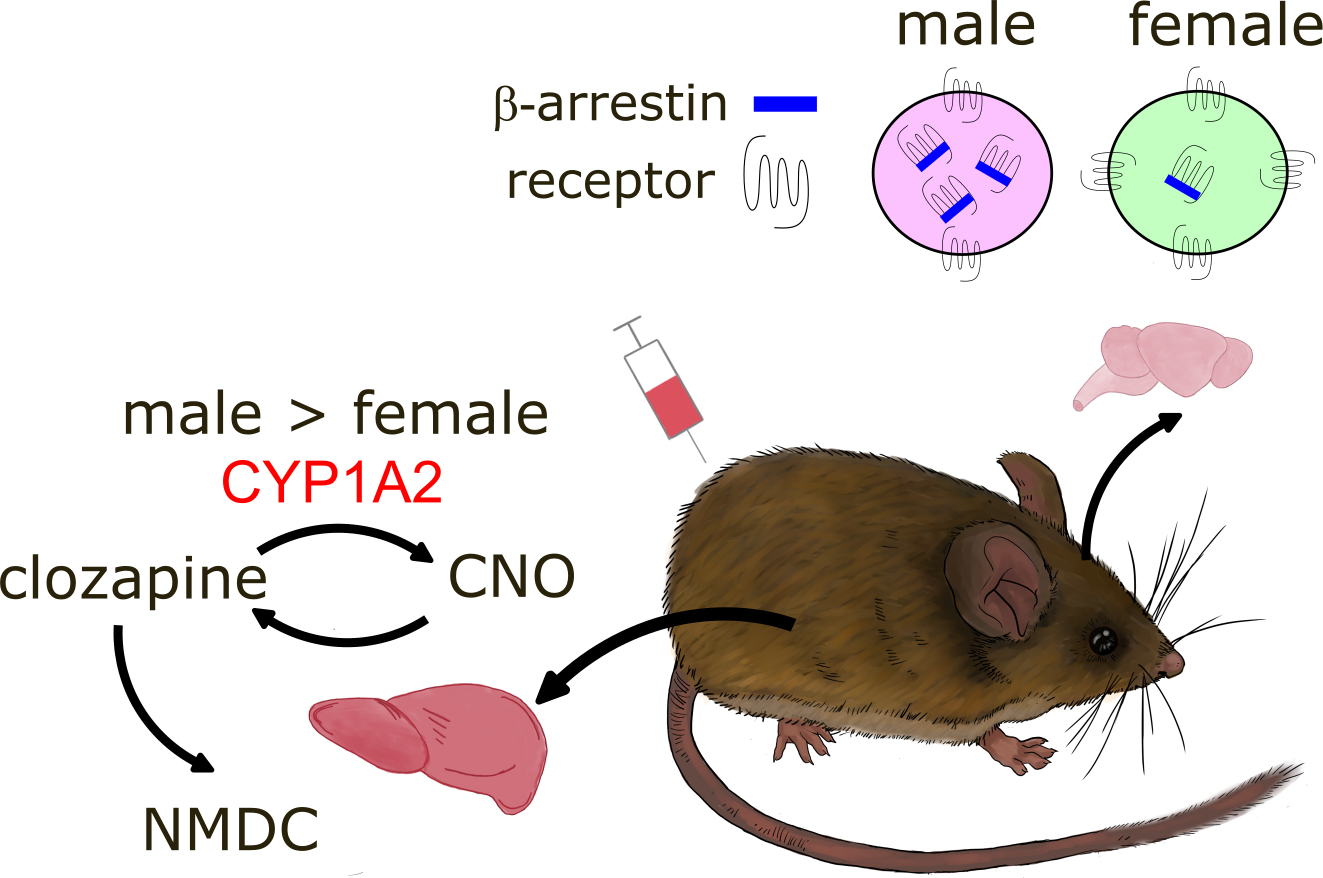
Metabolism of ligands within the liver is different in males versus females. CYP1A2, a key enzyme in the metabolism of CNO, has higher activity in males versus females, and thus lower dosages may be more optimal for females. Sex differences have also been identified in β-arrestin regulation of corticotrophin releasing hormone (CRH) receptors which, like most chemogenetic receptors, are G-protein-coupled receptors. After stress exposure, males showed more receptor internalization than females. If this effect generalized to females, chemogenetic stimulation would be expected to be weaker in females. Mouse drawing by Pei Luo.
A second generation of ligands for hM3Dq/hM4Di, compound 21 (C21) has been put forth as an alternative ligand [13]. C21 has better brain penetrance than CNO [14] but initial characterizations were performed primarily in male rodents [15]. There may be important sex differences in C21 efficacy. While C21 inactivated dopamine neurons expressing hM4Di at 0.5 mg/kg in both male and female mice, this same dose inactivated dopamine neurons in females that did not express hM4Di [16]. In males, 1 mg/kg of C21 inactivated dopamine neurons in mice that did not express hM4Di. Off-target effects of C21 could be mediated by serotoninergic 5-HT2 receptors [17] that have higher efficacy in female rodents [18]. Little is known about the metabolism of C21 and whether off target effects occur via direct C21 action or metabolites. Together, off-target effects of C21 may be more prevalent in females, so investigators should use the lowest dose possible.
2.2. Sex differences in signal transduction
There is little direct evidence for sex differences in muscarinic receptor signal transduction, but sex differences in β-arrestin regulation of G-protein-coupled receptors have been identified. In the locus corelleus (LC), stress enhanced the expression of β-arrestin in males but not females, and this was associated with increased internalization of corticotropin releasing hormone (CRH) receptor 1 in males [19]. Intriguingly, in CRH overexpressing mice, discharge rates of LC neurons were lower in males than females [20], suggesting that CRH receptor internalization reduces the sensitivity of male neurons to CRH. Muscarinic receptors can be internalized by β-arrestin [21], and the impact of internalization after repeated stimulation by CNO has been considered [22]. However, it is unknown whether chemogenetic receptors are more rapidly internalized in males than in females, possibly resulting in stronger effects of chemogenetic stimulation in females (Fig. 1).
Most studies using KORD have used only male rodents [23,24]. However, recent studies observed sex differences in effects of kappa opioid receptor ligands [25,26], even though their pharmacokinetics in males and females are similar [27,28]. In males, a key mechanism for the kappa antagonist norBNI to inhibit the action of kappa opioid receptors is the activation of c-Jun N-terminal kinase [29]. In females, norBNI fails to activate c-Jun N-terminal kinase in the brain [27]. While kappa agonists phosphorylate extracellular regulated kinase (ERK) in males, this effect was absent in females [30]. In females, high estradiol levels enhance activation of the inhibitory G protein-coupled receptor kinase 2. When G protein-coupled receptor kinase 2 was experimentally inhibited in female mice, kappa opioid receptor activation of ERK was restored. Thus, there are important sex differences in signal transduction of kappa opioid receptor. It is unknown if these mechanisms impact the performance of KORD. One study used KORD to examine cognitive function in ovariectomized female mice [31], which would reduce the influence of estrogens on G protein-coupled receptor kinase 2 signaling. A recent report used KORD to target prelimbic somatostatin neurons in an alcohol self-administration paradigm [32], and the authors found that Salvinorin B had similar effects on both neural activity and alcohol self-administration in male and female mice. Thus, while sex differences in signal transduction could affect the performance of KORD, at least some circuits appear to be unaffected. Future studies should consider the possibility that KORD-based inhibition of neural circuits could be less efficacious in females than in males.
2.3. Summary
Chemogenetic tools are useful for studying neural circuits, but investigators should be cautious when interpreting sex differences in chemogenetic studies. Although sex differences within this technique could reflect real mechanistic differences in the regulation of behavior, they could also be driven by sex differences in pharmacokinetics or signal transduction. Ligand dosages should be optimized separately for males and females, and investigators should assess potential off-target effects in both sexes rather than assuming an effective dose in males will behave similarly in females. Reporting male and female data separately (rather than pooling), will also help in the evaluation of the effectiveness of chemogenetic manipulations. If sex differences in a chemogenetic experiment outcomes are observed, in vivo or ex vivo assessments of neural activity in both sexes could help determine whether sex differences are driven by signal transduction or broader biological function. Overall, verifying sex differences with other methods and performing dose response curves with ligands will add rigor to the interpretation of chemogenetic data from males and females.
3. Optogenetics
Optogenetic approaches differ from chemogenetic methods in that these tools directly depolarize or hyperpolarize neurons to modulate their activity. Steroid hormones can modulate neuronal excitability and synaptic inputs, suggesting that optogenetic approaches could function differently in males and females.
3.1. Estrogens
One of the first discoveries of hormone-regulation of neuronal function was the regulation of dendritic spines. Increased spine density increases sensitivity to glutamatergic input, thus potentially exaggerating the impact of excitatory optogenetic stimulation and impeding inhibitory stimulation. Spine density in the CA1 increases during proestrus, when estradiol levels increase, and decreases during diestrus when estradiol levels decrease [33]. These changes are estrogen-dependent in hippocampus [34] and other brain areas [35,36]. Estradiol also enhances excitatory post-synaptic potentials and potentiation in CA1 pyramidal neurons in male [37] and female [38] rats. Some of these effects may be mediated by changes in spine morphology. Immature spines usually have fewer AMPA-type glutamate receptors, and these “silent synapses” are associated with weak or no postsynaptic depolarization [39]. Mushroom spines have wider spine heads that anchor AMPA receptors and facilitate depolarization. In the CA1, thin spines are more abundant during estrus while mushroom spines were more abundant during diestrus and proestrus [40]. Estrogens can also alter intrinsic membrane excitability, by increasing the sensitivity of CA1 pyramidal neurons to a depolarizing current [41,42]. In the dorsal striatum, medium spiny neurons in dorsal striatum also had decreased excitability in male rats compared to females [43]. Furthermore, evidence from other studies suggests that estradiol may influence inhibitory synaptic transmission. In vitro and in vivo studies with young and adult rats demonstrate that estradiol works to suppress GABAergic inhibition through sex-specific systems [44–46]. This suppression occurs through estrogen receptor α (ERα) that triggers activation of inositol tripohosphate (IP3), which exists at higher levels in females than males. This activates the IP3 receptor and ultimately leads to endocannabinoid release. Interestingly, this system is regulated via estradiol in females but not males [44]. When it comes to learning in the hippocampus, estradiol also suppresses inhibitory transmission onto CA1 pyramidal cells [45], which allows for estradiol to potentiate excitatory transmission. Interestingly, only females require cAMP-activated protein kinase. In the same study, it was found that females but not males required not only a release of internal calcium stores but also L-type channel activation, which facilitates extracellular calcium influx [46].
3.2. Androgens
Androgens, like estrogens, modulate both synaptic function and intrinsic neuronal excitability. However, androgens have more variable effects on neurophysiology and the basis for this variability is still unclear. The non-aromatizable androgen dihydrotestosterone (DHT, which binds androgen receptors) increases spine density in ex vivo hippocampal slices [47] and in vivo [48] with similar effects observed in medial PFC [49]. However, chronic DHT treatment reduced the efficacy of high concentrations of NMDA on irreversible depolarization [50]. The complexity of androgens on synaptic function extends to their effects on intrinsic excitability. In dorsal hippocampus, male gonadectomy reduced excitatory post-synaptic potential amplitudes [51] and action potentials generated from injected current spike induction [52]. These effects were reversed by testosterone treatment. Systemic administration of the androgen receptor antagonist flutamide also reduced measures of intrinsic excitability in dorsal hippocampus. Androgens can also have an organizational role in synaptic transmission. Although post-natal androgen treatment had no effect on hippocampal spine density in female rats, it sensitized females to stress in adulthood. Androgenized females exposed to restraint stress as adults had more apical and basal dendrites in CA1 compared to females that were treated with post-natal androgen [53]. Post-natal androgen treatment also alters effects of stress in an eyeblink conditioning task, reducing conditioned responses compared to normally cycling females [54] Androgens also modulate neuronal function in ventral hippocampus.
A population of ventral hippocampal CA1 neurons that project to nucleus accumbens (vHPC-NAc) was less excitable in male mice than in females [55]. Male gonadectomy increased the excitability of vHPC-NAc neurons and testosterone implants reversed this effect. Excitability also increased when vHPC-NAc neurons from males were treated with bath application of flutamide. Currently, it is unknown why androgens enhance neuronal excitability in dorsal hippocampus and decrease excitability in ventral hippocampus. One factor that could be involved is homeostatic scaling, which refers to the extent to which neurons adjust their intrinsic signaling properties to avoid hyper- or hypo-activity/excitation [56]. Homeostatic scaling can be achieved by altering excitatory inputs or through changing intrinsic membrane excitability. Thus, testosterone may increase the excitatory drive onto CA1 neurons while simultaneously reducing intrinsic membrane excitability as part of a homeostatic mechanism that regulates neuronal networks. While these mechanisms are not fully understood, androgens have important effects on neuronal activity that that vary by region, circuit, or cell type.
3.3. Progesterone
Similar to androgens and estrogens, progestins modulate neuronal activity but do so through different mechanisms. Rather than targeting dendritic spines or intrinsic membrane excitability, progestins rapidly inhibit neuronal activity by allosteric binding to GABAA receptors, particularly receptors containing the δ subunit in place of the γ subunit [57]. Intriguingly, receptors expressing the δ subunit are primarily located outside of the synapse where they mediate persistent, or tonic, inhibition [58]. Thus, increased tonic inhibition of neural activity would be expected to be present under conditions of high endogenous progesterone such as late diestrus or pregnancy. This is best described in rodent seizure models. For example, in late diestrus, there is increased tonic inhibition and reduced neuronal excitability in dentate gyrus granule cells [59]. Experimental knockdown of the δ GABAA subunit blocks this effect and increases seizure susceptibility under high progesterone conditions. These effects are absent in CA1 pyramidal cells where the expression of δ GABAA subunit is low [60]. Unlike estrogens or androgens, there is little evidence for progestin modulation of intrinsic excitability. Nevertheless, progesterone modulation of tonic inhibition could impact the outcomes of optogenetic stimulation experiments.
3.4. Summary
Gonadal steroids modulate several aspects of neuronal function, but the key question is whether these effects alter the performance of optogenetic tools. A recent paper suggests that at least some optogenetic manipulations are robust to fluctuations in hormone levels [61]. Excitatory optical stimulation of the motor cortex was used to induce spreading depolarization, a slow wave of neuronal and glial excitation that is used as a preclinical model for migraines. Optogenetic induction of spreading depolarization increased sensitivity to pain to a greater extent in females than males. However, no effect of the estrous stage on a spreading depolarization was observed, even though pain sensitivity was greater during proestrus or estrus in. Similarly, although spreading depolarization increased sensitivity to light, no differences were observed across the estrous cycle. Thus, in this model, while there are robust sex differences in susceptibility to spreading depolarization, the behavioral effects of optogenetic stimulation do not appear to be modulated by estrous cycle state. This may not be true for all systems.
Kisspeptin neurons in the hypothalamus drive pulsatile release of gonadotropin-releasing hormone, which in turn controls gonadal function [62]. Channelrhodopsin was expressed in kisspeptin neurons and blue light stimulation was applied at different stages of the estrous cycle [63]. During estrus, blue light stimulation increased the number of luteinizing hormone pulses. In contrast, when blue light was applied during diestrus, the number of luteinizing hormones pulses was reduced (Figure 2). These differences could be driven by progesterone, as optogenetic excitation of kisspeptin neurons induced surges of luteinizing hormone in the presence but not absence of progesterone [64]. While these data show that progestins can modulate the effects of optogenetic manipulations, the extent of these effects outside of the hypothalamus is unclear. Although there is little direct evidence for androgen effects on optogenetic stimulation, this has not been assessed directly.
Figure 2: Estrous cycle influences the effects of optogenetic stimulation of kisspeptin neurons.
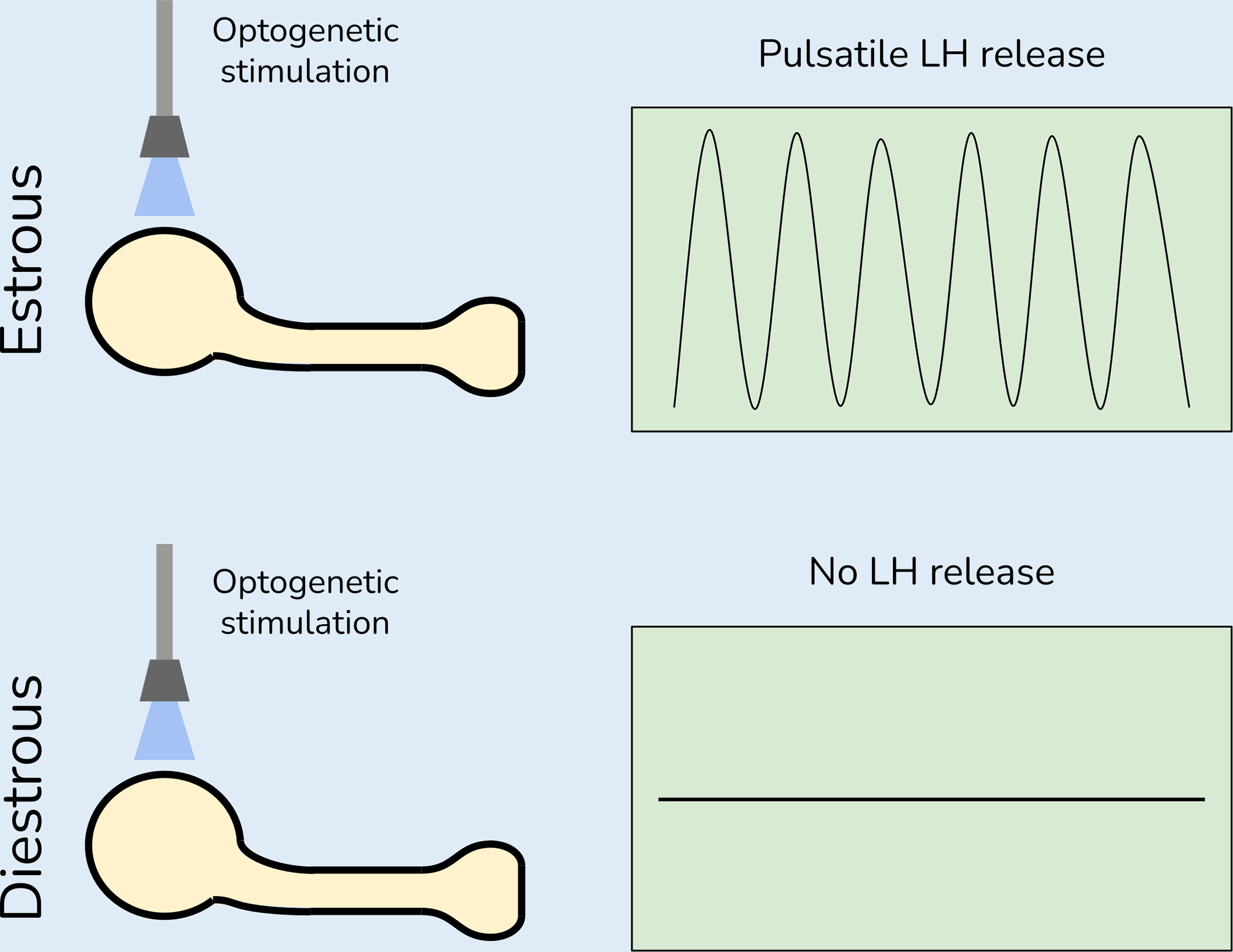
During estrus, circulating estrogens are elevated while progestin levels are decreasing, and optogenetic stimulation of kisspeptin neurons stimulates luteinizing hormone (LH) release. During diestrus estrogen levels are lower while progestin levels are higher, and optogenetic stimulation of kisspeptin neurons does not stimulate GnRH release.
4. Calcium imaging
Calcium imaging is a key method for monitoring neuronal activity [65]. Genetically encoded calcium indicators (such as GCaMP) allow for the targeting of specific cell populations in vivo using fiber photometry, 2-photon imaging, or miniature microscopes [66]. All methods assume that fluorescent signals track changes in intracellular calcium that track action potentials. Intracellular calcium levels can be modulated by gonadal hormones, which could influence the amplitude and fidelity of activity-dependent calcium signals.
4.1. Effects of gonadal hormones on calcium influx
In cultured hippocampus neurons, DHT treatment increased baseline calcium levels, and this effect was blocked by flutamide [67]. Differences in baseline levels might not impact calcium imaging applications that assess changes relative to a baseline (e.g. ΔF/F). More relevant were findings that DHT modulated glutamate-induced calcium responses. In neurons cultured from newborn rats DHT enhanced glutamate-induced calcium influx [68] but decreased calcium responses in cells harvested from older animals [69]. The latter outcome suggests that neurons exposed to androgens would produce blunted ΔF/F signals compared to neurons in the absence of androgens. A recent fiber photometry study also observed that male prepubertal gonadectomy enhanced calcium transients within bed nucleus of the stria terminalis [70]. Male California mice were randomly assigned to prepubertal castration or sham surgery and then exposed to social defeat as adults. Calcium imaging in the BNST showed that both castrated and intact males had increased ΔF/F to a threatening male target mouse. In contrast, only castrated males exhibited increased ΔF/F to a non-threatening male target mouse. These results suggest that the ventral BNST responds to threatening social contexts and that androgen exposure reduces neuronal excitability in less threatening contexts. A key question is whether the reduced calcium transients observed in castrated males were due to reduced neural activity or to direct effects of androgens on calcium influx. A separate dataset found that prepubertal castration increased c-fos expression within the BNST, suggesting that increased calcium transients in castrated males were driven by increased in neural activity. Even if androgens reduced calcium influx without altering the firing rates of neurons, the observation of reduced calcium transients would imply that neurotransmitter release would be reduced [71].
Estrogens also rapidly modulate intracellular calcium concentrations (Figure 3), but these effects appear to be dose dependent [72]. At lower picomolar concentrations, estradiol induces a rapid influx of intracellular calcium in cultured hippocampal cells [73]. This effect was blocked by pre-incubation with nifedipine, an L-type voltage-gated calcium channel antagonist. Similar effects of estradiol (at picomolar concentrations) were observed in cultured mouse midbrain neurons [74]. However, at higher nanomolar concentrations, estradiol reduced intracellular calcium concentrations in cultured cortical and these effects were reversed by L-type calcium channel activation [75]. These findings raise the difficult question of whether experimental concentrations of estradiol match physiological levels in vivo. Steroid concentrations in the brain can be much higher than in blood [76]. A recent analysis of estradiol concentrations in the bed nucleus of the stria terminalis reported concentrations in the 1 pg/10 mL range [77]. Although these measurements provide a useful estimate of brain estradiol content, they likely reflect a lower limit on estradiol concentrations at the synapse as neurons and astrocyte expressing aromatase are capable of synthesizing estradiol in close proximity to synapses [78].
Figure 3: Effects of estradiol on calcium influx:
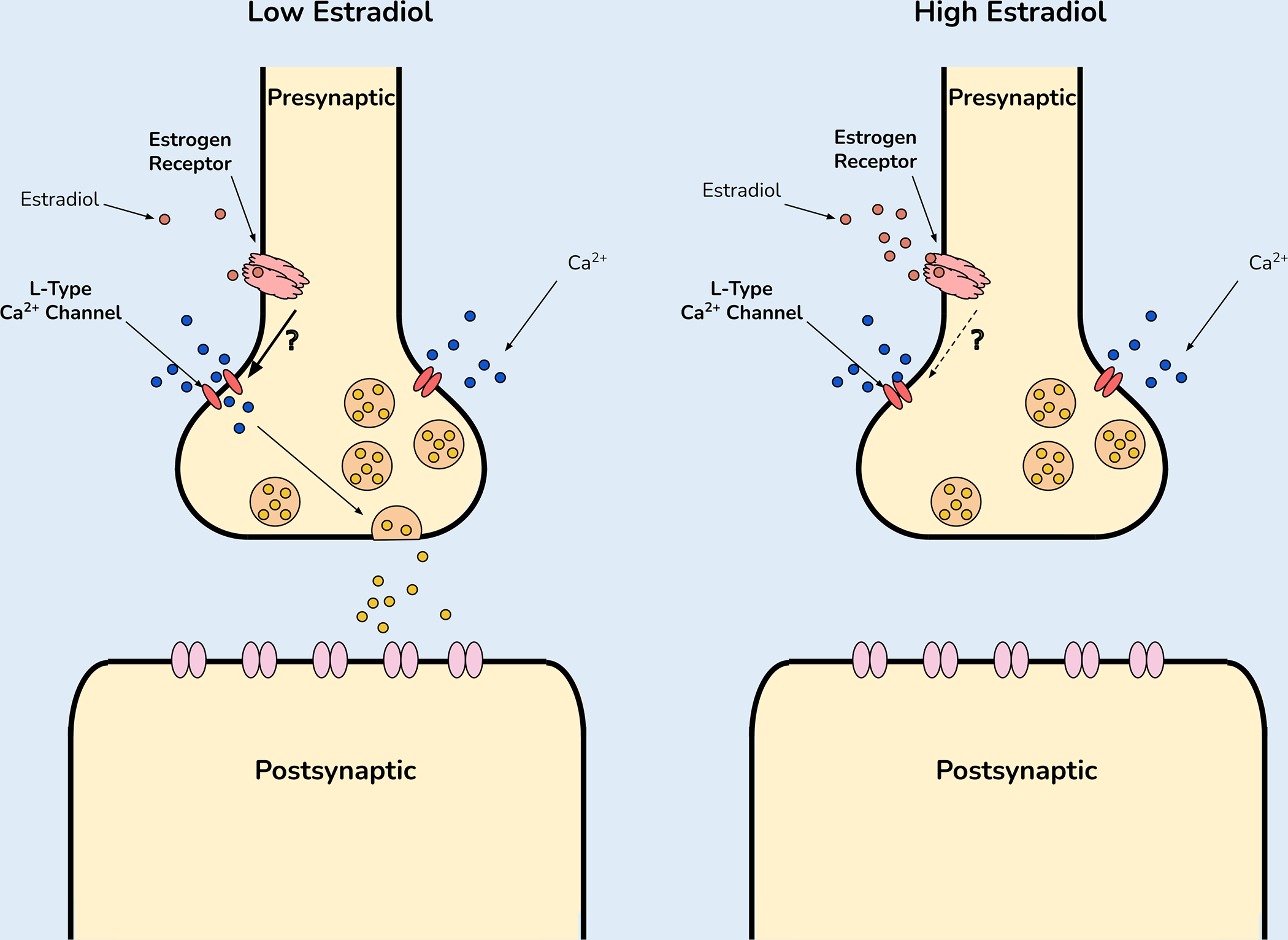
Cell culture studies find that low concentrations of estradiol facilitate calcium influx and that this effect is prevented by L-type calcium channel blockers. In contrast the opposite effect is observed at high estradiol concentrations. The mechanism for these concentration-dependent effects of estradiol is unknown.
Conclusions
Sex differences can influence the function of widely used neuroscientific tools. For chemogenetics, sex differences in ligand pharmacokinetics, signal transduction, and off-target effects of chemogenetic ligands are well established. For optogenetic tools, steroids can modulate synaptic plasticity and intrinsic excitability, but these tools may be less susceptible to sex differences in efficacy. There is also evidence that hormonal modulation of calcium channels could affect measures of neural activity via calcium imaging. However, since hormone-dependent effects on calcium influx would have functional consequences for neuronal signaling these effects would be physiologically relevant and likely not an artifact of the technique. An additional dimension not addressed in this review is the possibility that expression of genes on sex chromosomes (X and Y) could differentially impact neuronal signaling, and thus the function of neuroscience tools [79,80]. Together these findings suggest that chemogenetic, optogenetic, and calcium imaging tools may need separate optimization for use in males and females, and that caution should be taken to consider the role that sex hormones might play in the processes targeted by these tools.
Acknowledgements
This work supported by NIH R01MH121829-04S1 to VICS, R01MH111604 to AJR, and NSF IOS1937335 and NIH R01MH121829 to BCT.
References
- 1.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. : Natural neural projection dynamics underlying social behavior. Cell 2014, 157:1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, et al. : Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 2016, 164:617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peñagarikano O, Lázaro MT, Lu X-H, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, et al. : Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med 2015, 7:271ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CK, Sanchez MI, Hoerbelt P, Fenno LE, Malenka RC, Deisseroth K, Ting AY: A Molecular Calcium Integrator Reveals a Striatal Cell Type Driving Aversion. Cell 2020, 183:2003–2019.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kingsbury L, Huang S, Raam T, Ye LS, Wei D, Hu RK, Ye L, Hong W: Cortical Representations of Conspecific Sex Shape Social Behavior. Neuron 2020, 107:941–953.e7. * This paper uses microendoscopes to identify how social information is encoded in the medial prefrontal cortex and activity dependent tagging to target these cells with optogenetic manipulations that demonstrate their role in controlling preference behavior.
- 6.Shansky RM, Murphy AZ: Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci 2021, 24:457–464. [DOI] [PubMed] [Google Scholar]
- 7.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Shah PS, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, et al. : Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Developmental Dynamics 2017, 357:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, Sassano FM, Huang X-P, Zhu H, Urban DJ, et al. : A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 2015, 86:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson SG, Livingston M, Couchman L, Smith DJ, Connolly M, Miller J, Flanagan RJ, Heald AH: Sex differences in plasma clozapine and norclozapine concentrations in clinical practice and in relation to body mass index and plasma glucose concentrations: a retrospective survey. Ann Gen Psychiatry 2015, 14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson GD: Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt) 2005, 14:19–29. [DOI] [PubMed] [Google Scholar]
- 11.Fonsart J, Menet M-C, Declèves X, Galons H, Crété D, Debray M, Scherrmann J-M, Noble F: Sprague-Dawley rats display metabolism-mediated sex differences in the acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy). Toxicol Appl Pharmacol 2008, 230:117–125. [DOI] [PubMed] [Google Scholar]
- 12.Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, Weinshenker D: The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep 2018, 8:3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Choo H, Huang X-P, Yang X, Stone O, Roth BL, Jin J: The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci 2015, 6:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Kätzel D, Nissen W, Pekcec A: Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 2019, 9:4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson KJ, Khajehali E, Bradley SJ, Navarrete JS, Huang XP, Slocum S, Jin J, Liu J, Xiong Y, Olsen RHJ, et al. : DREADD Agonist 21 Is an Effective Agonist for Muscarinic-Based DREADDs in Vitro and in Vivo. ACS Pharmacol Transl Sci 2018, 1:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goutaudier R, Coizet V, Carcenac C, Carnicella S: Compound 21, a two-edged sword with both DREADD-selective and off-target outcomes in rats. PLoS One 2020, 15:e0238156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goutaudier R, Coizet V, Carcenac C, Carnicella S: DREADDs: The Power of the Lock, the Weakness of the Key. Favoring the Pursuit of Specific Conditions Rather than Specific Ligands. eNeuro 2019, 6:ENEURO.0171–19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanchard DC, Shepherd JK, De Padua Carobrez A, Blanchard RJ: Sex effects in defensive behavior: baseline differences and drug interactions. Neurosci Biobehav Rev 1991, 15:461–468. [DOI] [PubMed] [Google Scholar]
- 19.Bangasser DA, Curtis A, Reyes B a. S, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ: Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 2010, 15:877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangasser DA, Reyes B a. S, Piel D, Garachh V, Zhang X-Y, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ: Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry 2013, 18:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulin B, Butcher A, McWilliams P, Bourgognon J-M, Pawlak R, Kong KC, Bottrill A, Mistry S, Wess J, Rosethorne EM, et al. : The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc Natl Acad Sci U S A 2010, 107:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth BL: DREADDs for Neuroscientists. Neuron 2016, 89:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeters LM, Hinz R, Detrez JR, Missault S, De Vos WH, Verhoye M, Van der Linden A, Keliris GA: Chemogenetic silencing of neurons in the mouse anterior cingulate area modulates neuronal activity and functional connectivity. Neuroimage 2020, 220:117088. [DOI] [PubMed] [Google Scholar]
- 24.Taylor NE, Long H, Pei J, Kukutla P, Phero A, Hadaegh F, Abdelnabi A, Solt K, Brenner GJ: The rostromedial tegmental nucleus: a key modulator of pain and opioid analgesia. Pain 2019, 160:2524–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robles CF, McMackin MZ, Campi KL, Doig IE, Takahashi EY, Pride MC, Trainor BC: Effects of kappa opioid receptors on conditioned place aversion and social interaction in males and females. Behav Brain Res 2014, 262:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y-J, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen L-Y: Sex Difference in κ-Opioid Receptor (KOPR)-Mediated Behaviors, Brain Region KOPR Level and KOPR-Mediated Guanosine 5′-O-(3-[35S]Thiotriphosphate) Binding in the Guinea Pig. J Pharmacol Exp Ther 2011, 339:438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laman-Maharg A, Williams AV, Zufelt MD, Minie VA, Ramos-Maciel S, Hao R, Ordoñes Sanchez E, Copeland T, Silverman JL, Leigh A, et al. : Sex Differences in the Effects of a Kappa Opioid Receptor Antagonist in the Forced Swim Test. Front Pharmacol 2018, 9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH: Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 2014, 76:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C: Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem 2007, 282:29803–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, Johnson SD, Land BB, Chavkin C: Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. J Neurosci 2018, 38:8031–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuscher JJ, Taxier LR, Fortress AM, Frick KM: Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol Learn Mem 2018, 156:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dao NC, Brockway DF, Suresh Nair M, Sicher AR, Crowley NA: Somatostatin neurons control an alcohol binge drinking prelimbic microcircuit in mice. Neuropsychopharmacol 2021, 46:1906–1917. ** This paper used a kappa opioid receptor chemogenetics system to inhibit somatostatin neurons in prelimbic cortex to show that inhibition of these neurons reduced alcohol binge drinking in male and female mice. This suggests that at least some applications of chemogenetic tools work similarly in males and females.
- 33.Woolley C, Gould E, Frankfurt M, McEwen B: Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 1990, 10:4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gould E, Woolley CS, Frankfurt M, McEwen BS: Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 1990, 10:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K: Mode of Action and Functional Significance of Estrogen-Inducing Dendritic Growth, Spinogenesis, and Synaptogenesis in the Developing Purkinje Cell. J Neurosci 2007, 27:7408–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH: Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A 2007, 104:11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW: 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol 1999, 81:925–929. [DOI] [PubMed] [Google Scholar]
- 38.Córdoba Montoya DA, Carrer HF: Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res 1997, 778:430–438. [DOI] [PubMed] [Google Scholar]
- 39.Chidambaram SB, Rathipriya AG, Bolla SR, Bhat A, Ray B, Mahalakshmi AM, Manivasagam T, Thenmozhi AJ, Essa MM, Guillemin GJ, et al. : Dendritic spines: Revisiting the physiological role. Prog Neuropsychopharmacol Biol Psychiatry 2019, 92:161–193. [DOI] [PubMed] [Google Scholar]
- 40.González-Burgos I, Alejandre-Gómez M, Cervantes M: Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neurosci Lett 2005, 379:52–54. [DOI] [PubMed] [Google Scholar]
- 41.Wu WW, Adelman JP, Maylie J: Ovarian Hormone Deficiency Reduces Intrinsic Excitability and Abolishes Acute Estrogen Sensitivity in Hippocampal CA1 Pyramidal Neurons. J Neurosci 2011, 31:2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gervais NJ, Remage-Healey L, Starrett JR, Pollak DJ, Mong JA, Lacreuse A: Adverse Effects of Aromatase Inhibition on the Brain and Behavior in a Nonhuman Primate. J Neurosci 2019, 39:918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorris DM, Cao J, Willett JA, Hauser CA, Meitzen J: Intrinsic excitability varies by sex in prepubertal striatal medium spiny neurons. J Neurophysiol 2015, 113:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabatadze N, Huang G, May RM, Jain A, Woolley CS: Sex Differences in Molecular Signaling at Inhibitory Synapses in the Hippocampus. J Neurosci 2015, 35:11252–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang GZ, Woolley CS: Estradiol Acutely Suppresses Inhibition in the Hippocampus through a Sex-Specific Endocannabinoid and mGluR-Dependent Mechanism. Neuron 2012, 74:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain A, Huang GZ, Woolley CS: Latent Sex Differences in Molecular Signaling That Underlies Excitatory Synaptic Potentiation in the Hippocampus. J Neurosci 2019, 39:1552–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatanaka Y, Hojo Y, Mukai H, Murakami G, Komatsuzaki Y, Kim J, Ikeda M, Hiragushi A, Kimoto T, Kawato S: Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: Dependence on synaptic androgen receptor and kinase networks. Brain Res 2015, 1621:121–132. [DOI] [PubMed] [Google Scholar]
- 48.Kovacs EG, MacLusky NJ, Leranth C: Effects of testosterone on hippocampal CA1 spine synaptic density in the male rat are inhibited by fimbria/fornix transection. Neuroscience 2003, 122:807–810. [DOI] [PubMed] [Google Scholar]
- 49.Hajszan T, MacLusky NJ, Leranth C: Role of Androgens and the Androgen Receptor in Remodeling of Spine Synapses in Limbic Brain Areas. Horm Behav 2008, 53:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pouliot WA, Handa RJ, Beck SG: Androgen modulates N-methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells. Synapse 1996, 23:10–19. [DOI] [PubMed] [Google Scholar]
- 51.Smith MD, Jones LS, Wilson MA: Sex differences in hippocampal slice excitability: role of testosterone. Neuroscience 2002, 109:517–530. [DOI] [PubMed] [Google Scholar]
- 52.Islam MN, Sakimoto Y, Jahan MR, Ishida M, Tarif AMM, Nozaki K, Masumoto K-H, Yanai A, Mitsushima D, Shinoda K: Androgen Affects the Dynamics of Intrinsic Plasticity of Pyramidal Neurons in the CA1 Hippocampal Subfield in Adolescent Male Rats. Neuroscience 2020, 440:15–29. [DOI] [PubMed] [Google Scholar]
- 53.Dalla C, Whetstone AS, Hodes GE, Shors TJ: Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neuroscience Letters 2009, 449:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangasser DA, Shors TJ: The Bed Nucleus of the Stria Terminalis Modulates Learning after Stress in Masculinized But Not Cycling Females. J Neurosci 2008, 28:6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams ES, Manning CE, Eagle AL, Swift-Gallant A, Duque-Wilckens N, Chinnusamy S, Moeser A, Jordan C, Leinninger G, Robison AJ: Androgen-Dependent Excitability of Mouse Ventral Hippocampal Afferents to Nucleus Accumbens Underlies Sex-Specific Susceptibility to Stress. Biol Psychiatry 2020, 87:492–501. ** The authors showed that androgens reduce the excitability of ventral hippocampus neurons that project to the nucleus accumbens and that androgen regulation of these neurons controls stress-induced sucrose anhedonia.
- 56.Turrigiano GG, Nelson SB: Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 2004, 5:97–107. [DOI] [PubMed] [Google Scholar]
- 57.Reddy DS, Estes WA: Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol Sci 2016, 37:543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herd MB, Belelli D, Lambert JJ: Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther 2007, 116:20–34. [DOI] [PubMed] [Google Scholar]
- 59.Maguire JL, Stell BM, Rafizadeh M, Mody I: Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 2005, 8:797–804. [DOI] [PubMed] [Google Scholar]
- 60.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G: GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 2000, 101:815–850. [DOI] [PubMed] [Google Scholar]
- 61. Harriott AM, Waruinge A, Appiah-Danquah V, Berhanu L, Morais A, Ayata C: The effect of sex and estrus cycle stage on optogenetic spreading depression induced migraine-like pain phenotypes. J Headache Pain 2023, 24:85. ** Here the authors showed that an optogenetics-based model of migraine produced more severe effects on pain perception in females than males but that there were no differences in pain across the estrous cycle.
- 62.Lehman MN, Coolen LM, Goodman RL: Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010, 151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Voliotis M, Li XF, De Burgh RA, Lass G, Ivanova D, McIntyre C, O’Byrne K, Tsaneva-Atanasova K: Modulation of pulsatile GnRH dynamics across the ovarian cycle via changes in the network excitability and basal activity of the arcuate kisspeptin network. Elife 2021, 10:e71252. ** In this paper the authors showed that optogenetic stimulation of kisspeptin neurons induced GnRH release during estrous but not during diestrus, highlighting how gonadal hormones could impact the efficacy of optogenetic manipulations.
- 64.Lin X-H, Lass G, Kong L-S, Wang H, Li X-F, Huang H-F, O’Byrne KT: Optogenetic Activation of Arcuate Kisspeptin Neurons Generates a Luteinizing Hormone Surge-Like Secretion in an Estradiol-Dependent Manner. Front Endocrinol (Lausanne) 2021, 12:775233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, Svoboda K: Imaging calcium concentration dynamics in small neuronal compartments. Sci STKE 2004, 2004:pl5. [DOI] [PubMed] [Google Scholar]
- 66.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. : Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 2009, 6:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foradori CD, Werner SB, Sandau US, Clapp TR, Handa RJ: Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience 2007, 149:155–164. [DOI] [PubMed] [Google Scholar]
- 68.Nunes JL, McCarthy MM: Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience 2009, 158:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zup SL, Edwards NS, McCarthy MM: Sex- and age-dependent effects of androgens on glutamate-induced cell death and intracellular calcium regulation in the developing hippocampus. Neuroscience 2014, 281:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wright EC, Zakharenkov HC, Godoy AS, Lake AA, Prince ZD, Sekar S, Culkin HI, Luo PX, Ramirez AV, Dwyer T, et al. : Sexual differentiation of neural mechanisms of stress sensitivity during puberty. bioRxiv; 2023. ** In this paper prepubertal castration increased calcium transients in the bed nucleus of the stria terminalis (BNST) in non-threatening contexts compared to sham surgery males. Since prepubertal castration also increased c-fos expression in the BNST this effect is likely due to changes in activity rather than androgen modulation of calcium channels.
- 71.Kavalali ET: The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 2015, 16:5–16. [DOI] [PubMed] [Google Scholar]
- 72.Vega-Vela NE, Osorio D, Avila-Rodriguez M, Gonzalez J, García-Segura LM, Echeverria V, Barreto GE: L-Type Calcium Channels Modulation by Estradiol. Mol Neurobiol 2017, 54:4996–5007. [DOI] [PubMed] [Google Scholar]
- 73.Wu TW, Wang JM, Chen S, Brinton RD: 17b-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprot. Neurosci 2005, 135:59–72. [DOI] [PubMed] [Google Scholar]
- 74.Beyer C, Raab H: Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. European Journal of Neuroscience 1998, 10:255–262. [DOI] [PubMed] [Google Scholar]
- 75.Sanchez JC, Lopez-Zapata DF, Pinzon OA: Effects of 17beta-estradiol and IGF-1 on L-type voltage-activated and stretch-activated calcium currents in cultured rat cortical neurons. Neuro Endocrinol Lett 2014, 35:724–732. [PubMed] [Google Scholar]
- 76.Cornil CA: Rapid Regulation of Brain Oestrogen Synthesis: The Behavioural Roles of Oestrogens and their Fates. Journal of Neuroendocrinology 2009, 21:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Bournonville M-P, De Bournonville C, Vandries LM, Nys G, Fillet M, Ball GF, Balthazart J, Cornil CA: Rapid changes in brain estrogen concentration during male sexual behavior are site and stimulus specific. Sci Rep 2021, 11:20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Remage-Healey L, Saldanha CJ, Schlinger BA: Estradiol Synthesis and Action at the Synapse: Evidence for ?Synaptocrine? Signaling. Front Endocrin 2011, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnold AP: Mouse Models for Evaluating Sex Chromosome Effects that Cause Sex Differences in Non-Gonadal Tissues. Journal of Neuroendocrinology 2009, 21:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X, Grisham W, Arnold AP: X chromosome number causes sex differences in gene expression in adult mouse striatum. European Journal of Neuroscience 2009, 29:768–776. [DOI] [PubMed] [Google Scholar]


