Abstract
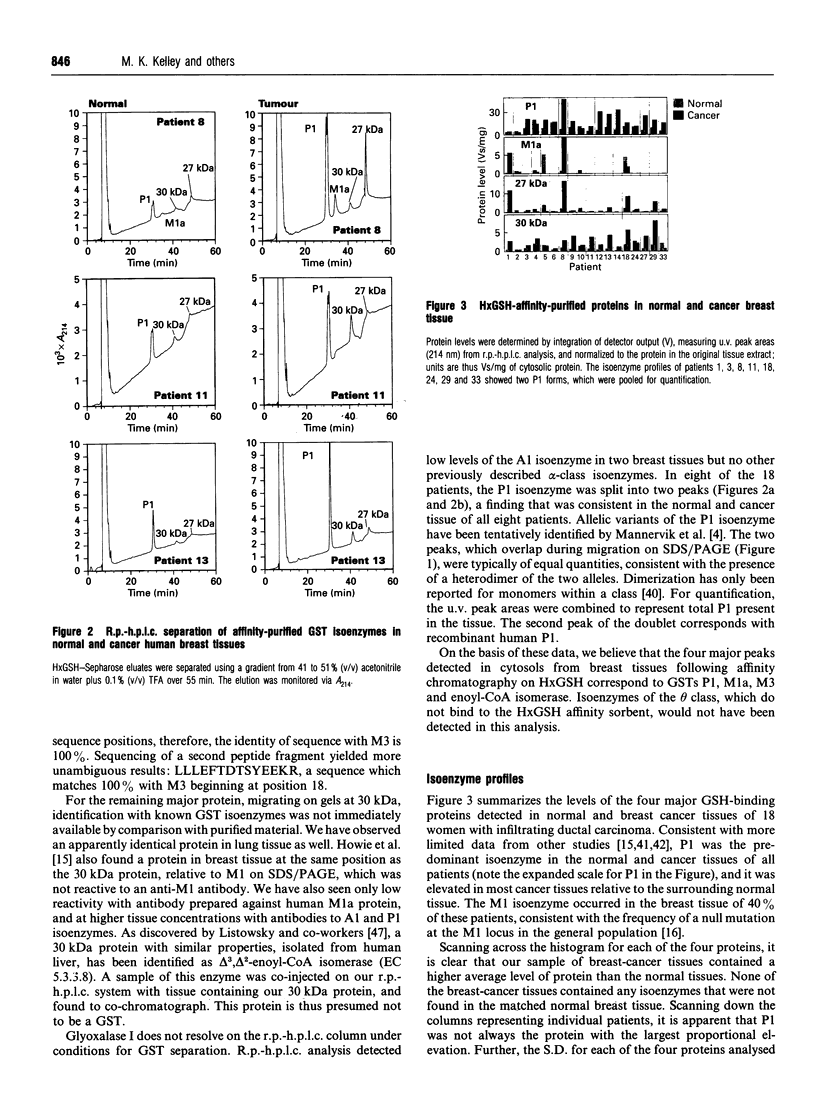
The determination of GST levels in blood has been proposed to a marker of tumour burden in general, whereas level of the P1 isoenzyme has been identified as a prognostic factor for breast-cancer patients receiving no adjuvant chemotherapy. Particular glutathione S-transferase (GST) isoenzymes differ in their substrate specificity, however, and their presence or absence might therefore account for the resistance of tumours to particular chemotherapeutic drugs, as already established for cultured cell lines. Determination of the GST isoenzyme profile of a cancer tissue could have prognostic value in the selection of treatment if the levels of expression/activity show a degree of variation comparable with that exhibited by actual patient responses. Using reversed-phase h.p.l.c. to quantify affinity-isolated GSTs, we have analysed full isoenzyme profiles in the first large sample of matched normal and cancer human tissues (18 breast-cancer patients). In no patients did the tumour tissues express any isoenzymes that were not found in normal breast tissue. In addition to the GSTs, another enzyme, identified as enoyl-CoA isomerase, was regularly found in breast tissue cytosol following elution from a hexyl-glutathione affinity column. In most cases, the average level of GST was substantially elevated in the cancer tissues above the levels in normal breast tissue from the same patient. Furthermore, the relative levels of the isoenzymes were substantially more variable in the cancer samples than in the normal breast tissue, providing a plausible mechanism for the well established variable response to treatment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black S. M., Beggs J. D., Hayes J. D., Bartoszek A., Muramatsu M., Sakai M., Wolf C. R. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem J. 1990 Jun 1;268(2):309–315. doi: 10.1042/bj2680309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E., Takahashi Y., Abramovitz M., Peretz M., Listowsky I. A distinct human testis and brain mu-class glutathione S-transferase. Molecular cloning and characterization of a form present even in individuals lacking hepatic type mu isoenzymes. J Biol Chem. 1990 Jun 5;265(16):9188–9193. [PubMed] [Google Scholar]
- Castro V. M., Kelley M. K., Engqvist-Goldstein A., Kauvar L. M. Glutathione analogue sorbents selectively bind glutathione S-transferase isoenzymes. Biochem J. 1993 Jun 1;292(Pt 2):371–377. doi: 10.1042/bj2920371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro V. M., Söderström M., Carlberg I., Widersten M., Platz A., Mannervik B. Differences among human tumor cell lines in the expression of glutathione transferases and other glutathione-linked enzymes. Carcinogenesis. 1990 Sep;11(9):1569–1576. doi: 10.1093/carcin/11.9.1569. [DOI] [PubMed] [Google Scholar]
- Chow N. W., Whang-Peng J., Kao-Shan C. S., Tam M. F., Lai H. C., Tu C. P. Human glutathione S-transferases. The Ha multigene family encodes products of different but overlapping substrate specificities. J Biol Chem. 1988 Sep 15;263(26):12797–12800. [PubMed] [Google Scholar]
- Clapper M. L., Hoffman S. J., Carp N., Watts P., Seestaller L. M., Weese J. L., Tew K. D. Contribution of patient history to the glutathione S-transferase activity of human lung, breast and colon tissue. Carcinogenesis. 1991 Oct;12(10):1957–1961. doi: 10.1093/carcin/12.10.1957. [DOI] [PubMed] [Google Scholar]
- Di Ilio C., Del Boccio G., Aceto A., Casaccia R., Mucilli F., Federici G. Elevation of glutathione transferase activity in human lung tumor. Carcinogenesis. 1988 Feb;9(2):335–340. doi: 10.1093/carcin/9.2.335. [DOI] [PubMed] [Google Scholar]
- Fisher B. The evolution of paradigms for the management of breast cancer: a personal perspective. Cancer Res. 1992 May 1;52(9):2371–2383. [PubMed] [Google Scholar]
- Flatgaard J. E., Bauer K. E., Kauvar L. M. Isozyme specificity of novel glutathione-S-transferase inhibitors. Cancer Chemother Pharmacol. 1993;33(1):63–70. doi: 10.1007/BF00686025. [DOI] [PubMed] [Google Scholar]
- Gilbert L., Elwood L. J., Merino M., Masood S., Barnes R., Steinberg S. M., Lazarous D. F., Pierce L., d'Angelo T., Moscow J. A. A pilot study of pi-class glutathione S-transferase expression in breast cancer: correlation with estrogen receptor expression and prognosis in node-negative breast cancer. J Clin Oncol. 1993 Jan;11(1):49–58. doi: 10.1200/JCO.1993.11.1.49. [DOI] [PubMed] [Google Scholar]
- Hao X. Y., Widersten M., Ridderström M., Hellman U., Mannervik B. Co-variation of glutathione transferase expression and cytostatic drug resistance in HeLa cells: establishment of class Mu glutathione transferase M3-3 as the dominating isoenzyme. Biochem J. 1994 Jan 1;297(Pt 1):59–67. doi: 10.1042/bj2970059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., McLellan L. I., Kerr L. A., Peacock S. D., Neal G. E. Ethoxyquin-induced resistance to aflatoxin B1 in the rat is associated with the expression of a novel alpha-class glutathione S-transferase subunit, Yc2, which possesses high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem J. 1991 Oct 15;279(Pt 2):385–398. doi: 10.1042/bj2790385. [DOI] [PMC free article] [PubMed] [Google Scholar]
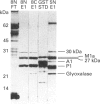
- Howie A. F., Forrester L. M., Glancey M. J., Schlager J. J., Powis G., Beckett G. J., Hayes J. D., Wolf C. R. Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissues. Carcinogenesis. 1990 Mar;11(3):451–458. doi: 10.1093/carcin/11.3.451. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Hayes J. D. Characterization of a human class-Theta glutathione S-transferase with activity towards 1-menaphthyl sulphate. Biochem J. 1992 Sep 15;286(Pt 3):929–935. doi: 10.1042/bj2860929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmich S., Vanderveer L. A., Tew K. D. Evidence for a glycoconjugate form of glutathione S-transferase pI. Int J Pept Protein Res. 1991 Jun;37(6):565–571. doi: 10.1111/j.1399-3011.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyttle M. H., Aaron D. T., Hocker M. D., Hughes B. R. Construction of affinity sorbents utilizing glutathione analogs. Pept Res. 1992 Nov-Dec;5(6):336–342. [PubMed] [Google Scholar]
- Lyttle M. H., Satyam A., Hocker M. D., Bauer K. E., Caldwell C. G., Hui H. C., Morgan A. S., Mergia A., Kauvar L. M. Glutathione-S-transferase activates novel alkylating agents. J Med Chem. 1994 May 13;37(10):1501–1507. doi: 10.1021/jm00036a016. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Awasthi Y. C., Board P. G., Hayes J. D., Di Ilio C., Ketterer B., Listowsky I., Morgenstern R., Muramatsu M., Pearson W. R. Nomenclature for human glutathione transferases. Biochem J. 1992 Feb 15;282(Pt 1):305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- O'Dwyer P. J., LaCreta F., Nash S., Tinsley P. W., Schilder R., Clapper M. L., Tew K. D., Panting L., Litwin S., Comis R. L. Phase I study of thiotepa in combination with the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991 Nov 15;51(22):6059–6065. [PubMed] [Google Scholar]
- Peters W. H., Wormskamp N. G., Thies E. Expression of glutathione S-transferases in normal gastric mucosa and in gastric tumors. Carcinogenesis. 1990 Sep;11(9):1593–1596. doi: 10.1093/carcin/11.9.1593. [DOI] [PubMed] [Google Scholar]
- Petrini M., Conte A., Caracciolo F., Sabbatini A., Grassi B., Ronca G. Reversing of chlorambucil resistance by ethacrynic acid in a B-CLL patient. Br J Haematol. 1993 Oct;85(2):409–410. doi: 10.1111/j.1365-2141.1993.tb03187.x. [DOI] [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S., Walsh E. S., Godwin A. K., Tew K. D. Cloning and characterization of human colon glyoxalase-I. J Biol Chem. 1993 Mar 15;268(8):5661–5667. [PubMed] [Google Scholar]
- Ross V. L., Board P. G. Molecular cloning and heterologous expression of an alternatively spliced human Mu class glutathione S-transferase transcript. Biochem J. 1993 Sep 1;294(Pt 2):373–380. doi: 10.1042/bj2940373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidegård J., Vorachek W. R., Pero R. W., Pearson W. R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea T. C., Claflin G., Comstock K. E., Sanderson B. J., Burstein N. A., Keenan E. J., Mannervik B., Henner W. D. Glutathione transferase activity and isoenzyme composition in primary human breast cancers. Cancer Res. 1990 Nov 1;50(21):6848–6853. [PubMed] [Google Scholar]
- Singh S. V., Haque A. K., Ahmad H., Medh R. D., Awasthi Y. C. Glutathione S-transferase isoenzymes in human lung tumors. Carcinogenesis. 1988 Sep;9(9):1681–1685. doi: 10.1093/carcin/9.9.1681. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Campbell E. A., Hirata Y., Takayama T., Listowsky I. A basis for differentiating among the multiple human Mu-glutathione S-transferases and molecular cloning of brain GSTM5. J Biol Chem. 1993 Apr 25;268(12):8893–8898. [PubMed] [Google Scholar]
- Takahashi Y., Hirata Y., Burstein Y., Listowsky I. Delta 3, delta 2-enoyl-CoA isomerase is the protein that copurifies with human glutathione S-transferases from S-hexylglutathione affinity matrices. Biochem J. 1994 Dec 15;304(Pt 3):849–852. doi: 10.1042/bj3040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew K. D., Bomber A. M., Hoffman S. J. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res. 1988 Jul 1;48(13):3622–3625. [PubMed] [Google Scholar]
- Vince R., Daluge S., Wadd W. B. Studies on the inhibition of glyoxalase I by S-substituted glutathiones. J Med Chem. 1971 May;14(5):402–404. doi: 10.1021/jm00287a006. [DOI] [PubMed] [Google Scholar]
- Vorachek W. R., Pearson W. R., Rule G. S. Cloning, expression, and characterization of a class-mu glutathione transferase from human muscle, the product of the GST4 locus. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4443–4447. doi: 10.1073/pnas.88.10.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Spurr N. K., Hayes J. D., Wolf C. R. Deduced amino acid sequence, gene structure and chromosomal location of a novel human class Mu glutathione S-transferase, GSTM4. Biochem J. 1993 Apr 1;291(Pt 1):41–50. doi: 10.1042/bj2910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen B., Bogaards J. J., Peters W. H., Blaauboer B., van Bladeren P. J. Quantification of human hepatic glutathione S-transferases. Biochem J. 1990 Aug 1;269(3):609–613. doi: 10.1042/bj2690609. [DOI] [PMC free article] [PubMed] [Google Scholar]