Abstract
Background
Chlorambucil has been used for patients with primary biliary cirrhosis as it possesses immunosuppressive properties. But it is unknown whether it benefits or harms these patients.
Objectives
To evaluate the beneficial and any harmful effects of chlorambucil for primary biliary cirrhosis patients.
Search methods
Eligible trials were identified by searching the Cochrane Hepato‐Biliary Group Controlled Trials Register (March 2012), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 2), MEDLINE (1946 to March 2012), EMBASE (1974 to March 2012), Science Citation Index EXPANDED (1900 to March 2012), The Chinese Biomedical Database (1976 to March 2012), The Chinese Medical Current Contents (1994 to March 2012), The China Hospital Knowledge Database (1994 to March 2012), and a database of ongoing trials (http://www.controlled‐trials.com/mrct/) (accessed 6 March 2012). The reference lists of the retrieved publications and review articles were also read through, and pharmaceutical companies known to produce chlorambucil were contacted.
Selection criteria
Randomised clinical trials, irrespective of language, year of publication, and publication status, comparing chlorambucil at any dose versus placebo, no intervention, another active drug, or one dose of chlorambucil with another dose.
Data collection and analysis
We planned to assess continuous data with mean differences (MD), and dichotomous outcomes with relative risk (RR), both with 95% confidence intervals (CI). As we only identified one trial, Fisher's exact tests were employed.
Main results
Only one randomised trial was identified and included in the review. The bias risk in the trial was high. The trial compared chlorambucil versus no intervention in 24 patients with primary biliary cirrhosis. Fisher's exact test did not show a significant reduction of mortality when comparing chlorambucil with no treatment (0/13 (0%) versus (2/11 (18.2%); P = 0.20). There was no significant difference regarding adverse events for chlorambucil compared with no treatment, but all patients receiving chlorambucil experienced adverse events (13/13 (100%) versus (3/11 (27%); P = 0.1). According to the authors of the trial, chlorambucil led to a significant improvement in mean serum levels of bilirubin (P < 0.05), albumin (P < 0.05), immunoglobulin M (P < 0.01), serum aspartate aminotransferase activity (P < 0.01), and hepatic inflammatory infiltrates (P < 0.01).
Authors' conclusions
There is not sufficient evidence to support or reject the use of chlorambucil for patients with primary biliary cirrhosis. Chlorambucil may show benefit in some unvalidated surrogate outcome measures (for example, serum bilirubin and immunoglobulin M levels). Chlorambucil is, however, connected with a number of adverse events. Bone marrow suppression should be noted in particular. Further randomised clinical trials are necessary to assess the benefits and harms of chlorambucil in this indication.
Keywords: Humans; Chlorambucil; Chlorambucil/therapeutic use; Immunosuppressive Agents; Immunosuppressive Agents/therapeutic use; Liver Cirrhosis, Biliary; Liver Cirrhosis, Biliary/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Chlorambucil for patients with primary biliary cirrhosis
Primary biliary cirrhosis is an autoimmune disease of the liver. Chlorambucil has been used for patients with primary biliary cirrhosis as it possesses immunosuppressive properties. This review aimed to assess the beneficial or harmful effects of chlorambucil for primary biliary cirrhosis. The authors identified only one randomised trial, with 24 participants included. This trial compared chlorambucil with no intervention. The trial is small and at a high risk of bias, which suggests that the results may not be reliable. Meta‐analyses were not possible because of the inclusion of one trial only. Fisher's exact test and t‐test were used instead. Chlorambucil was not associated with significantly lower mortality when compared with no intervention. All patients on chlorambucil experienced adverse events, especially bone marrow suppression. Chlorambucil led to a significant improvement in mean serum levels of bilirubin, albumin, immunoglobulin M, serum aspartate aminotransferase activity, and hepatic inflammatory infiltrates. However, these outcomes are unvalidated surrogate outcomes for patient‐relevant outcomes. This means that improvement of these biochemistry measures cannot be taken as proof of improvement of patient‐relevant outcomes. It remains unclear whether chlorambucil can be supported or rejected for use in patients with primary biliary cirrhosis.
Background
Description of the condition
Primary biliary cirrhosis (PBC) is a chronic liver disease that is characterised by destruction of the epithelium of the small septal and interlobular bile ducts. It primarily affects middle‐aged women (40 to 60 years old). The incidence of primary biliary cirrhosis among adults (more than 20 years old) is about two to three in 100,000 persons per year in both Europe and the United States. The prevalence of the disease varies widely in different geographical regions (Kim 2002; Sood 2004). It is thought that primary biliary cirrhosis is caused by a complex interplay between genetic and environmental risk factors (Gershwin 2008). Some environmental factors (for example, micro‐organisms or xenobiotics) may initiate autoimmune mechanisms in combination with the effects of genetic predisposition that leads to immunopathological damage of small bile duct, and eventually to liver fibrosis, cirrhosis, and failure (Kaplan 2005). Antimitochondrial autoantibodies (AMA) are the characteristic serologic hallmark of primary biliary cirrhosis, found in 90% to 95% of patients and less than 1% of normal controls (Gershwin 1987; Invernizzi 1997).
The most common symptoms of primary biliary cirrhosis are fatigue and pruritus (Goldblatt 2002; Bergasa 2005). More than 50% of patients are asymptomatic during the first stage of the disease, but they do not show a better prognosis than symptomatic patients (Prince 2004). The diagnosis is based on abnormal biochemical indicators of cholestasis (mainly raised activity of alkaline phosphatases), AMA‐positivity, and exclusion of other causes of chronic cholestatic liver disease. Histologic evidence of impaired bile duct lobular and nonsuppurative destructive cholangitis can help the diagnosis in some patients (Lindor 2009).
Over the past decades, there have been many attempts to improve the management of primary biliary cirrhosis, and it is still one of the major indications for liver transplantation worldwide (Gong 2004a; Gong 2004b; Prince 2005; Gong 2007a; Gong 2007b; Gong 2008; Giljaca 2010).
Description of the intervention
Over the years, many drugs have been used to try to treat primary biliary cirrhosis. These are immunomodulatory drugs as well as other agents such as D‐penicillamine (Dickson 1985; Neuberger 1985; Gong 2004a), azathioprine (Christensen 1985; Gong 2007b), colchicine (Poupon 1996; Gong 2004b), cyclosporin A (Wiesner 1990; Gong 2007a), methotrexate (Kaplan 1991; Giljaca 2010), and prednisolone (Mitchison 1992; Prince 2005). However, their usage is limited (Verma 1999). A bile acid ursodeoxycholic acid (UDCA) is the most extensively used drug among them. It is also the only FDA‐approved drug in patients with primary biliary cirrhosis. A Cochrane Hepato‐Biliary Review did not demonstrate any benefit of UDCA on mortality or liver transplantation (Gong 2008), and UDCA did not improve pruritus, fatigue, autoimmune conditions, liver histology, or portal pressure (Gong 2008). The few observed beneficial effects such as improved biochemical variables, like serum bilirubin, ascites, and jaundice, were uncertain (Gong 2008). The use of UDCA is also associated with weight gain and costs (Gong 2008).
Chlorambucil is an alkylating agent. It is indicated in the treatment of chronic lymphatic (lymphocytic) leukaemia, and malignant lymphomas including lymphosarcoma, giant follicular lymphoma, and Hodgkin's disease but it has also been used for treatment of patients with primary biliary cirrhosis.
How the intervention might work
Autoimmune mechanisms are involved in the pathogenesis of primary biliary cirrhosis. Activated T‐lymphocytes attack and destroy epithelial cells in the small intralobular bile ducts of genetically susceptible individuals. Chlorambucil has a high selective inhibition of lymphoid tissue, which can quickly and clearly lead to lymphoid tissue atrophy and to inhibition of antibody synthesis, at a lower dose than than those that cause toxicity. Thus, chlorambucil is a potential agent to modify the immunologic irregularities in primary biliary cirrhosis. It is often combined with glucocorticoids in the therapy of immune‐mediated diseases (Miller 1997).
Why it is important to do this review
Chlorambucil may delay the progression of primary biliary cirrhosis and thereby improve serum bilirubin, serum aspartate aminotransferase activity, and albumin levels. Also, it can decrease immunoglobulin M levels and may improve inflammatory cell infiltration in treated patients (Hoofnagle 1986). However, one review article states that chlorambucil is not ideal because of its toxicity (Kaplan 1997). We could find no meta‐analyses or systematic reviews studying the beneficial or harmful effects of chlorambucil in patients with primary biliary cirrhosis.
Objectives
To evaluate the beneficial or harmful effects of chlorambucil for primary biliary cirrhosis patients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised clinical trials, with no language, year of publication, or publication status restrictions. We planned to also include quasi‐randomised clinical and observational studies for the assessment of harm.
Types of participants
Any adult (age > 18 years) with primary biliary cirrhosis regardless of sex or race. Participants with primary biliary cirrhosis were defined by at least two of the following criteria: ‐ elevated serum activity of alkaline phosphatases (or other markers of intrahepatic cholestasis); ‐ antimitochondrial autoantibodies (AMA) positive; ‐ histologic evidence of nonsuppurative destructive cholangitis and destruction of interlobular bile ducts.
Types of interventions
Administration of per oral chlorambucil at any doses compared with placebo, no intervention, another active drug, or one dose of chlorambucil with another dose. Co‐ interventions were allowed if used equally in both intervention groups.
Types of outcome measures
Primary outcomes
All‐cause mortality and hepatic‐related mortality.
Number of patients undergoing liver transplantation.
Adverse events: any adverse events correlated to use of chlorambucil or other related treatment, such as bone marrow suppression, anaemia, leukopenia, neutropenia, thrombocytopenia, pancytopenia; nausea, vomiting, or diarrhoea; tremors, muscular twitching, and hallucinations; skin rash, urticaria, angioneurotic edema; interstitial pneumonia, drug fever, etc. The adverse events were subdivided into non‐serious adverse events as well as serious adverse events according to the International Conference on Harmonisation Good Clinical Practice (ICH‐GCP) guidelines (ICH‐GCP 1997). Serious adverse events are defined as any event that led to death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or congenital anomaly or birth defect, or any important medical event which might have jeopardized the patient or required intervention to prevent it.
Health‐related quality of life (using a validated instrument), such as patient‐reported health outcomes.
Secondary outcomes
Fatigue: number of patients with fatigue.
Pruritus: number of patients with pruritus.
Other clinical symptoms or signs: number of patients with jaundice or who have developed jaundice, hepatomegaly, xanthoma, ascites, sicca complex, variceal bleeding, hepatic encephalopathy, or hepatorenal syndrome.
Liver biochemistry: serum (s)‐alkaline phosphatases (ALP); s‐bilirubin; s‐gamma‐glutamyltransferase (ɤ‐GT); s‐aspartate aminotransferase (AST); s‐alanine aminotransferase (ALT); s‐albumin; s‐cholesterol (total); plasma immunoglobulin M; s‐copper, etc.
Liver biopsy findings: worsening of liver histological stage or score.
Costs.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (March 2012), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 2), MEDLINE (1946 to March 2012), EMBASE (1974 to March 2012), and Science Citation Index EXPANDED (1900 to March 2012) (Royle 2003). We also searched The Chinese Biomedical Database (CBM‐CD) (1976 to March 2012), The Chinese Medical Current Contents (CMCC‐CD) (1994 to March 2012), The China Hospital Knowledge Database (CHKD) (1994 to March 2012), and the database of ongoing trials (at www.controlled‐trials.com/mrct/) (accessed 6 March 2012).
We searched the databases using the search strategies given in Appendix 1.
Searching other resources
Further trials were sought through scanning of reference lists of the included trials and (systematic) reviews and meta‐analysis related to the topic. In addition, we contacted the pharmaceutical company (GlaxoSmithKline (GSK)) involved in the production of chlorambucil for information about any published, unpublished, or ongoing trials, at http://www.gsk‐clinicalstudyregister.com/) (accessed 6 March 2012).
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2012) for data collection and analysis.
Selection of studies
Two authors (WX Li and CHR Shi) independently cross‐checked the search results, including titles, abstracts, and full texts, according to the inclusion and exclusion criteria. Disagreements were resolved by discussion. In case of disagreements, a third author (X Yan) had to decide whether the trial should be included or not.
Data extraction and management
Two authors (WX Li and AP Zhang) independently extracted the data below from the included trials. Disagreements were resolved in consultation with a third author (CHR Shi).
1. General information: language of publication; authors; article title; journal title, year of publication, volume, issue, and page numbers. 2. Study design: design type, predetermined sample size, generation of randomisation sequence, allocation concealment method, blinding of information, statistical methods, and handling losses to follow‐up. 3. Participants: sample size, diagnostic criteria, baseline characteristics (eg, age, sex), inclusion criteria, exclusion criteria, and study settings. 4. Intervention: duration of treatment, dose, co‐intervention, control. 5. Outcome measures: observed outcome measures as published at the end of treatment.
Assessment of risk of bias in included studies
The bias risk of the randomised clinical trials was assessed following the guidelines of the Cochrane Hepato‐Biliary Group (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Higgins 2011).
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards and throwing dice are adequate if performed by an independent adjudicator.
Uncertain risk of bias: the trial is described as randomised, but the method of sequence generation was not specified.
High risk of bias: the sequence generation method is not, or may not be, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate patients, are inadequate and will be excluded for the assessment of benefits but not for assessment of harms.
Allocation concealment
Low risk of bias: allocation was controlled by a central and independent randomisation unit, sequentially numbered, opaque and sealed envelopes, or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment.
Uncertain risk of bias: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised. Quasi‐randomised studies will be excluded for the assessment of benefits but not for assessment of harms.
Blinding of participants, personnel, and outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to introduce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data were unlikely to make treatment effects depart from plausible values, or proper methods had been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data were likely to induce bias on the estimate of effect.
High risk of bias: the crude estimate of effects (eg, complete case estimate) was clearly biased due to the underlying reasons for missing data, and the methods used to handle missing data were unsatisfactory.
Selective outcome reporting
Low risk of bias: predefined or clinically relevant and reasonably expected outcomes (mortality, adverse events, quality of life, and liver transplant) were reported on.
Uncertain risk of bias: not all predefined or clinically relevant and reasonably expected outcomes were reported on or were not reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Other sources of bias
Low risk of other bias: the trial appeared to be free of other components that could put it at risk of bias.
Uncertain risk of other bias: the trial seemed to or seemed not to be free of other components that put it at risk of bias.
High risk of other bias: there were other factors in the trial that could put it at risk of bias, eg, for‐profit involvement, authors had conducted trials on the same topic etc.
Trials with 'low risk of bias' were considered to be those trials that were assessed as trials having 'low risk of bias' in all of the individual domains specified above. Trials with 'high risk of bias' were considered to be those trials that were assessed as trials having 'uncertain risk of bias' or 'high risk of bias' in one or more of the specified individual domains.
Measures of treatment effect
Measurement data were reported as mean difference (MD) and count data were reported as relative risk (RR) for statistical analysis, with 95% confidence intervals (CIs).
Unit of analysis issues
Randomised clinical trials with cross‐over or cluster designs may raise statistical problems. If, in the future, we identify randomised trials that are cross‐over trials, then data from the first period only will be considered for the review.
Dealing with missing data
We described the numbers and reasons for dropouts and withdrawals in all trial groups. We used the intention‐to‐treat principle for our analysis.
Assessment of heterogeneity
We planned to explore the presence of statistical heterogeneity by using the Chi2 test with significance set at P < 0.10 and to measure the extent of heterogeneity with the I2 statistic (Higgins 2003).
Assessment of reporting biases
The presence of publication bias was planned to be assessed by funnel plots (Egger 1997).
Data synthesis
The Review Manager 5 statistical software (RevMan 2011) provided by The Cochrane Collaboration was to be used for statistical analysis. We planned to perform both fixed‐effect and random‐effects model analyses with the significance level set at P < 0.05. We planned to report the fixed‐effect model results when there was no difference between the results of the two models.
Subgroup analysis and investigation of heterogeneity
We had planned the following subgroup analyses.
1. Trials with low risk of bias compared to trials with high risk of bias. 2. Different doses of chlorambucil. 3. Difference of clinical stage of primary biliary cirrhosis. 4. Difference of duration of treatment.
Sensitivity analysis
We planned to perform sensitivity analyses for testing the robustness of conclusions.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified a total of 146 references through electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 2), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 3), MEDLINE (n = 20), EMBASE (n = 116), Science Citation Index Expanded (n = 5), The Chinese Biomedical CD Database (n = 0), The Chinese Medical Current Contents (CMCC‐CD) (n = 0), The China Hospital Knowledge Database (CHKD) (n = 0), and the database of ongoing trials (www.controlled‐trials.com/mrct/) (n = 0). No additional studies were retrieved from the references listed in relevant reviews, meta‐analysis, included articles, and pharmaceutical companies.
We excluded 143 duplicates, reviews, and clearly irrelevant references. We identified three potentially relevant references. Of the three retrieved publications, two fulfilled the inclusion criteria of the present review (Hoofnagle 1984; Hoofnagle 1986). However, they reported on the same trial. Hoofnagle 1984 was only published as an abstract and it was a preliminary report to Hoofnagle 1986. The third study was an editorial on azathioprine, colchicine, and chlorambucil for primary biliary cirrhosis, so we excluded it. No quasi‐randomised clinical or observational studies were found in order to extract data on harm.
Included studies
The identified randomised trial was a prospective clinical trial assessing the efficacy and safety of chlorambucil in patients with primary biliary cirrhosis compared with no intervention.
Patients
The sample size of the trial was 24 patients. The mean age of the patients was 47 years (range 34 to 63 years), and 23 patients were females. All patients had chronic liver disease symptoms (23 pruritus, 23 fatigue, 6 jaundice) and had been symptomatic for a mean of 2.4 years (range 0.4 to 6 years).
Interventions
The patients were randomly assigned to chlorambucil (n = 13) or no therapy (n = 11). The two groups were comparable with regard to clinical, biochemical, and hepatic histologic indices. The initial dose was 10 mg per day for 10 days, then the dose was adjusted to 0.5 to 4 mg (mean 2 mg) per day according to the results of the white blood cell count and the platelet count. In most of the treated patients, the yearly average decrease in lymphocyte count was from 56% to 63%, and the decrease in polymorphonuclear cell counts was from 25% to 46%. The mean lymphocyte count was initially 2.2 × 10‐3/mm3, a year later it was 0.8 × 10‐3/mm3, and two years later it was 0.9 × 10‐3/mm3. The mean polymorphonuclear cell count was initially 4.8 × 10‐3/mm3, a year later it was 3.6 × 10‐3/mm3, and two years later it was 2.9 × 10‐3/mm3. There was no decrease in the white blood cell count in the control group. The platelet count decreased in the treated patients (the initial mean platelet count was 297600/mm3 and during therapy it ranged between 159100 to 215900/mm3).
Biochemical testing was re‐examined every year, and liver biopsies were repeated at years one, two, and four after randomisation.
Both groups of patients continued on their other routine medications.
Follow‐up
Only one patient from the no intervention group was lost from follow‐up during the third year. The mean duration of follow‐up was 52 months. All chlorambucil‐treated patients had some degree of bone marrow suppression (for example, decrease in white blood cell count). Severe bone marrow suppression led to permanent discontinuation of chlorambucil in four patients.
Outcome measures
The outcome measures were overall survival; peripheral blood counts; changes in biochemical variables; and changes of hepatic histological indices.
Further details are shown in the table 'Characteristics of included studies'.
Excluded studies
The excluded publication was an editorial (Kaplan 1987).
Risk of bias in included studies
See: 'Risk of bias' table in 'Characteristics of included studies'.
Effects of interventions
Mortality
In the chlorambucil group, 0/13 (0%) patients died versus 2/11 (18.2%) patients in the no treatment group. The reasons for the deaths were reported. The two patients from the control group died of hepatic failure. With Fisher's exact test, the two‐tailed P = 0.20, which means that the chlorambucil group was not associated with significantly lower mortality as compared with the no intervention group.
Liver transplantation
This outcome was not reported.
Adverse events
One hundred per cent of the participants (13/13) in the chlorambucil group versus 27% (3/11) in the no treatment group experienced adverse events. With Fisher's exact test, the two‐tailed P = 0.0957, and hence no significant difference in the adverse events between the chlorambucil and no treatment groups was seen. In addition, four treated patients and three no treatment patients had severe systemic infection, but they all recovered during hospitalisation or by using systemic antibiotic treatment. All chlorambucil‐treated patients had decreased white blood cell counts and platelet counts; in four patients the drop in the platelet count was classified as serious. In addition, two patients had menopause, two developed oral herpes simplex, and one patient had localised herpes zoster.
Quality of life
This outcome was not reported.
Clinical findings
The included trial did not report on any clinical findings. However, the authors stated that treatment with chlorambucil was not associated with fatigue, anorexia, nausea, diarrhoea, bitter taste, or hair loss.
Number of patients with fatigue
This outcome was not reported.
Number of patients with pruritus
This outcome was not reported.
Biochemical changes
Following the authors' report, chlorambucil led to a significant improvement in mean serum levels of bilirubin (P < 0.05), albumin (P < 0.05), immunoglobulin M (P < 0.01), and serum aspartate aminotransferase activity (P < 0.01) compared with no treatment at the second year or two years later. Serum alkaline phosphatases showed no statistically significant change in either group during the course of the evaluation.
Liver biopsy findings
In the chlorambucil group, the average intensity of the hepatic inflammatory cell infiltrate decreased significantly from year 0 to years 1 and 2 (P < 0.01). Six out of 10 (60%) treated patients had a marked decrease in inflammatory infiltrates in comparison with 3/10 (30%) untreated patients. For the average degree of hepatic fibrosis and stage of primary biliary cirrhosis, there was no statistically significant improvement in the chlorambucil group versus the no treatment group.
Costs
This outcome was not reported.
Discussion
Summary of main results
We could only identify one small randomised clinical trial examining the beneficial and harmful effects of chlorambucil for primary biliary cirrhosis patients. The chlorambucil group did not differ significantly regarding mortality compared with the no intervention group. The authors of the trial stated that chlorambucil seemed to improve the mean serum levels of bilirubin, albumin, immunoglobulin M, and serum aspartate aminotransferase activity compared with no treatment. Serum alkaline phosphatases did not change in either group. These biochemical outcomes may be used in the evaluation of primary biliary cirrhosis evolution or prognosis (Dickson 1989; Pasha 1997; Kaplan 2002; Alempijevic 2009) but it is questionable whether the biomarkers can be used as proxies for a clinical benefit of the intervention (Baker 2003; Gluud 2005).
The included trial observed significant effects on the degree of hepatic histological inflammation, but the average degree of hepatic fibrosis and stage of primary biliary cirrhosis had no statistically significant improvement in the chlorambucil group versus the no intervention group. Primary biliary cirrhosis is a pathological process starting with portal inflammation which progresses towards liver fibrosis and cirrhosis, and eventually liver failure. Chlorambucil in this small trial might not have slowed down the progression towards the cirrhotic stage or other more advanced stages. As the included trial had so few patients included and a short follow‐up, it is impossible to determine if chlorambucil could delay the natural progression of primary biliary cirrhosis.
The included trial did not report on any clinical features, such as fatigue and pruritus. The significance of the biochemical and histological changes identified are uncertain, probably due to the small sample size of this trial. Thus, the chance of demonstrating or excluding any benefit of the intervention is almost nil.
Meta‐analyses were not possible as there is only one trial that fulfilled the inclusion criteria of this review. The result on adverse events obtained through the Fisher's exact test shows that the use of chlorambucil is not significantly different from the no treatment group. It must be noted, however, that bone marrow suppression is of concern as it leads to an increased risk of infection from the drop of white blood cells.
The one included trial with 24 patients did not report any results on the number of patients undergoing liver transplantation, health‐related quality of life, and costs. In addition, the methodological quality of the trial is low, which makes it impossible to make any firm conclusion on the benefits or harms of chlorambucil.
Overall completeness and applicability of evidence
We have been unable to draw any conclusion about the benefits or harms of chlorambucil in patients with primary biliary cirrhosis.
Quality of the evidence
The identified randomised trial had a very small sample size and a high risk of bias (Figure 1; Figure 2). This opens the possibility for both random errors and systematic errors (Keus 2009).
1.
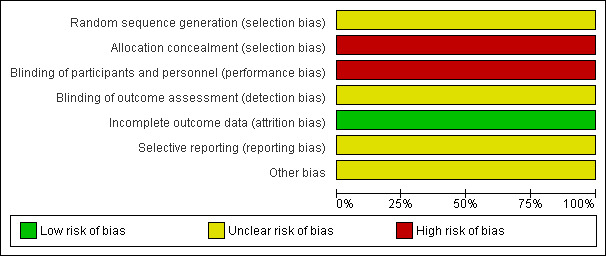
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Potential biases in the review process
The existence of only one randomised trial, the lack of grey literature (for example, presentations, unpublished data, government reports, and other traditional or non‐traditional sources of evidence), the incomplete number of outcomes and the lack of standard deviations in the included trial lead to the limitations of this review. We cannot exclude publication bias. We do not think we have been biased during the review process, and all decisions were reached through consensus between at least two authors.
Agreements and disagreements with other studies or reviews
Chlorambucil has been used to treat many diseases, such as chronic lymphocytic leukaemia (Robak 2000; Knauf 2009), low grade non‐Hodgkin's lymphoma (Chisesi 1991; Taylor 2006), Waldenstrom's macroglobulinaemia (Kyle 2000; Johnson 2005), breast cancer (Senn 1997), idiopathic glomerulonephritis (Nand 1997), membranous nephropathy (Ponticelli 1995), systemic sclerosis (Clements 1993), ovarian cancer (Tattersall 1992), children's idiopathic nephrotic syndrome (Niaudet 1992), and rheumatoid arthritis (Cannon 1985). In several studies, chlorambucil achieved beneficial effects to a certain extent and did not clearly show more adverse events (for example, infection) compared with other drugs (Niaudet 1992; Robak 2000; Knauf 2009).
The exact cause of primary biliary cirrhosis is unknown, but the dynamics of the disease resemble the group of 'autoimmune diseases'. The natural history and progression of the disease varies greatly among individual patients (Ishibashi 2011). Some patients live long without any symptoms while other patients present with jaundice and develop hepatic failure in the early phases of the disease. The bile acid ursodeoxycholic acid (UDCA) is the only FDA‐approved drug in patients with primary biliary cirrhosis, but it is uncertain if UDCA has beneficial effects on mortality or liver transplantation (Gong 2008). Some patients do not respond to UDCA, and have advanced histologic disease at presentation. These patients require different or adjuvant treatment. However, cyclosporin A (Gong 2007a), methotrexate (Giljaca 2010), D‐penicillamine (Gong 2004a), and azathioprine (Gong 2007b) have no significant effects on survival or progression of the disease (cirrhosis development), and cause more adverse events than placebo. No convincing evidence exists to support or refute the use of glucocorticosteroids (Prince 2005) and colchicine (Gong 2004b) in patients with primary biliary cirrhosis.
A case report (Hughes 1983) suggested that four months of chlorambucil treatment improved the clinical symptom (for example, pruritus and jaundice), laboratory parameters, and hepatic granulomas of patients with sarcoidosis presenting as biliary cirrhosis. In the Israel 1991 trial, chlorambucil treatment was a useful alternative in patients with severe sarcoidosis that was unresponsive, or had developed tolerance, to corticosteroids. Severe hepatic sarcoidosis and primary biliary cirrhosis may have similar clinical and pathologic findings. This is why researchers have hypothesised that chlorambucil may also be an useful adjunct in the treatment of primary biliary cirrhosis when the disease is unresponsive to UDCA or to glucocorticosteroids or other interventions.
We cannot rule out a possible role of chlorambucil in a subset of primary biliary cirrhosis patients with a very aggressive disease and in whom adverse effects would not be a major problem. But based on the available evidence, we cannot identify any evidence to support or reject the use of chlorambucil for patients with primary biliary cirrhosis.
Further research should focus on understanding the pathogenic process of the disease and finding an effective medical treatment for patients with primary biliary cirrhosis.
Authors' conclusions
Implications for practice.
Based on the evidence from one trial with 24 participants only, we cannot come to any firm conclusions on the clinical importance of chlorambucil for primary biliary cirrhosis. Adverse events may be frequent, and hence we cannot advocate the use of chlorambucil for patients with primary biliary cirrhosis outside randomised clinical trials.
Implications for research.
Only 24 patients have been investigated in this review. There is insufficient evidence to support chlorambucil for primary biliary cirrhosis. Therefore, we recommend that placebo‐controlled randomised trials should be considered. Such trials ought to be conducted and repeated accordingly to the CONSORT guidelines.
Acknowledgements
We primarily extend our acknowledgements to the patients who took part in the trial and the investigators who designed and conducted the reviewed trial. We thank all the people who were involved in the preparation of this review, especially to Sarah Louise Klingenberg and Dimitrinka Nikolova for the expert assistance that they have provided.
Review Peer Reviewers: Pietro Invernizzi, Italy; Luit Penninga, Denmark. Contact Editor: Ronald L Koretz, USA; Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | March 2012. | (chlorambucil* OR chloroambucil* OR Chloraminophen* OR chlorbutin* OR chlorobutin* OR ambochlorin OR amboclorin OR elcloril OR ecloril OR leukeran OR leukersan OR linfolizin OR linfolysin OR pepstatin) AND ('primary biliary cirrhosis' OR PBC) |
| The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 2, 2012. | #1 MeSH descriptor Chlorambucil explode all trees #2 chlorambucil* OR chloroambucil* OR Chloraminophen* OR chlorbutin* OR chlorobutin* OR ambochlorin OR amboclorin OR elcloril OR ecloril OR leukeran OR leukersan OR linfolizin OR linfolysin OR pepstatin #3 (#1 OR #2) #4 MeSH descriptor Liver Cirrhosis, Biliary explode all trees #5 primary biliary cirrhosis OR PBC 666 #6 (#4 OR #5) #7 (#3 AND #6) |
| MEDLINE (Ovid SP) | 1946 to March 2012. | 1. exp Chlorambucil/ 2. (chlorambucil* or chloroambucil* or Chloraminophen* or chlorbutin* or chlorobutin* or ambochlorin or amboclorin or elcloril or ecloril or leukeran or leukersan or linfolizin or linfolysin or pepstatin).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 3. 1 or 2 4. exp Liver Cirrhosis, Biliary/ 5. (primary biliary cirrhosis or PBC).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 6. 4 or 5 7. 3 and 6 |
| EMBASE(Ovid SP) |
1974 to March 2012. | 1. exp chlorambucil/ 2. (chlorambucil* or chloroambucil* or Chloraminophen* or chlorbutin* or chlorobutin* or ambochlorin or amboclorin or elcloril or ecloril or leukeran or leukersan or linfolizin or linfolysin or pepstatin).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp primary biliary cirrhosis/ 5. (primary biliary cirrhosis or PBC).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. 3 and 6 |
| SCI‐EXPANDED (http://apps.isiknowledge.com) |
1900 to March 2012. |
#1 TS=(chlorambucil* or chloroambucil* or Chloraminophen* or chlorbutin* or chlorobutin* or ambochlorin or amboclorin or elcloril or ecloril or leukeran or leukersan or linfolizin or linfolysin or pepstatin) #2 TS=(primary biliary cirrhosis or PBC) # 3 #2 AND #1 |
| Chinese Biochemical CD Database(CBM‐CD) | 1976 to March 2012. | cirrhosis AND (chlorambucil* or chloroambucil* or Chloraminophen* or chlorbutin* or chlorobutin* or ambochlorin or amboclorin or elcloril or ecloril or leukeran or leukersan or linfolizin or linfolysin or pepstatin) / |
| Chinese Medical Current Contents (CMCC‐CD) | 1994 to March 2012. | Same as CBM‐CD |
| China Hospital Knowledge Database (CHKD) | 1994 to March 2012. | Same as CBM‐CD |
| the database of ongoing trials (at http://www.controlled‐trials.com/mrct/) | March 6, 2012. | ‘Chlorambucil’ AND ‘primary biliary cirrhosis’ or (‘leukeran’ AND ‘primary biliary cirrhosis’) or (‘Amboclorin’ AND ‘primary biliary cirrhosis’) or (‘elcloril’ AND ‘primary biliary cirrhosis’) or (‘linfolizin’ AND ‘primary biliary cirrhosis’) or (‘pepstatin’ AND ‘primary biliary cirrhosis’) |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hoofnagle 1986.
| Methods | Prospective, randomised, clinical trial. | |
| Participants | Inclusion criteria:
Patients were between 30 and 65 years old and had symptomatic primary biliary cirrhosis. The diagnosis of primary biliary cirrhosis was based on: the presence of marked elevations in serum alkaline phosphatase and compatible liver biopsy histopathology. Exclusion criteria: Patients with a history of ascites, oesophageal variceal haemorrhage, or hepatic decompensation (bilirubin more than 12 mg/dl, prothrombin time more than 3 s prolonged). Characteristics of the included patients: N = 24 (intervention:13; control: 11). Mean age: 47 years. Proportion of females: 95.8%. Race: 21 white, 1 black, 2 Asian. All patients had symptoms attributable to their chronic liver disease: 23 pruritus, 23 fatigue, 6 jaundice. Twenty‐two patients had positive serum mitochondrial antibody. The serum alkaline phosphatase levels in all patients were elevated more than three‐fold. |
|
| Interventions | Experimental group: chlorambucil.
Initial dose of chlorambucil: 10 mg/day for 10 days followed by mean 2 mg/day. The dose was further adjusted to keep the lymphocyte count at half of pretreatment levels. The drug dose was halved if the white blood count was less than 3000/mm3 or the platelet count was less than 100000/mm3.
Chlorambucil was stopped if leucopenia or thrombocytopenia persisted at a dose of only 0.5 mg/day. Control group: no treatment. Co‐intervention: the two groups of patients were continued on their other routine medications. |
|
| Outcomes | Mortality. Adverse events. Changes in biochemical indices (eg, bilirubin, alkaline phosphatase, aspartate aminotransferase). Changes in immunoglobulins and mitochondrial antibody levels. Changes in the intensity of hepatic inflammation, the degree of hepatic fibrosis, and the hepatic histologic stage of primary biliary cirrhosis. |
|
| Notes | Language: English. Study settings: United Kingdom. Sample size calculations: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | High risk | Patients were randomised to the chlorambucil therapy or the untreated control group using a set of coded envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the participants nor the personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial only indicated that the liver biopsy histology outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants and reasons for dropouts in the trial were described. |
| Selective reporting (reporting bias) | Unclear risk | We did not find the trial protocol. |
| Other bias | Unclear risk | Not reported, we could not find whether the trial funded by the industry. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kaplan 1987 | Editorial, not a clinical trial. |
Differences between protocol and review
Two additional Chinese databases (Chinese Medical Current Contents (CMCC‐CD), China Hospital Knowledge Database (CHKD), and a database of ongoing trials (at http://www.controlled‐trials.com/mrct/) were also searched in this review.
Two bias risk domains were removed as the domains 'baseline imbalance' and 'early stopping of trials' are not routinely judged when assessing the risk of bias in an included trial of a systematic review.
We had planned to use RevMan 5 for statistical analysis. However, as only one trial fulfilled the inclusion criteria of our review, and as the trial was small, we could not use RevMan. Instead, we used Fischer's exact test to calculate and present our data.
Contributions of authors
Obtain copies of studies: WX Li. Select which studies to include: WX Li, CHR Shi, and X Yan. Extract data from studies: WX Li, AP Zhang, and CHR Shi. Enter data into RevMan: WX Li and AP Zhang. Carry out the analysis: WX Li and X Yan. Interpret the analysis: WX Li and X Yan. Draft the final review: WX Li and X Yan. Updates of the review: WX Li and X Yan.
Sources of support
Internal sources
-
First Hospital of Lanzhou University, Not specified.
Lanzhou University, China
External sources
No sources of support supplied
Declarations of interest
None known
New
References
References to studies included in this review
Hoofnagle 1986 {published data only}
- Hoofnagle JH, Davis GL, Schafer DF, Peters M, Avigan MI, Pappas SC, et al. Randomized trial of chlorambucil for primary biliary cirrhosis. Gastroenterology 1986;91(6):1327‐34. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Davis GL, Schafer DF, Peters MG, Avigan MI, Hanson RG, et al. Randomized trial of chlorambucil for primary biliary cirrhosis [AASLD abstract]. Hepatology 1984;4(5):1062. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kaplan 1987 {published data only}
- Kaplan MM. Another treatment for primary biliary cirrhosis. Gastroenterology 1987;92(1):255‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Alempijevic 2009
- Alempijevic T, Krstic M, Jesic R, Jovanovic I, Sokic Milutinovic A, Kovacevic N, et al. Biochemical markers for non‐invasive assessment of disease stage in patients with primary biliary cirrhosis. World Journal of Gastroenterology 2009;15(5):591‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2003
- Baker SG, Kramer BS. A perfect correlate does not a surrogate make. BMC Medical Research Methodology 2003;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergasa 2005
- Bergasa NV. The pruritus of cholestasis. Journal of Hepatology 2005;43(6):1078‐88. [DOI] [PubMed] [Google Scholar]
Cannon 1985
- Cannon GW, Jackson CG, Samuelson CO Jr, Ward JR, Williams HJ, Clegg DO. Chlorambucil therapy in rheumatoid arthritis: clinical experience in 28 patients and literature review. Seminars in Arthritis and Rheumatism 1985;15(2):106‐18. [DOI] [PubMed] [Google Scholar]
Chisesi 1991
- Chisesi T, Congiu M, Contu A, Coser P, Moretti L, Porcellini A, et al. Randomized study of chlorambucil (CB) compared to interferon (alfa‐2b) combined with CB in low‐grade non‐Hodgkin's lymphoma: an interim report of a randomized study. Non‐Hodgkin's Lymphoma Cooperative Study Group. European Journal of Cancer 1991;Suppl 4:31‐3. [DOI] [PubMed] [Google Scholar]
Christensen 1985
- Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, et al. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology 1985;89(5):1084‐91. [DOI] [PubMed] [Google Scholar]
Clements 1993
- Clements P, Lachenbruch P, Furst D, Paulus H. The course of skin involvement in systemic sclerosis over three years in a trial of chlorambucil versus placebo. Arthritis and Rheumatism 1993;36(11):1575‐9. [DOI] [PubMed] [Google Scholar]
Dickson 1985
- Dickson ER, Fleming TR, Wiesner RH, Baldus WP, Fleming CR, Ludwig J, et al. Trial of penicillamine in advanced primary biliary cirrhosis. New England Journal of Medicine 1985;312(16):1011‐5. [DOI] [PubMed] [Google Scholar]
Dickson 1989
- Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology 1989;10(1):1‐7. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gershwin 1987
- Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. Journal of Immunology 1987;138(10):3525‐31. [PubMed] [Google Scholar]
Gershwin 2008
- Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology 2008;47(2):737‐45. [DOI] [PubMed] [Google Scholar]
Giljaca 2010
- Giljaca V, Poropat G, StimacD, Gluud C. Methotrexate for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD004385.pub3] [DOI] [PubMed] [Google Scholar]
Gluud 2005
- Gluud C. Mortality from cirrhosis: lack of progress over the last 35 years. Gut 2005;54(11):1523‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2012
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2012, Issue 2. Art. No.: LIVER.
Goldblatt 2002
- Goldblatt J, Taylor PJ, Lipman T, Prince MI, Baragiotta A, Bassendine MF, et al. The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology 2002;122(5):1235‐41. [DOI] [PubMed] [Google Scholar]
Gong 2004a
- Gong Y, Klingenberg SL, Gluud C. D‐penicillamine for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004789.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gong 2004b
- Gong Y, Gluud C. Colchicine for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004481.pub2] [DOI] [PubMed] [Google Scholar]
Gong 2007a
- Gong Y, Christensen E, Gluud C. Cyclosporin A for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005526.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gong 2007b
- Gong Y, Christensen E, Gluud C. Azathioprine for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006000.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gong 2008
- Gong Y, Huang ZB, Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD000551.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoofnagle 1984
- Hoofnagle JH, Davis GL, Schafer DF, Peters MG, Avigan MI, Hanson RG, et al. Randomized trial of chlorambucil for primary biliary cirrhosis [AASLD abstract]. Hepatology 1984;4(5):1062. [DOI] [PubMed] [Google Scholar]
Hughes 1983
- Hughes GS Jr, Kataria YP, O'Brien TF Jr. Sarcoidosis presenting as biliary cirrhosis: treatment with chlorambucil. Southern Medical Journal 1983;76(11):1440‐2. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Invernizzi 1997
- Invernizzi P, Crosignani A, Battezzati PM, Covini G, Valle G, Larghi A, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody‐positive and ‐negative primary biliary cirrhosis. Hepatology 1997;25(5):1090‐5. [DOI] [PubMed] [Google Scholar]
Ishibashi 2011
- Ishibashi H, Komori A, Shimoda S, Ambrosini YM, Gershwin ME, Nakamura M. Risk factors and prediction of long‐term outcome in primary biliary cirrhosis. Internal Medicine 2011;50(1):1‐10. [DOI] [PubMed] [Google Scholar]
Israel 1991
- Israel HL, McComb BL. Chlorambucil treatment of sarcoidosis. Sarcoidosis 1991;8(1):35‐41. [PubMed] [Google Scholar]
Johnson 2005
- Johnson SA, Owen RG, Oscier DG, Leblond V, Levy V, Jaeger U, et al. Phase III study of chlorambucil versus fludarabine as initial therapy for Waldenstrom's macroglobulinemia and related disorders. Clinical Lymphoma 2005;5(4):294‐7. [DOI] [PubMed] [Google Scholar]
Kaplan 1991
- Kaplan MM, Knox TA. Treatment of primary biliary cirrhosis with low‐dose weekly methotrexate. Gastroenterology 1991;101(5):1332‐8. [DOI] [PubMed] [Google Scholar]
Kaplan 1997
- Kaplan MM. The use of methotrexate, colchicine, and other immunomodulatory drugs in the treatment of primary biliary cirrhosis. Seminars in Liver Disease 1997;17(2):129‐36. [DOI] [PubMed] [Google Scholar]
Kaplan 2002
- Kaplan MM. Primary biliary cirrhosis: past, present, and future. Gastroenterology 2002;123(4):1392‐4. [DOI] [PubMed] [Google Scholar]
Kaplan 2005
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. New England Journal of Medicine 2005;353(12):1261‐73. [DOI] [PubMed] [Google Scholar]
Keus 2009
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [DOI] [PubMed] [Google Scholar]
Kim 2002
- Kim WR, Brown RS Jr, Terrault NA, El‐Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology 2002;36(1):227‐42. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Knauf 2009
- Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. Journal of Clinical Oncology 2009;27(26):4378‐84. [DOI] [PubMed] [Google Scholar]
Kyle 2000
- Kyle RA, Greipp PR, Gertz MA, Witzig TE, Lust JA, Lacy MQ, et al. Waldenström's macroglobulinaemia: a prospective study comparing daily with intermittent oral chlorambucil. British Journal of Haematology 2000;108(4):737‐42. [DOI] [PubMed] [Google Scholar]
Lindor 2009
- Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology 2009;50(1):291‐308. [DOI] [PubMed] [Google Scholar]
Miller 1997
- Miller E. The use of cytotoxic agents in the treatment of immune‐mediated diseases of dogs and cats. Seminars in Veterinary Medicine and Surgery (small animal) 1997;12(3):157‐60. [DOI] [PubMed] [Google Scholar]
Mitchison 1992
- Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three‐year results. Journal of Hepatology 1992;15(3):336‐44. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Nand 1997
- Nand N, Das R, Jaswal TS. Evaluation of efficacy of methylprednisolone, prednisolone and chlorambucil in idiopathic glomerulonephritis. Journal of the Indian Medical Association 1997;95(6):163‐5, 174. [PubMed] [Google Scholar]
Neuberger 1985
- Neuberger J, Christensen E, Portmann B, Caballeria J, Rodes J, Ranek L, et al. Double blind controlled trial of d‐penicillamine in patients with primary biliary cirrhosis. Gut 1985;26(2):114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niaudet 1992
- Niaudet P. Comparison of cyclosporin and chlorambucil in the treatment of steroid‐dependent idiopathic nephrotic syndrome: a multicentre randomized controlled trial. The French Society of Paediatric Nephrology. Pediatric Nephrology 1992;6(1):1‐3. [DOI] [PubMed] [Google Scholar]
Pasha 1997
- Pasha TM, Dickson ER. Survival algorithms and outcome analysis in primary biliary cirrhosis. Seminars in Liver Disease 1997;17(2):147‐58. [DOI] [PubMed] [Google Scholar]
Ponticelli 1995
- Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, et al. A 10‐year follow‐up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney International 1995;48(5):1600‐4. [DOI] [PubMed] [Google Scholar]
Poupon 1996
- Poupon RE, Huet PM, Poupon R, Bonnand AM, Nhieu JT, Zafrani ES. A randomized trial comparing colchicine and ursodeoxycholic acid combination to ursodeoxycholic acid in primary biliary cirrhosis. UDCA‐PBC Study Group. Hepatology 1996;24(5):1098‐103. [DOI] [PubMed] [Google Scholar]
Prince 2004
- Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut 2004;53(6):865‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prince 2005
- Prince M, Christensen E, Gluud C. Glucocorticosteroids for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003778.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robak 2000
- Robak T, Bloński JZ, Kasznicki M, Blasińska‐Morawiec M, Krykowski E, Dmoszyńska A, et al. Cladribine with prednisone versus chlorambucil with prednisone as first‐line therapy in chronic lymphocytic leukemia: report of a prospective, randomized, multicenter trial. Blood 2000;96(8):2723‐9. [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Senn 1997
- Senn HJ, Maibach R, Castiglione M, Jungi WF, Cavalli F, Leyvraz S, et al. Adjuvant chemotherapy in operable breast cancer: cyclophosphamide, methotrexate, and fluorouracil versus chlorambucil, methotrexate, and fluorouracil ‐ 11‐year results of Swiss Group for Clinical Cancer Research trial SAKK 27/82. Journal of Clinical Oncology 1997;15(7):2502‐9. [DOI] [PubMed] [Google Scholar]
Sood 2004
- Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in Victoria, Australia: High prevalence in migrant populations. Gastroenterology 2004;127(2):470‐5. [DOI] [PubMed] [Google Scholar]
Tattersall 1992
- Tattersall MH, Swanson CE, Solomon HJ. Long‐term survival with advanced ovarian cancer: an analysis of 5‐year survivors in the Australian trial comparing combination versus sequential chlorambucil and cisplatin therapy. Gynecologic Oncology 1992;47(3):292‐7. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor PR, White JM, Prescott RJ, Angus B, Galloway MJ, Jackson GH, et al. The addition of oral idarubicin to a chlorambucil/dexamethasone combination has a significant impact on time to treatment failure but none on overall survival in patients with low grade non‐Hodgkin's lymphoma: Results of the Scotlandand Newcastle Lymphoma Group randomized NHL VIII trial. Leukemia & Lymphoma 2006;47(11):2321‐30. [DOI] [PubMed] [Google Scholar]
Verma 1999
- Verma A, Jazrawi RP, Ahmed HA, Northfield TC. Prescribing habits in primary biliary cirrhosis: a national survey. European Journal of Gastroenterology & Hepatology 1999;11(8):817‐20. [DOI] [PubMed] [Google Scholar]
Wiesner 1990
- Wiesner RH, Ludwig J, Lindor KD, Jorgensen RA, Baldus WP, Homburger HA, et al. A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. New England Journal of Medicine 1990;322(20):1419‐24. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


