Abstract
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia seen in cardiovascular departments. Treatments include medical interventions and catheter ablation. Due to uncertainties in medical therapies for AF, and the need to continue sinus rhythm, ablation has been recently considered as a viable alternative. Many new ablation methods based on pulmonary vein isolation (PVI) have been developed.
Objectives
The primary objective of this review was to assess the beneficial and harmful effects of catheter ablation (CA) in comparison with medical treatment in patients with paroxysmal and persistent AF. The secondary objective was to determine the best regimen of CA.
Search methods
Searches were run on The Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library Issue 3 2009, MEDLINE (1950 to August 2009), EMBASE (1980 to August 2009), the Chinese Biomedical Literature Database (1978 to August 2009) and the CKNI Chinese Paper Database (1994 to 2009) . Several journals published in Chinese were also handsearched.
Selection criteria
Randomised controlled trials (RCTs) in people with paroxysmal and persistent AF treated by any type of CA method. Two reviewers independently selected the trials for inclusion.
Data collection and analysis
Assessments of risk of bias were performed by two reviewers, and relative risk (RR) and 95% confidence intervals (CI) were used for dichotomous variables. Meta‐analysis were performed where appropriate.
Main results
A total of 32 RCTs (3,560 patients) were included. RCTs were small in size and of poor quality.
CA compared with medical therapies: seven RCTs indicated that CA had a better effect in inhibiting recurrence of AF [RR 0.27; 95% CI 0.18, 0.41)] but there was significant heterogeneity. There was limited evidence to suggest that sinus rhythm was restored during CA (one small trial: RR 0.28, 95% CI 0.20‐0.40), and at the end of follow‐up (RR 1.87, 95% CI 1.31‐2.67; I2=83%). There were no differences in mortality (RR, 0.50, 95% CI 0.04 to 5.65), fatal and non‐fatal embolic complication (RR 1.01, 95% CI 0.18 to 5.68) or death from thrombo‐embolic events (RR 3.04, 95% CI 0.13 to 73.43).
Comparisons of different CAs; 25 RCTs compared CA of various kinds. Circumferential pulmonary vein ablation was better than segmental pulmonary vein ablation in improving symptoms of AF (p<=0.01) and in reducing the recurrence of AF (p<0.01). There is limited evidence to suggest which ablation method was the best.
Authors' conclusions
There is limited evidence to suggest that CA may be a better treatment option compared to medical therapies in the management of persistent AF. This review was also unable to recommend the best CA method.
Keywords: Humans, Anti‐Arrhythmia Agents, Anti‐Arrhythmia Agents/therapeutic use, Atrial Fibrillation, Atrial Fibrillation/drug therapy, Atrial Fibrillation/mortality, Atrial Fibrillation/prevention & control, Atrial Fibrillation/surgery, Catheter Ablation, Catheter Ablation/adverse effects, Catheter Ablation/methods, Catheter Ablation/mortality, Pulmonary Veins, Pulmonary Veins/surgery, Quality of Life, Randomized Controlled Trials as Topic, Secondary Prevention, Treatment Outcome
Plain language summary
Catheter ablation would be an alternative to inhibit recurrence of paroxysmal or persistent atrial fibrillation
Atrial fibrillation is a common arrhythmic disease where the heart beats rapidly and irregularly. This can occur for separate brief or long episodes (paroxysmal) or it may become continuous (persistent). This review's aim was to establish whether catheter ablation was better than medical therapies to control heart rate or rhythm for paroxysmal and persistent AF. If catheter ablations were found to be better, the aim was to determine which ablation method was superior to the other. In catheter ablation, a thin tube is passed through a vein to the heart through which instruments can target the misfiring parts of the tissue that control the hearts rhythm. A total of thirty two randomised controlled trials (RCTs) were included in this review. Catheter ablation may be superior to medical treatment but the data is inconclusive in inhibiting recurrence of AF. Embolic complications were commonly caused by catheter ablation. Although these complications and death rate of catheter ablation were similar to that of medical therapies, catheter ablation may cause adverse events of radiation exploration. We were also unable to determine which catheter ablation technique was the best as most RCTs were small scale. Evidence from RCTs cannot yet support catheter ablation as the first line of treatment for paroxysmal and persistent AF.
Background
Description of the condition
Atrial fibrillation is the most common cardiac arrhythmia seen in cardiovascular departments. Prevalence of AF has been increasing in the past decades, is most prevalent among the elderly and hospitalisation for AF poses a great public health burden (Wattigney 2003). Evidence suggests that the incidence of AF increases from 3.8% among men in their 50s to 9% in the general population over 70 years of age (Kannel 1982). Existing cardiovascular disease is correlated to AF. Thromboembolism is the most common complication of AF. Patients with persistent or permanent AF have a higher risk of cerebral embolism (Bernhardt 2006). Moreover, AF usually coexists with heart failure (Hoppe 2006). The increase of complications may worsen prognosis, and AF itself could increase mortality of cardiovascular diseases and the risk of stroke (Crandall 2009).
There are three kinds of therapies to deal with AF, namely medical therapies, surgical treatment and catheter ablation (Ma 2006). Medical treatments include antiarrhythmic drugs and anticoagulant agents. Antiarrhythmic drugs, including class IA, IC, and III antiarrhythmic agents, are used for cardioversion (Meinertz 2011). In previous studies which compared rate with rhythm control in treating AF, all cause mortality, cardiovascular mortality, incidence of heart failure, thromboembolic complications, and quality of life were not significantly different between treatment groups (Gelder 2002; Wyse 2002). For some patients, to maintain sinus rhythm was difficult (STAF 2003 ) since the application of these medical therapies was limited by their contraindications (Karin 2004; Taylor 2010). A new class III antiarrhythmic agent, dronedarone could effectively prevent recurrence of AF with less adverse events (Singh 2007). In managing AF, anticoagulant agents reduce incidence of stroke in AF (Yamashita 2011) and all cause mortality (Bordin 2003). A clinical practice guideline suggests that warfarin and aspirin are more efficacious than placebo for primary stroke prevention (ORs 0.30; 95%CI 0.19 to 0.48 and 0.68; 95%CI 0.46, 1.02 respectively) (Snow 2003) and it is recommended that adjusted warfarin dose be used for AF patients without contraindication (Garnier 2004). For primary prevention of stroke in non‐valvular AF patients, about minor 25 strokes and about 12 disabling or fatal strokes could be prevented yearly for every 1,000 AF patients given oral anticoagulants (Aguilar 2005). Adverse bleeding events are a main limitation of the use of warfarin (Bechtel 2011) and it has been suggested that aspirin is a safer alternative compared to warfarin (Lip 2011). Other strategies include atrial defibrillators and direct current cardioversion. Atrial or dual chamber pacing could prevent bradycardia‐induced dispersion of repolarization and suppression of atrial premature beats, and maintain atrial‐ventricular synchrony. An implantable atrial defibrillator may also be considered, however, its efficacy and safety remains limited in clinical practice (Tracy 2006). Electrial cardioversion is a strategy of rhythm control, which does not result in any greater reduction in mortality than rate control strategies, and may increase the risk of stroke (Mead 2005). Due to many uncertainties in therapies for AF, and the need to continue sinus rhythm, ablation has been recently considered as a viable alternative.
Catheter ablation for AF
Unlike surgical ablation (Cochrane Systematic Review: Hassantash 2008) which was developed based on the Maze operation (Ma 2006), catheter ablation is performed in catheter laboratories and is undertaken by trained cardiologists. Over a 100 genes may play an important role in the initiation or continuation of AF (Ohki 2005), however, the discovery of the molecular mechanism has not reduced the incidence of AF to date (Cha 2004). Recently, more macroscopic structural disorders such as atrial fibrosis have been discovered (Cha 2004). Functional re‐entry has been the popular theory since 1960s (Moe 1964). Atrial remodelling, including three crossed stages, namely electrical remodelling, contractile remodelling, and structural remodelling (Wijffels 1995), are supposed to be the cause of AF. Remodelling theory establishes the foundation of initiation of AF. The pulmonary vein and ligament of Marshall could be responsible for the rapid activation of AF (Wu 2001). In 1998, Haissaguerre et al. discovered that tacho‐impulse conducted by ectopic focus in pulmonary veins and atrium could induce atrial fibrillation (Haïssaguerre 1998). Ectopic focus ablation of these sites could cure AF, which could easily conduct a conclusion that, isolation between pulmonary veins and atrium would obstruct electric conduction so as to cure AF (Haïssaguerre 1998). Later, circumferential or segmental pulmonary vein isolation (CPVI and SPVI) were developed in 2000 and Pappone et al reported on circumferential pulmonary vein ablation (CPVA) guided by CARTO system, whose ablating site was in the left atrium other than in the pulmonary veins (Pappone 2000; Pappone 2001). CPVA seems have a higher success rate than CPVI or SPVI as it not only isolates the electric connection between pulmonary veins and the left atrium, but also partially ablates the substrate of left atrium. Based on this, many additional ablation of left atrium ablation were designed to increase the success rate (Oral 2002). Furthermore, combined approaches were used to improve the prognosis of both kinds of AF (Li 2008). Catheter ablation was also supposed to improve cardiac function of patients with AF (Lutomsky 2008). However, the effect of additional left atrial ablation based on CPVA or PVI remained controversial (Sawhney 2010). The atrial electrogram is difficult locate precisely, and the region of atrial electrogram varies from patient to patient (Nademanee 2007). Many new catheter ablation methods, as complex fractionated atrial electrograms (CFAEs) and autonomic ganglia ablation, remain under developed (Li 2011). The classifications of ablation techniques are given in Table 1. The different approaches are usually applied alternatively in paroxysmal or persistent AF.
1. Classfications of catheter ablation.
| Type of AF | Basal technics | Skills | Combined Skills |
| Paroxysmal | PVI: targeted at ablation of the muscular sleeves | Ostial PVI | |
| Circular ostials PVI | |||
| Segmental PVI | |||
| PV ablation (Circumferential PV ablation, CPVA) | |||
| Circumferential PV isolation (CPVI) | |||
| Ablation of complex fractionated atrial electrograms (CFAE) | |||
| Ablation of autonomic plexuses | |||
| Combined approaches | PVI plus ablation of vein of Marshall | ||
| PVI plus ablation of superior vena cava | |||
| Inducibility guided additional ablation targeting the substrate | |||
| Adding Mitral isthmus line | |||
| Adding roofline | |||
| PVI plus CFAE | |||
| Combinations of more than 2 techniques | |||
| Double/single Lasso technique | |||
| PV antrum isolation | PV antrum isolation guided by ICE | ||
| Wide anatomic circumferential ablation: encircling the PVs within the left atrium | |||
| Persistent | PVI alone | ||
| Linear lesions | Mitral isthmus ablation | ||
| Roofline ablation | |||
| Ablation of anterior left atrial line | |||
| Combined approaches | PVI plus Mitral ablation | ||
| PVI plus roofline ablation | |||
| PVI plus ablation of anterior left atrial line | |||
| Stepwise ablation techniques | Ablation of focal source in addition to PV foci | ||
| Double atrium ablation |
PV: Pulmonary vein; PVI: pulmonary vein isolation; ICE: Intracardiac Echocardiography.
Catheter ablation can cause some adverse events. Left atrial catheter ablation was reported to promote vasoconstriction of right coronary artery and cause variant angina (Yamashita 2007). Thirty one percent of patients were reported to develop left atrial tachycardia after left atrial catheter ablation with additional ablation lines, compared with only 16% of patients who underwent left atrial catheter ablation alone (Hashimoto 2007). Pulmonary vein stenosis was the most common complication after pulmonary vein electrical isolation. Other complications include phrenic nerve injury (Sacher 2007), thrombotic events (especially cerebral embolism) (Padanilam 2006), and haemorrhagic events (Tang 2006). However, three‐dimensional guiding systems would possibly help to prevent advert events.
The ACC/AHA ESC guidelines suggest that CA may be a second line of treatment for patients who have failed or are intolerant to anti‐arrhythmic medical therapies where anti‐arrhythmic drug therapy is the first line for paroxysmal and persistent AF (Tracy 2006). However, the success of preventing recurrences is low to moderate and a considerable proportion of individuals will discontinue the medication due to side effects. Therefore, there is a need to evaluate the benefits and harms of catheter ablation technique in treating AF.
Objectives
The primary objective of this review was to assess the beneficial and harmful effects of catheter ablation compared with medical therapies in treating patients with paroxysmal and persistent AF. The secondary objective was to determine the best regimen of catheter ablation.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials were included irrespective of blinding, publication status, or language. Quasi‐randomised trials and historically controlled clinical trials were excluded.
Types of participants
Male or female patients, of any age or ethnic origin, who were suffering from paroxysmal and persistent AF.
AF was defined as a supraventricular tachyarrhythmia characterised by uncoordinated atrial activation with consequent deterioration of atrial mechanical function. Episodes of AF lasting less than or equal to 7 days were defined as paroxysmal AF, while sustained episodes lasting more than 7 days were defined as persistent AF. The definitions were according to the ACC/AHA/ESC Guidelines for the Management of Patients With AF in 2001(Ryden 2001). Chronic AF were included in persistent AF, but those patients diagnosed with permanent AF, which was failed by cardioversion or had been foregone, were excluded. Studies using more specific diagnostic criteria were included.
Types of interventions
Catheter ablation was defined as to ablation local myocardial cell by inducing catheters and radiofrequent currents so as to inhibit the re‐entrant cycle or reduce the focal zone and cure the tachycardia (Ma 2006). Any type of catheter ablation, including pulmonary vein electrical isolation, superior vena cava isolation, left atrium posterior wall ablation, crista terminalis ablation, coronary sinus ostium ablation, interatrial septum ablation and 'ligament of Marshall ablation', were included. Catheter ablation for atrial flutter were excluded.
Types of outcome measures
The primary outcomes after treatment (at completion of regimen and at maximum follow up) were:
recurrence of AF (either electrophysiological or clinical recurrence of AF; trials using more specific diagnostic criteria of recurrent AF were taken into consideration);
fatal and non‐fatal embolic complications (including stroke and other thromboembolic events);
all cause mortality;
death of thrombo‐embolic events.
The secondary outcome measures were:
improvement of symptoms of AF (symptoms including palpitation, tachypnoea, chest stuffiness);
sinus rhythm restored during the procedure;
sinus rhythm at last follow up;
other complications (e.g. pericardial effusion, pulmonary vein stenosis); and
quality of life.
Search methods for identification of studies
Electronic searches
The Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (Issue 3 2009), MEDLINE (1950 to August 2009), EMBASE (1980 to August 2009), and the Chinese BioMedical Literature Database (1978 to August 2009) were searched. See Appendix 1 for details of the search strategies. The CKNI Chinese Paper Database (from 1994 to 2009) was also searched. No language or date restrictions were applied.
Searching other resources
Handsearches
The following journals published in Chinese were searched: Journal of Clinical Cardiology; Chinese Journal of Hypertension; Chinese Journal of Arrhythmia; and Chinese Journal of Circulation. Conference proceedings in Chinese, relevant to this topic, were handsearched from 2000 to August 2009.
Additional searches
The reference lists of identified trials and review articles were checked in order to find randomised trials not identified by the electronic searches or handsearches. Ongoing trials were searched through the National Research Register (NRR) Archive (http://www.nihr.ac.uk/Pages/NRRArchive), and the web site www.controlled‐trials.com, and grey literature through the database of Open Grey (http://www.opengrey.eu/).
Data collection and analysis
Selection of trials for inclusion
Two reviewers (HC and JW) independently selected the trials by reading the titles and abstracts of the citations. Any potentially eligible studies were retrieved for further identification according to the pre‐specified selection criteria. Any disagreements were resolved by discussion with a third reviewer (JL).
Assessment of methodological quality
We followed the guidance provided by the Cochrane Handbook (Higgins 2011).
Allocation concealment
'Yes': low risk of bias. Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study.
'Unclear': randomisation stated but no information on method used was available.
'No': high risk of bias. Methods of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated investigators or participants could influence intervention group.
Blinding
Blinding of investigators, participants, outcome assessors and data analysts was assessed. Blinding was judged to be 'inadequate' if the treatment group could be identified in > 20% of participants due to side effects of treatment.
Incomplete outcome data
'Yes': low risk of bias. Specifically reported by authors that intention‐to‐treat analysis was undertaken which was confirmed at study assessment stage. If analysis was not clearly stated but it was confirmed at study assessment stage, it would also be granted a judgement of 'Yes'. 'No': high risk of bias. No intention‐to‐treat analysis was reported and no confirmation at study assessment stage. If the analysis was not clearly stated, or if it was stated but there was no confirmation it had taken place at the study assessment stage, it would also be judged as 'inadequate'. The percentages of participants excluded or lost to follow‐up were reported where possible. Further, we recorded whether included RCTs intention‐to‐treat analysis (Gluud 2001).
Data extraction
Data were extracted independently by two reviewers (HC and JW) by using a self‐developed data extraction form. Papers not in Chinese, English, Japanese, or German were translated with the help of the Cochrane Heart Group. The following characteristics and data were extracted from each included trial: primary author; study design and quality; mean age, gender, and ethnic origin of patients; number of randomised patients and number lost during follow‐up; patient inclusion and exclusion criteria; dosage and duration of interventions, outcome measures; and number and type of adverse events.
Data on the number of patients with each outcome, by allocated treatment group, irrespective of compliance or follow‐up, were sought to allow an intention‐to‐treat analysis. If the above data were not available in the trial reports, further information would be sought by correspondence with the principal investigator.
Data synthesis
The dichotomous data were presented as relative risk (RR) and continuous outcomes as difference in means, both with 95% confidence intervals (CI). Intention‐to‐treat analysis were performed where possible. For dichotomous outcomes, we included patients with incomplete or missing data in a sensitivity analysis by counting them as treatment failures to explore the possible effect of loss to follow up on the findings (a 'worst‐case' scenario). We also performed meta‐analysis within comparisons where individual trials compared the same experimental intervention with the same control intervention. Heterogeneity were analysed using a chi‐squared test and the I² test (Higgins 2011). I² values of 25%, 50%, and 75% correspond to low, medium, and high levels of heterogeneity, respectively. A fixed‐effect meta‐analysis were performed if there is no statistical significant heterogeneity among data from all trials. Otherwise, a random‐effects meta‐analysis were performed. RevMan 5.1 were used to carry out the meta‐analysis.
We intended to display the results as comparisons of:
catheter ablation versus medical therapies.
Subsequent to this
catheter ablation of all kinds versus no intervention or placebo;
types of catheter ablation versus other types;
catheter ablation of all kinds versus rhythm control or rate control medical regimens
If sufficient randomised clinical trials were identified, we planned to perform sensitivity analyses according to their methodological quality:
trials with adequate versus inadequate concealment of allocation;
trials with or without double‐blinding;
trials with or without intention‐to‐treat analysis; and
trials with adequate versus inadequate generation of allocation sequence.
Furthermore, if sufficient randomised clinical trials were identified, we planned to perform the following subgroup analyses:
patients with other diseases; (e.g..diabetes mellitus, or other diseases can not directly cause AF)
children versus adults;
different types of populations;
male versus female; and
the time that sinus rhythm was maintained.
We examined the potential biases (Vickers 1998) according to Egger 1997. A funnel plot was performed by RevMan 5.1 to examine publication bias.
Results
Description of studies
The searches found 1186 records, after duplicates had been removed. 1146 papers were excluded as they were reviews, non‐human research, controlled studies, or RCTs comparing different ablative methods. Of the remaining 40 full texts that were selected initially and read through, 32 RCTs, 3,560 participants, were finally included and analysed in the review.( Figure 1). The other eight RCTs were excluded for reasons depicted in the characteristics of excluded studies. Only two records were found by searching the grey literature database OpenGrey, but neither of them were included as they were not related to catheter ablation for treating paroxysmal or persistent atrial fibrillation.
1.
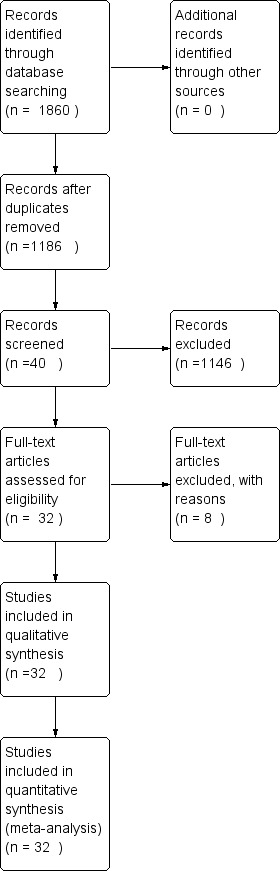
Flow diagram.
1. Catheter ablation compared with medical therapies
A total of seven RCTs, 767 cases, were eligibly included in comparing effects of catheter ablation with that of medical treatment. Among these, 365 cases were randomised to the catheter ablation, and 382 to medical treatment group.
Patients in three RCTs did not discontinue their antiarrhythmics before ablation procedure (Calo 2006; Fassini 2005; Rajappan 2009). Four RCTs did not describe medical therapeutics (Liu02 2006; Marrouche 2007; Oral 2003; Rajappan 2009). Patients in two RCTs were not given antiarrhythmics before ablation procedure (Katritsis 2004; Pappone 2004). For one RCT, class I antiarrhythmics, amiodarone, and sotalol was discontinued for one day and restarted the following day after ablation (Arentz 2007). In the remaining RCTs, antiarrhythmics except for amiodarone were discontinued for three to five half‐lives before ablation. Anticoagulation (heparin) were applied before ablation to a target of international normalized ratio (INR) of 2 to 3.
A total of six patients were lost of follow‐up or withdrew after randomisation; 0.26% in the catheter ablation group, and 0.27% in medical treatment group. In two RCTs, patients were given circumferential pulmonary vein ablation (CPVA) (Oral 2006; Pappone 2006). In another RCT, patients were given cavo‐tricuspid and left inferior pulmonary vein (PV)‐mitral isthmus ablation plus CPVA (Stabile 2006). In three RCTs, pulmonary vein isolation (PVI) was used as ablation methods. Among these three RCTs where patients received PVI in treatment group (Forleo 2009; Jaïs 2008; Wazni 2005), one included paroxysmal and persistent AF patients with type 2 diabetes (Jaïs 2008). In the last RCT, the patients in treatment group received double atrium ablation (Krittayaphong 2003). Left atrium linear ablation isolated the ostia of PVs and a line connecting the circular line with the mitral annulus, and right atrium linear ablation was performed between the tricuspid valve ring and ischmus region. The common medical treatment used was amiodarone; others included sotalol, flecainide, propafenone and other class IC antiarrhythmic drugs. Six patients were died (Stabile 2006; Wazni 2005), of which four patients were in medical treatment and two in catheter ablation group. There were insufficient trials with small patients numbers to undertake sensitivity or subgroup analysis.
2. Comparison of different catheter ablation methods
Twenty‐five RCTs with 2,793 patients were included which compared different catheter ablation methods. The mean age ranged from 50‐60 years old.The catheter ablation methods were various and we had to classify the methods by their purposes. The ablation methods included: CPVA, circumferential pulmonary vein isolation (CPVI), segmental pulmonary vein ablation (SPVA), segmental pulmonary vein isolation (SPVI), PVI, left atrial circumferential ablation (LACA), LACA plus additional ablations in posterior left atrium between two encircling lesions and in mitral isthmus, isolation of large or small areas near pulmonary veins, anterior approach, posterior approach, complex fractionated atrial electrogram (CFAE), or only ablation of superior pulmonary veins. Comparisons were made between:
CPVA and SPVA.
CPVI and SPVI.
CPVA and CPVA plus additional ablation (including PVI).
Superior pulmonary veins ablation and four pulmonary veins ablation.,
LACA and LACA plus additional ablation.,
PVI and LACA.
Large and small areas ablation near pulmonary veins.,
CFAE plus PVI and PVI.
Arrhythmogenic PVI and all PVI.
Eleven RCTs included paroxysmal AF patients (Deisenhofer 2009; Dixit 2008; Hocini 2005; Karch 2005; Katritsis 2004; Oral 2003; Oral 2004; Pappone 2004; Sheikh 2006; Wang 2008; Zhang 2007), four RCTs included persistent AF patients (Calo 2006; Nilsson 2006; Oral 2009; Willems 2006), four included patients with paroxysmal and persistent AF (Arentz 2007; Fassini 2005; Pontoppidan 2009; Verma 2007), one included paroxysmal or chronic AF patients (Lee 2000). The remaining four RCTs did not describe what kind of AF patients were included (Haïssaguerre 2004; Liu 2006; Liu02 2006; Wazni 2003). Only two RCTs reported patients lost to follow‐up (Deisenhofer 2009; Dixit 2008). The total lost rate was 0.21% (Dixit 2008; Deisenhofer 2009). There were insufficient trials with small patients numbers to undertake sensitivity or subgroup analysis.
Risk of bias in included studies
1. Catheter Ablation compared with medical therapies
Selection bias: Three RCTs comparing catheter ablation for rhythm control with medical treatment were randomised by computer (Forleo 2009; Stabile 2006; Wazni 2005). For the remaining papers, randomised methods were not described.
Blinding: None of the RCTs described blinding.
Incomplete outcome addressed: Incomplete data were not addressed in all RCTs.
Funding of trials: Three RCTs were supported by research funds (Krittayaphong 2003; Oral 2006; Wazni 2005).
Publication bias: Seven RCTs were included in a funnel plot (Forleo 2009; Jaïs 2008; Krittayaphong 2003; Oral 2006; Pappone 2006; Stabile 2006; Wazni 2005). The funnel plot was symmetrical, which indicated the publication bias (Figure 2).
2.
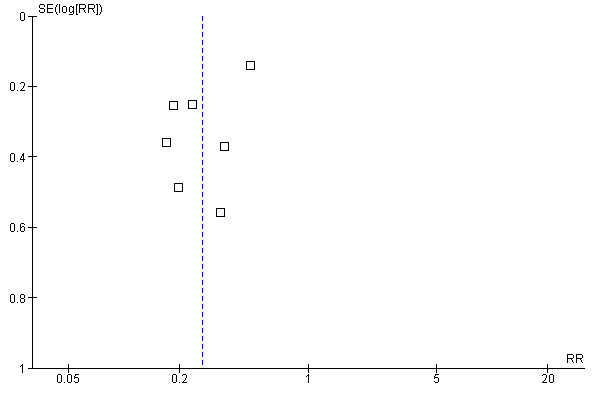
Funnel plot of comparison: 5 Recurrence of AF in comparing CA with Medicines, outcome: 5.1 recurrence of AF.
There were no time lag bias and duplicate publication bias identified. We researched papers from MEDLINE, EMBASE, and also databases in Chinese so as to reduce the location biases, citation biases, and language biases. We searched grey literatures from SIGLE database which could reduce the outcome reporting biases. Our judgements about each risk of bias item was presented in Figure 3 and Figure 4.
3.
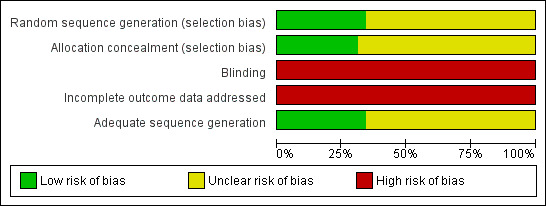
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
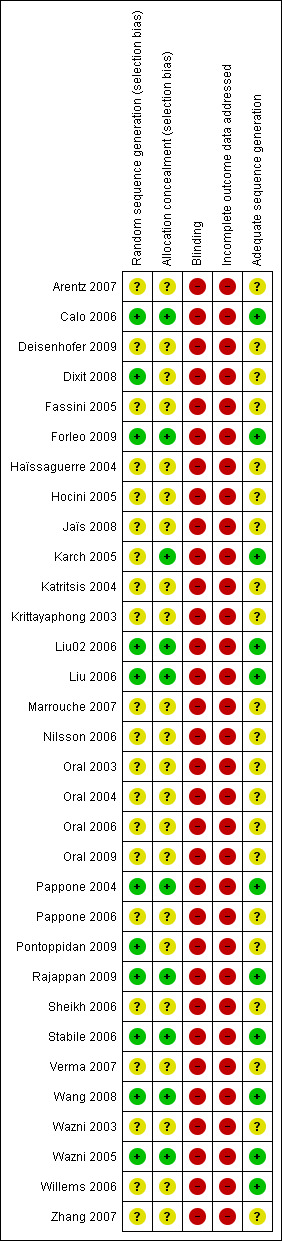
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2. Comparison of different catheter ablation methods
Selection bias: Five RCTs randomised their patients by computer‐generated list (Calo 2006; Liu 2006; Liu02 2006; Pappone 2004; Wang 2008). Two randomised the patients by randomisation code which was concealed in the sealed envelopes (Karch 2005; Rajappan 2009). One RCT randomly arranged the patients with two by two factorial table (Dixit 2008). One claimed 'block randomisation' (Pontoppidan 2009). One RCTs arranged their patients by randomisation number tables but did not depict the methods allocation concealment (Willems 2006). For other papers the randomised methods were not described.
Blinding: One RCT used single blinding (patients) (Wang 2008). The remaining RCTs did not describe blinding.
Incomplete outcome addressed: Incomplete data were not addressed in any of the RCTs.
Funding of trials: Authors of four RCTs were supported by research funds or speaker fees (Karch 2005; Oral 2009; Verma 2007; Zhang 2007).
There were no time lag bias and duplicate publication bias identified. We researched papers from MEDLINE, EMBASE, and also databases in Chinese, so as to reduce the location biases, citation biases, and language biases. We searched grey literatures from SIGLE database which could reduce the outcome reporting biases. Our judgements about each risk of bias item was presented in Figure 3 and Figure 4.
Effects of interventions
1. Catheter Ablation compared with Medical treatment
Primary outcomes
Recurrence of AF
Seven RCTs compared recurrence of AF between catheter ablation and medical treatment (Forleo 2009; Jaïs 2008; Krittayaphong 2003; Oral 2006; Pappone 2006; Stabile 2006; Wazni 2005): 79/ 379 (20.8%) patients in the catheter ablation group and 288/381 (75.6%) patients in the medical treatment group had recurrence of AF at the end of follow up: RR 0.27; 95%CI 0.18, 0.41, but there was significant heterogeneity [Analysis 1.1].
1.1. Analysis.
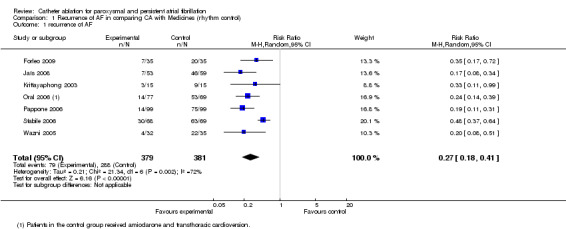
Comparison 1 Recurrence of AF in comparing CA with Medicines (rhythm control), Outcome 1 recurrence of AF.
Fatal and non‐fatal embolic complication
Two small studies compared different ablation techniques with medical treatment (Krittayaphong 2003; Stabile 2006). Among 167 participants, 2/83 (2.4%) in the catheter ablation group and 2/84 (2.4%) in the medical treatment group had embolic complications. The incidence of fatal and non‐fatal embolic complications between both groups was insignificant: RR was 1.01 (95% CI 0.18‐5.68) [Analysis 2.1].
2.1. Analysis.

Comparison 2 Fatal or non‐fatal embolic complications in comparing CA with Medicines (rhythm control), Outcome 1 fatal or non‐fatal embolic complications.
Mortality
One small scale RCT (n=137) reported on mortality (Stabile 2006): 1.5% (1/68) of patients in the catheter ablation group and 2.9% (2/ 69) in the medical treatment group died. There were no significant differences. [Analysis 3.1].
3.1. Analysis.
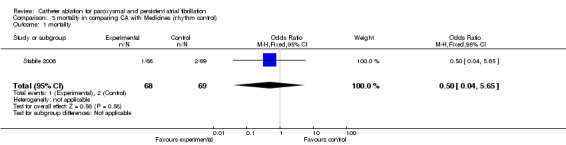
Comparison 3 mortality in comparing CA with Medicines (rhythm control), Outcome 1 mortality.
Death of thrombo‐embolic events
One small scale RCT (n=137) reported that 1/ 68 (1.5%) patient in the catheter ablation group had stroke during the procedure and died of a brain haemorrhage nine months later (Stabile 2006). None of 69 patients in controlled group had a fatal thrombo‐embolic events during the research period. The difference between both group were insignificant. [Analysis 4.1].
4.1. Analysis.
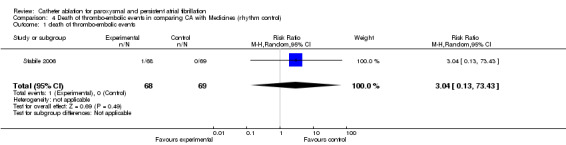
Comparison 4 Death of thrombo‐embolic events in comparing CA with Medicines (rhythm control), Outcome 1 death of thrombo‐embolic events.
Secondary outcomes
Improvement of symptoms of AF
Two RCTs compared CPVA or PVI with amiodarone (Oral 2006; Pappone 2006). The data could not be combined in meta‐analysis as two studies used different effect measurements. In Oral's study, among patients who remained in sinus rhythm, the symptom severity score was 17+/‐4 at baseline and 6+/‐2 points at 12 months after CPVA (p<0.001). Among patients who had recurrent AF or atrial flutter, the symptom severity score was 17+/‐4 at baseline and 12+/‐4 at 12 months after CPVA (p=0.02). A total of fourteen patients (about 16%) reported recurrence of AF soon after ablation procedure, three of whom were asymptomatic (Pappone 2006). Seventy‐five patients (75.8%) in the medical treatment group suffered recurrence of AF, twenty of whom were asymptomatic (Oral 2006).
One RCT compared PVI and linear ablation of right atrium with amiodarone in reducing frequency of symptoms (Krittayaphong 2003). There was no significant reduction between both groups.
Sinus rhythm restored during the procedure
One RCT reported sinus rhythm restored during the procedure: 84% (85/ 99) patients in the catheter ablation group compared with 24% (24/ 99) in the medical therapy group during the same period of observation (Pappone 2006). The difference was statistically significant; RR 0.28, 95% CI, 0.20‐0.40. [Analysis 5.1].
5.1. Analysis.
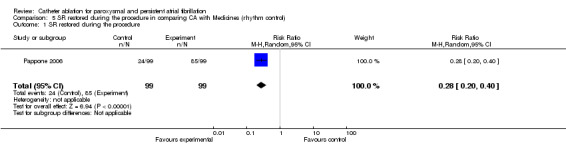
Comparison 5 SR restored during the procedure in comparing CA with Medicines (rhythm control), Outcome 1 SR restored during the procedure.
Sinus rhythm at last follow‐up
Four small RCTs recorded sinus rhythm restored at last follow up (Forleo 2009; Jaïs 2008; Oral 2006; Pappone 2006): 229/264 in catheter ablation group and 128/262 in medical treatment therapy group restored sinus rhythm at last follow‐up; RR 1.87 (95% CI 1.31, 2.67) but heterogeneity was significant; I2 83% [Analysis 6.1].
6.1. Analysis.
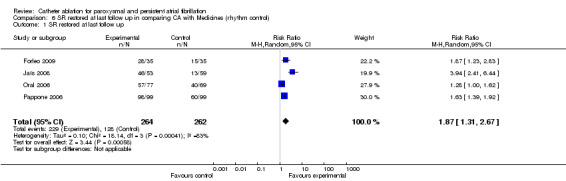
Comparison 6 SR restored at last follow up in comparing CA with Medicines (rhythm control), Outcome 1 SR restored at last follow up.
Other complications
Three patients in medical treatment group (0.8%) suffered sinus dysfunction or sick sinus syndrome (Krittayaphong 2003; Oral 2006), one patient in catheter ablation group (0.3%) had sick sinus syndrome (Oral 2006). Four patients in medical treatment group (1.0%) had bradycardia (Forleo 2009; Wazni 2005). Three patients in catheter ablation group suffered pulmonary vein (PV) stenosis (Jaïs 2008; Wazni 2005). The percentage of PV stenosis in catheter ablation group was 0.8%.
Quality of life
Wazni et al. evaluated a short‐form 36 sub‐scale, among which the general health, physical functioning, bodily pain, social functioning favoured the catheter ablation group (p<0.001, p=0.001, p=0.004 and p=0.004 respectively) (Wazni 2005). In the RCT that included type 2 diabetes patients (Forleo 2009), the mean change of quality of life scores (SF‐36) were greater in PVI group than that of medical therapy group (p<0.05).
One RCT compared physical and mental scores Jaïs 2008. At one year follow‐up, physical and mental component summary scores of the catheter ablation group were significantly higher than those of medical treatment group (p=0.01). Additionally, symptom severity decreased in the ablation group compared with the medical treatment group (p=0.001).
2. Comparison of different catheter ablation methods
Primary outcomes
Recurrence of AF
CPVA compared to SPVA
Four RCTs compared CPVA to SPVA, however, as the outcomes and interventions differed we were unable to combine the data for meta‐analysis (Karch 2005; Liu 2006; Nilsson 2006; Oral 2003).
One RCT (n=100) compared the effects of inhibiting recurrence of AF in CPVA and SPVA (Karch 2005). Eight patients in CPVA group (16%) and eight in SPVA group (16%) had documented recurrence of atrial tachyarrhythmia (AT). Nine in CPVA (18%) and one in SPVA group (2%) were observed with atypical atrial flutter (AFL). The incidence of AFL was statistically different between both groups (p<0.01). However, this paper did not report recurrence of AF.
The recurrence of AF was significant when comparing SPVA with CPVA (Oral 2003). The difference was significant. RR 3.25; (95%CI was 1.16 to 9.12) (Analysis 7.1).
7.1. Analysis.

Comparison 7 Recurrence of AF in comparing SPVA with CPVA, Outcome 1 Recurrence of AF.
One RCT (n=100) compared CPVI and SPVI (Liu 2006) in which 17 patients who underwent SPVI (34%) and 20 who underwent CPVI (40%) had recurrence of atrial tachyarrhythmia. Late recurrence of AF was discovered in one patient in each group (2%, respectively).
One RCT (n=100) compared circumferential extra‐ostial PVI with segmental ostial PVI (Nilsson 2006). After the first ablation procedure, 84 had recurring symptomatic AF (84%) but which group these patients belonged to was not reported.
Left atrial ablation compared to bi‐atrial ablation
One RCT (n=80)compared left atrial with bi‐atrial ablation (Calo 2006). The bi‐atrial ablation group had a lower recurrence of AF (p=0.034).
PVI, CPV(A)I, or left atrium ablation compared to ablation plus additional linear ablation
Five RCTs were classified in this section (Oral 2004; Pappone 2004; Pontoppidan 2009; Sheikh 2006; Wang 2008).
One RCT (n=100) compared PVI and PVI plus left atrium linear ablation (Sheikh 2006). Nine patients in PVI group and five in PVI plus left atrium linear ablation group had recurring AF. Another RCT (n=280) compared CPVA and modified CPVA in observing recurrence of AF (Pappone 2004). In this study modified CPVA included two additional ablation lines in the posterior left atrium connecting the contra‐lateral superior and inferior PVs and along the mitral isthmus between the inferior aspect of the left‐sided encircling ablation line and the mitral annulus. The recurrence of AF was reported is 14.3% (41 cases) in CPVA group and 12.9% (36 cases) in modified CPVA group respectively (p=0.57).
One RCT (n=149) compared cavo‐tricuspid isthmus block (CTIB) in addition to CPVA with CPVA alone (Pontoppidan 2009). AF recurrence was documented in 34% in CPVA plus CTIB group and 32% in CPVA alone group after the procedure (p=0.71). In 12 months of follow‐up, 45/68 patients in the CPVA plus CTIB group and 39/75 in the CPVA alone group had recurring AF. When a intension‐to‐treat analysis was performed 50/73 patients (68.5%) in the CPVA plus CTIB group and 40/76 (52.6%) in the CPVA alone group had recurred AF. The difference between both groups was significant (p=0.05) Analysis 8.1.
8.1. Analysis.
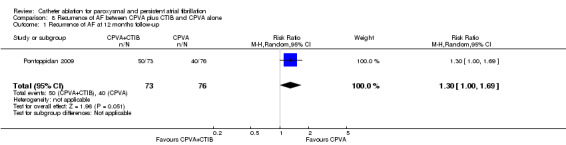
Comparison 8 Recurrence of AF between CPVA plus CTIB and CPVA alone, Outcome 1 Recurrence of AF at 12 months follow‐up.
One RCT (n=106) compared CPVI plus superior vena cava isolation (SVCI) and CPVI alone (Wang 2008). At the end of follow‐up (4.0+/‐2.2 months in CPVI and SCVI group, 4.6+/‐2.3 months in CPVI alone group), eight patients in each group (15.1%) recurred AF and the difference was insignificant.
The recurrence AF was insignificant when comparing LACA to encircle the left‐ and right‐sided PVs (67% vs. 86%, p=0.02) (Oral 2004).
PV‐left atrium junction ablation compared to PV‐left atrium junction ablation combined with cavo‐tricuspid isthmus ablation
The recurrence AF rate was insignificant when comparing PV‐left atrium junction disconnection alone to PV‐left atrium junction disconnection combined with cavo‐tricuspid isthmus ablation (35% vs. 30%, p = NS) (Wazni 2003).
CPVA compared to CPVA plus PVI
CPVA alone had a higher rate of recurrence of AF (36.4%) when comparing to that of CPVA plus PVI (23.3%) (p<0.01) (Zhang 2007).
Superior PVs ablation compared to four‐PV ablation
One RCT (n=52) compared superior PVs ablation and four PVs ablation (Katritsis 2004). Nine in superior PVs ablation group (33.3%) and eight in four‐PV ablation group (32%) recurred AF, and the difference between two groups were statistically insignificant (p = 0.54).
Small area isolation compared to large area isolation around PVs in CPVA
Small isolation area was limited to ostial segmental ablation. Large isolation area was defined as the circumferential ablation was performed on the posterior wall more than 1cm and on the anterior wall more than 0.5cm away from the PV ostia. Small area isolation had a higher rate of recurrence of AF when comparing to large area isolation (Arentz 2007). Twenty‐eight patients in the small area group (50.9%) and fifteen in the large area group (27.3%) had recurrence of AF (p=0.02) (Analysis 9.1).
9.1. Analysis.

Comparison 9 Recurrence of AF between small and large area PVI, Outcome 1 recurrence of AF.
CFAE in addition to pulmonary vein antrum isolation (PVAI) compared to pulmonary vein antrum isolation alone
Two RCTs compared CFAE plus PVAI and PVAI alone (Oral 2009; Verma 2007). Thirty‐four patients in CFAE plus PVAI (22.7%) and twenty‐four in the PVAI group (16%) had recurring AF at the end of follow‐up, the difference was insignificant; RR 1.39 (95% CI was 0.70 to 2.75) [Analysis 10.1]. For paroxysmal AF patients, recurrence occurred in 8/60 cases in the PVAI group (13.3%) and in 9/60 cases in the CFAE plus PVAI group (15%) respectively (p=0.39) (Verma 2007). For persistent and permanent AF patients, 25/90 patients in the CFAE plus PVAI group (27.8%) and 21/90 in the PVAI group (23.3%) had recurrence of AF (p=0.53) [Analysis 10.2].
10.1. Analysis.

Comparison 10 Comparison of recurrence of AF between CFAE plus PVAI and PVAI alone, Outcome 1 Recurrence of AF.
10.2. Analysis.
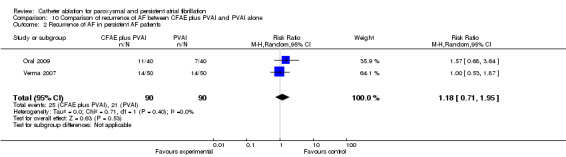
Comparison 10 Comparison of recurrence of AF between CFAE plus PVAI and PVAI alone, Outcome 2 Recurrence of AF in persistent AF patients.
Fatal and non‐fatal embolic complication
No differences in fatal and non‐fatal thrombo‐embolic events were observed when comparing CPVA and SPVA (p=0.61) (Karch 2005), SPVI and CPVI (Liu 2006), and circumferential extra‐ostial PVI with segmental ostial PVI (Nilsson 2006).
Two RCTs compared embolic complications between PVI and PVI plus left atrium linear ablation (Fassini 2005; Karch 2005). Two of 142 (1.4%) patients in PVI group and 0/145 with left atrium ablation addition to PVI group had embolic complications. The difference of incidence of embolic complication was insignificant (p=0.33); RR 3.05 (95% CI was 0.32 to 28.93) (Analysis 11.1).
11.1. Analysis.
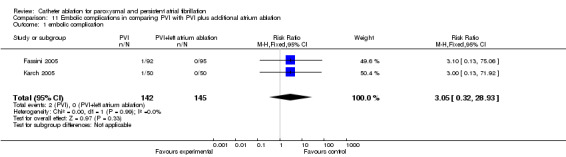
Comparison 11 Embolic complications in comparing PVI with PVI plus additional atrium ablation, Outcome 1 embolic complication.
In one RCT (n=106) which compared CPVI and superior vena cava isolation (SVCI) with CPVI alone (Wang 2008), one patient (0.9%) suffered stroke after ablation but it was not clear which group this patient was belonged to.
One RCT (n=100) reported one stroke event (2%) in the modified CPVA group (Liu 2006). Modified CPVA in the RCT was defined as segmental PV ostia ablation in addition to CPVA. There was no similar complications in CPVA alone group (0%).
In the RCT (n=105) that compared arrhythmogenic PVI and all PVI, one patient in four PVI group (1.9%) suffered left‐sided weakness forty‐five minutes after ablation and was diagnosed with stroke after ablation but recovered within twenty‐four hours. (Dixit 2008)
Mortality
In the RCT (n=105) that compared arrhythmogenic PVI and all PVI, one patient in four PVI group died (1.9%) two weeks after ablation (Dixit 2008).
Death of thrombo‐embolic events
There was no death of thrombo‐embolic events reported.
Secondary outcomes
Improvement of symptoms of atrial fibrillation
Two RCTs compared CPVA and SPVA in improvement of symptoms of AF, both of which followed up for six months. In Karch's study, 27 cases in CPVA group (54%) and 41 cases in SPVA group (82%) were free from arrhythmia‐related symptoms (p<0.01) (Karch 2005). Another reported that 67% cases who underwent SPVA and 89% of those who underwent CPVA were free from symptomatic AF (p=0.01) (Oral 2003).
One RCT (n=83) compared PVI and PVI plus left linear ablation (Sheikh 2006). No significant difference was observed. One RCT (n=52) compared superior PVs ablation and four PVs ablation (Katritsis 2004). Nineteen patients in superior PVs ablation group (70.4%) and 19 in the four PVs ablation group (76%) improved symptomatically at the end of follow‐up, but the differences were not significant.
Sinus rhythm restored during the procedure
Three RCTs reported sinus rhythm restored during the ablation procedure, among which two compared PVI and left atrium linear ablation in addition to PVI (Sheikh 2006; Willems 2006).The data from the first two RCTs were combined (Analysis 12.1). Of the 162 patients who were included, 36 in the PVI group and 61 in the PVI plus left atrium linear ablation group had restored sinus rhythm during the ablation procedure: RR 0.60 (95% CI 0.46‐0.78).
12.1. Analysis.
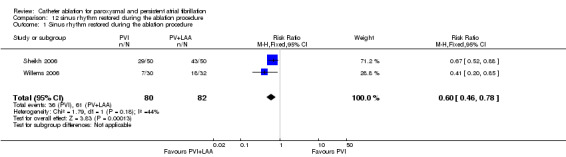
Comparison 12 sinus rhythm restored during the ablation procedure, Outcome 1 Sinus rhythm restored during the ablation procedure.
Sinus rhythm at last follow‐up
CPVA compared to SPVA
Two RCTs compared CPVA and SPVA (Karch 2005; Nilsson 2006). In Karch's study, 21 patients in CPVA group and 33 in SPVA group were in sinus rhythm in six months follow up (Karch 2005). The difference was statistically significant (P<0.01). One RCT compared circumferential extra‐ostial PVI with segmental ostial PVI (Nilsson 2006). At the end of twelve months follow‐up, 31% of patients in the segmental ostial PVI group and 57% in the circumferential extra‐ostial PVI group were free of symptomatic AF without anti‐arrhythmic medicines (p=0.02).
Left atrial ablation compared to bi‐atrial ablation
One RCT (n=80) compared left atrial ablation with bi‐atrial ablation (Calo 2006). Bi‐atrial ablation had a higher rate of restoring sinus rhythm than left atrial ablation at the end of six months follow up (15% vs. 39%, p=0.022).
PVI compared to PVI plus additional left atrium linear ablation
Six RCTs compared PVI and PVI plus additional left atrium linear ablation (Fassini 2005; Haïssaguerre 2004; Hocini 2005; Oral 2004; Sheikh 2006; Willems 2006).
LACA was performed to encircle the left and right‐sided PVs, which is a similar method used in PVI. Of the total patient population, 67% of the patients in LACA group and 86% in the additional ablation group were free of AF at the end of follow‐up (p=0.05) (Oral 2004). At one year follow‐up, the maintenance of stable sinus rhythm was significantly higher in PVD and MIL group as compared to PVD alone group (71+/‐5% vs. 53+/‐5%, P=0.01) (Fassini 2005). The data of the remaining four RCTs were combined and meta‐analysed (Analysis 13.1):104/160 patients in the PVI group (65%), and 135/162 in the PVI plus additional linear ablation group (83.3%) were in sinus rhythm at the end of follow‐up: RR 0.78 (0.60, 1.02).
13.1. Analysis.
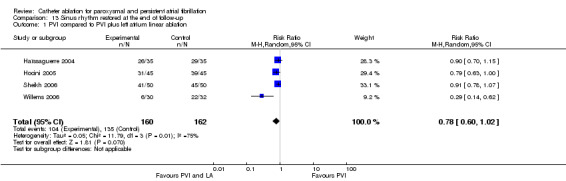
Comparison 13 Sinus rhythm restored at the end of follow‐up, Outcome 1 PVI compared to PVI plus left atrium linear ablation.
One RCT (n=106) compared CPVI plus superior vena cava isolation (SVCI) with CPVI alone (Wang 2008). At the end of 12 months follow‐up, 49 patients in the CPVI plus SVCI (94.2%) and 50 in the CPVI group (92.6%) remained in sinus rhythm (p=0.73).
CPVA compared to modified CPVA
One RCT (n=110) compared CPVA with modified CPVA (Liu02 2006). Another RCT compared CPVA with CPVA plus PVI aim to see if PVI was necessary to the CPVA (Zhang 2007). PVI followed by CPVA could be seen as modified CPVA. The data from these two RCTs were combined (Analysis 13.2); 68/83 patients in CPVA group (81.9%) and 54/80 patients in M‐CPVA group (67.5%) restored sinus rhythm at the end of follow‐up: RR was 1.17 (0.80, 1.70), p=0.41.
13.2. Analysis.
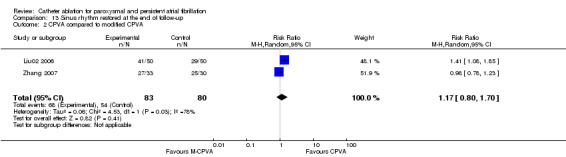
Comparison 13 Sinus rhythm restored at the end of follow‐up, Outcome 2 CPVA compared to modified CPVA.
Superior PVs ablation compared to four‐PV ablation
The restore of sinus rhythm was insignificant when comparing superior PVs ablation with four‐PV ablation in one year follow‐up (p=0.51) (Katritsis 2004) (Analysis 14.1).
14.1. Analysis.
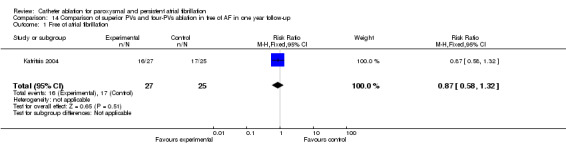
Comparison 14 Comparison of superior PVs and four‐PVs ablation in free of AF in one year follow‐up, Outcome 1 Free of atrial fibrillation.
Small isolation areas compared to large isolation areas
One RCT (n=110) compared small with large isolation areas about PVs (Arentz 2007). In 15+/‐4 months follow‐up, 27/55 patients in small areas group (49.1%) and 37/55 in large areas group (67.3%) remained free of AF or AT. The success rate was higher in paroxysmal AF (19 patients in small areas and 23 in large areas, 54.3% vs. 71.9%, p=0.1) than in persistent AF (eight in small areas and 14 in large areas, 40% vs. 60.9%, p=0.16). But the difference between paroxysmal AF and persistent AF was also statistically insignificant.
Another RCTs (n=149) compared cavo‐tricuspid isthmus block (CTIB) in addition to CPVA and CPVA alone (Pontoppidan 2009). At 12 months of follow up, 31/68 patients in CPVA plus CTIB group (45.6%) and 37/75 in CPVA group (49.3%), sinus rhythm was attained (p=0.64).
CFAE in addition to PV(A)I compared to PV(A)I alone
One RCT compared CFAE in addition to PVI with PVI alone (Deisenhofer 2009). Forty patients in the CFAE plus PVI group (80%) and 34 in the PVI group (70.8%) remained sinus rhythm at long‐term follow‐up. The difference was non‐significant.
Another RCT (n=119) compared CFAE plus PVAI and PVAI alone (Oral 2009). At the end of follow‐up, 30/35 patients in CFAE plus PVAI group (60%) and 34/50 of fifty patients in PVAI group (68%) remained sinus rhythm. The difference between both groups were insignificant (p=0.95) (Analysis 15.1).
15.1. Analysis.
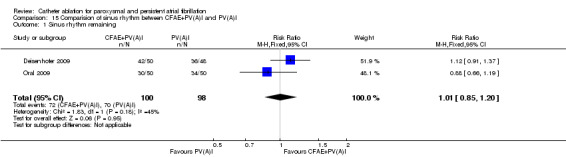
Comparison 15 Comparision of sinus rhythm between CFAE+PV(A)I and PV(A)I, Outcome 1 Sinus rhythm remaining.
Arrhythmogenic PVI compared to all PVI
One RCT (n=105) compared arrhythmogenic PVI with all PVIs (Dixit 2008). Forty‐seven of 52 in arrhythmogenic PVI group (90.4%) and 48/83 patients in all PVI group (90.6%) were AF control at the end of 17+/‐5 months follow‐up. The AF control rate was similar between both groups (p=0.483).
Other complications
CPVI compared to SPVI
In the study (n=100) that compared CPVI and SPVI, one in each group (2%) had asymptomatic right superior PV stenosis (Liu 2006).
CPVA compared to SPVA
Twenty‐two patients in CPVA (44%) and five in SPVA (10%) suffered pericardial effusion, and the difference was significant between two groups (p<0.01). Three patients in CPVA and six in SPVA complicated with pulmonary vein stenosis (p=0.48). (Sheikh 2006)
CPVI compared to CPVI plus linear ablation
One retroperitoneal haematoma and one haemothorax were observed in additional left atrial ablation group. The difference between both groups was insignificant (Calo 2006).
Femoral artery pseudo‐aneurysm occurred in one patient in the CPVI group (1.9%) and two patients in the CPVI plus SVCI group (3.8%) (Wang 2008).
PVI compared to PVI plus left atrium linear ablation
Four main complications were reported, namely cardiac tamponade, pericardial effusion, PV stenosis, and phrenic nerve injury. Only one RCT (n=90) reported one patient (2.2%) developed asymptomatic stenosis of the left superior PV, and one patient (2.2%) developed right phrenic nerve injury (Hocini 2005). One RCT (n=100) reported the complication of pericardial effusion (Sheikh 2006). One patient (2%) underwent PVI developed pericardial effusion, which was not severe and resolved with medicines. Three RCTs reported the incidences of cardiac tamponade (Fassini 2005; Sheikh 2006; Willems 2006). The data were combined and meta‐analysed. One of one hundred and seventy‐two patients (0.6%) in PVI group and two of one hundred and seventy‐seven patients (1.1%) in PVI plus left atrium linear ablation group suffered cardiac tamponade (Analysis 16.1). There were no statistical differences about the incidence of cardiac tamponade between both groups [RR=0.74 (0.15, 3.71), p=0.71].
16.1. Analysis.

Comparison 16 Insidence of cardiac tamponade, Outcome 1 Cardiac tamponade.
CPVA compared to modified CPVA
One patients (2%) in CPVA group developed pericardial tamponade (P=0.50) ). One patients (2%) in CPVA group suffered severe haematoma in femoral venous (p=0.50) . Two patients (4%) underwent modified CPVA had asymptomatic single PV stenosis (p=0.29).(Liu02 2006)
Superior PVs ablation compared to four‐PVs ablation
Two patients (8%) in four‐PV ablation group and none (0%) in superior PVs ablation group had asymptomatic PV stenosis (p=0.27) (Katritsis 2004) (Analysis 17.1).
17.1. Analysis.
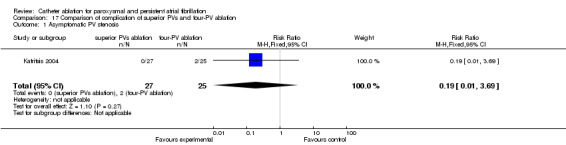
Comparison 17 Comparison of complication of superior PVs and four‐PV ablation, Outcome 1 Asymptomatic PV stenosis.
PV‐left atrium junction ablation compared to PV‐left atrium junction ablation combined with cavo‐tricuspid isthmus ablation
One patient in each group (1.7% in PV‐left atrium junction ablation group, and 2% in PV‐left atrium junction ablation plus cavo‐tricuspid isthmus ablation group) had moderate asymptomatic PV stenosis (p=0.89) (Wazni 2003) (Analysis 18.1).
18.1. Analysis.
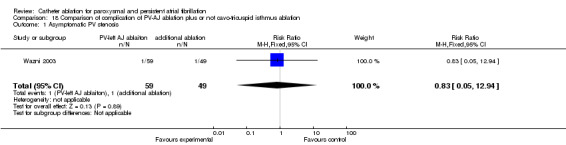
Comparison 18 Comparison of complication of PV‐AJ ablation plus or not cavo‐tricuspid isthmus ablation, Outcome 1 Asymptomatic PV stenosis.
CFAE in addition to PVAI compared to PVAI alone
One patient in CFAE in addition to PVAI had an internal jugular haematoma (1%), and two (2%) in PVAI group complicated by a large haematoma at the femoral venous site. (Verma 2007). Oral 2009 reported two patients who had transient pericarditis (1.7%) and one patient had a small pericardial effusion (0.8%). Another two vascular complications, a self‐limited extraperitoneal bleed and a femoral arteriovenous fistula, were reported (0.8% respectively). However, the authors did not report which group the adverse events were complicated by.
Arrhythmogenic PVI compared to all PVI
One patient (1.9%) in all PVI group had left atrial oesophageal fistula (Dixit 2008). One patient (1.9%) in arrhythmogenic PVI group suffered arteriovenous fistula, and another one (1.9%) in the same group had haematoma in the right neck.
There were no sick sinus syndrome or bradycardia reported. Nine RCTs reported patients with PV stenosis ( Arentz 2007; Deisenhofer 2009; Hocini 2005; Karch 2005; Katritsis 2004; Liu 2006; Liu02 2006; Rajappan 2009; Wazni 2003). A total of 23 patients was reported with PV stenosis, and the percentage was 0.8%.
Quality of life
There were no RCTs comparing various catheter ablation methods in improving the quality of life of AF patients.
Discussion
The systematic review was designed to assess the beneficial and harmful effects of catheter ablation in comparison with medical therapies in patients with paroxysmal and persistent AF. We also sought to determine which catheter ablation technique was superior to the other. Overall, the RCTs were small in size and of poor quality. For our first comparison, we combined results from seven RCTs which indicated that catheter ablation may have a better effect in inhibiting recurrence of AF but there was significant heterogeneity. There was limited evidence to suggest that sinus rhythm was restored but heterogeneity was high. There were no differences in outcomes such as mortality, fatal and non‐fatal embolic complication or death from thrombo‐embolic events. For the second comparison 25 RCTs compared catheter ablation of various kinds. Circumferential pulmonary vein ablation proved marginally better than segmental pulmonary vein ablation in improving symptoms of AF and in reducing the recurrence of AF. Additional ablation to PVI, circumferential pulmonary vein isolation and left atrium ablation were no better in inhibiting recurrence of AF than any other catheter ablation.
Hetergeneity: where possible we undertook pooled analysis and heterogeneity was high in these analysis, particularly those of restored sinus rhythm at last follow up and recurrence of AF. In the first comparison, the I2 statistic was 83% and when we excluded Jais's trial in the analysis, the I2 statistic decreased to 46%. In Forleo's study, the authors used PVI in treatment group, Class Ia/b/c, amiodarone, beta‐blockers and CCBs in control group (Forleo 2009). Jaïs et al. used similar measurements in both groups (Jaïs 2008). Oral et al. used CPVA comparing to amiodarone alone (Oral 2006). And Pappone et al. compared CPVA to amiodarone, flecainide, or sotalol, either as single drugs or in combination (Pappone 2006). Forleo et al. included patients with type 2 diabetes (Forleo 2009). Oral and Pappone included chronic atrial fibrillation (Oral 2006; Pappone 2006). Only Jaïs et al. included 'pure' paroxysmal atrial fibrillation patients (Jaïs 2008). Furthermore, the patients in Jaïs's study were younger than other three RCTs. So it was possible that the significant heterogeneity had been induced by two factors: age and category of AF. For the second comparison; 'recurrence of AF', we found that if Stabile's study was excluded in the analysis, the I2 statistic decreased from 72% to 0%. After reviewing all seven RCTs, we found that only Stabile et al. used additional atrial ablation after CPVA in treatment group (Stabile 2006) while additional ablation in left atrium was not performed in other studies. However the recurrence rate in the treatment group was higher than those results reported by other authors. So we cautiously suggest that additional atrial ablation (Cavo‐tricuspid and left inferior pulmonary vein‐mitral isthmus ablation) after CPVA may not have an effect on reducing recurrence of AF. This is also true for the direct comparison of CPVA plus additional atrial ablation and CPVA.
Adverse events: there were no differences between catheter ablation and medical treatment in death, fatal and non‐fatal embolic complications. Peripheral vascular complications, pericardial effusion, cardiac tamponade, cerebral embolisms, and pulmonary vein stenosis underlie the main adverse events of catheter ablation. A cumulative complication rate of 3.9% was reported by a multi‐centra trial in Italy (Bertaglia 2007). Atrial oesophageal fistula was one of the fatal complications of catheter ablation, even though its incidence rate was low (Morady 2005). As the clinicians explored radiation, the relationship of quantum and harmfulness should be carefully calculated so as to protect clinicians and patients. Radiation related adverse events were not evaluated in the RCTs. A multicenter survey reported that death occurred in one of 1,000 AF patients who received catheter ablation treatment (Cappato 2009). The main reason of death was tamponade, stroke, atrial oesophageal fistula. A recently published meta‐analysis also made a conclusion that, comparing to medical therapy, catheter ablation could reduce the recurrence of atrial tachyarrhythmia of AF without increasing adverse events (Bonanno 2010). Although this review included AF patients of all types, their result was similar to that of our review. However, the author suggested that as most trials were performed by experts from high‐volume centres, and anti‐arrhythmia treatments were co‐committed with catheter ablation, the results of catheter ablation were possibly over‐estimated.
Quality of life: the SF‐36 was mostly used to evaluate the quality of life in our RCTs. Our results indicate that CA could improve the aspects of quality of life of patients (Forleo 2009; Wazni 2005) with paroxysmal AF (Reynolds 2010), or persistent AF (Lu 2009). Those AF patients with asymptomatic arrhythmia would possibly acquire more benefit by accepting CA than with symptomatic arrhythmia (Pontoppidan02 2009).
Cost‐effectiveness of catheter ablation was not supported by RCT evidence (McKenna 2009). Reynolds et al included a published clinical trial and registry data and developed a Markov disease simulation model to estimate the quality‐adjusted life expectancy. The study concluded that catheter ablation could improve quality of life and avoid future health costs (Reynolds 2009). In another study, the cost‐effectiveness of catheter ablation for paroxysmal AF, catheter ablation was reported to be cost‐effective (Andrikopoulos 2009).
Catheter ablation: There were a variety of ablation methods used. The concrete ablation strategies included: (1) PV isolation under the electrophysiological mapping systems. A mapping catheter should be put inside the PVs before the ablation of the anticipate PVs potentials. (2) Left atrium ablation under the 3 dimension guidance system. Reconstruction of left atrium model could be performed by CARTO or EnSite system. The systems could also guide the movement of catheters in the left atrium. PVs ostia and left atrium liner ablation should be performed. This ablation method did not require fully isolation of PVs, in stead of which it reduced the potentials voltage to lower than 0.1mV. (3) Circumferential PVs isolation (CPVI). This ablation method required fully isolation of PVs, and the isolating area included PVs ostia and left atrium transition zone. (4) Other ablation methods included complex fractionated atrial electrograms (CFAE) and ganglial ablation in left atrium. Circumferential pulmonary vein ablation was the basis of catheter ablation in AF. The recurrence rate was lower in CPVA group than that of SPVA (Oral 2003). Pappone et al. had improved its success rate and reduce the procedural time by navigating with the Stereo taxis system (Pappone02 2006; Pappone 2007). There were many RCTs comparing different catheter ablation methods in our review, however, these RCTs were small scale with considerable heterogeneity. There was little evidence available to compare different methods of catheter ablation.The effect of large area isolation was better than small area isolation on preventing recurrence of AF. This conclusion was resulted from a single small scale RCT (Arentz 2007). Large area PV isolation seemed to become a trend in clinical practice. CFAE‐targeted ablation for AF was demonstrated to be effective in maintaining sinus rhythm. Five‐year survival rate was about 92% after CFAE ablation (Nademanee 2008). CFAE sites were difficult to be discovered during the ablation procedure. But this method has been considered as an effective additional ablation method to PVI (Verma 2008).
Even though many ablation methods were developed, the recurrence rate of AF remains high (Ma 2006; Morady 2005). Many patients may need at least two ablation procedures to reduce the recurrence rate of AF (Ouyang 2005). A new technology, autonomic ganglia ablation, was supposed to be more effective than CPVA (Lemola 2008), but no RCT has been undertaken to date.
Authors' conclusions
Implications for practice.
There is limited evidence to suggest that catheter ablation may be a better treatment option compared to medical therapies in management of persistent AF. This review was also unable recommend the best catheter ablation method.
Implications for research.
Economic endpoint outcomes should be considered in future RCTs when comparing catheter ablation with anti‐arrhythmic medicines. If future research suggests catheter ablation could be considered as the first line treatment, the relationship of quantum and harmfulness of radiation should be made clear. One the other hand, larger scale RCTs could be designed to determine which ablation method would be the best in inhibiting recurrence of AF, restoring sinus rhythm, and improving the patients' quality of life.
Acknowledgements
We thank Margaret Burke, Liz Bickerdike, Claire Williams and other staff of the Cochrane Heart Group for providing search strategies, full text and great help with the review. We also thank all participants in the clinical trials for their contribution to our knowledge of catheter ablation for the treatment of paroxysmal and persistent atrial fibrillation. We appreciate Ms. Liao JieYing for reading the review and plain language summary and providing suggestion as a non‐medical person.
Appendices
Appendix 1. Search strategies
CENTRAL on The Cochrane Library
#1 MeSH descriptor atrial fibrillation this term only #2 atrial in All Text #3 atrium in All Text #4 Auricular next Fibrillat* in All Text #5 MeSH descriptor tachycardia this term only #6 MeSH descriptor Tachycardia, Paroxysmal this term only #7 MeSH descriptor Tachycardia, Supraventricular this term only #8 tachycardia* in All Text #9 tachyarrhythmia* in All Text #10 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #11 MeSH descriptor catheter ablation this term only #12 ablat* in All Text #13 (#11 or #12) #14 (#10 and #13)
MEDLINE on Ovid
1 Atrial Fibrillation/ 2 atrial fibrillation.tw. 3 atrium fibrillation.tw. 4 auricular fibrillation.tw. 5 atrial tachycardia$.tw. 6 or/1‐5 7 Catheter Ablation/ 8 (catheter and (ablat$ or isolat$)).tw. 9 (transcatheter and (ablat$ or isolat$)).tw. 10 or/7‐9 11 6 and 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 Randomized controlled trials/ 15 random allocation/ 16 double blind method/ 17 single‐blind method/ 18 or/12‐17 19 exp animal/ not humans/ 20 18 not 19 21 clinical trial.pt. 22 exp Clinical trials as topic/ 23 (clin$ adj25 trial$).ti,ab. 24 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 25 placebos/ 26 placebo$.ti,ab. 27 random$.ti,ab. 28 research design/ 29 or/21‐28 30 29 not 19 31 20 or 30 32 31 and 11
EMBASE on Ovid
1 Heart Atrium Fibrillation/ 2 Heart Atrium Arrhythmia/ 3 atrial fibrillation.tw. 4 atrium fibrillation.tw. 5 auricular fibrillation.tw. 6 atrial tachycardia$.tw. 7 or/1‐6 8 Catheter Ablation/ 9 (catheter and (ablat$ or isolat$)).tw. 10 (transcatheter and (ablat$ or isolat$)).tw. 11 or/8‐10 12 7 and 11 13 clinical trial/ 14 random$.tw. 15 randomized controlled trial/ 16 double blind procedure/ 17 factorial$.ti,ab. 18 (crossover$ or cross‐over$).ti,ab. 19 (double$ adj blind$).ti,ab. 20 (singl$ adj blind$).ti,ab. 21 assign$.ti,ab. 22 allocat$.ti,ab. 23 volunteer$.ti,ab. 24 Crossover Procedure/ 25 Single Blind Procedure/ 26 controlled clinical trial/ 27 or/13‐26 28 27 and 12
Search strategy for Chinese BioMedical Literature Database
(心房颤动【主题词】OR 心房颤动【题目】OR 房颤【题目】OR 心房颤动【摘要】OR 房颤【摘要】)AND ((消融【题目】OR 射频消融【题目】OR 导管消融【题目】)OR (消融【摘要】OR 射频消融【摘要】OR 导管消融【摘要】))
CNKI Chinese Paper Database
(心房颤动【题目】OR 房颤【题目】)AND (射频【题目】OR 射频消融【题目】OR 消融【题目】)
Data and analyses
Comparison 1. Recurrence of AF in comparing CA with Medicines (rhythm control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 recurrence of AF | 7 | 760 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.18, 0.41] |
Comparison 2. Fatal or non‐fatal embolic complications in comparing CA with Medicines (rhythm control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 fatal or non‐fatal embolic complications | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.18, 5.68] |
Comparison 3. mortality in comparing CA with Medicines (rhythm control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 mortality | 1 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 5.65] |
Comparison 4. Death of thrombo‐embolic events in comparing CA with Medicines (rhythm control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 death of thrombo‐embolic events | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [0.13, 73.43] |
Comparison 5. SR restored during the procedure in comparing CA with Medicines (rhythm control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SR restored during the procedure | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.20, 0.40] |
Comparison 6. SR restored at last follow up in comparing CA with Medicines (rhythm control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SR restored at last follow up | 4 | 526 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.31, 2.67] |
Comparison 7. Recurrence of AF in comparing SPVA with CPVA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of AF | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.16, 9.12] |
Comparison 8. Recurrence of AF between CPVA plus CTIB and CPVA alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of AF at 12 months follow‐up | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.00, 1.69] |
Comparison 9. Recurrence of AF between small and large area PVI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 recurrence of AF | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.13, 3.09] |
Comparison 10. Comparison of recurrence of AF between CFAE plus PVAI and PVAI alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of AF | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.70, 2.75] |
| 2 Recurrence of AF in persistent AF patients | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.71, 1.95] |
Comparison 11. Embolic complications in comparing PVI with PVI plus additional atrium ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 embolic complication | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.32, 28.93] |
Comparison 12. sinus rhythm restored during the ablation procedure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sinus rhythm restored during the ablation procedure | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.78] |
Comparison 13. Sinus rhythm restored at the end of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PVI compared to PVI plus left atrium linear ablation | 4 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.02] |
| 2 CPVA compared to modified CPVA | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.80, 1.70] |
Comparison 14. Comparison of superior PVs and four‐PVs ablation in free of AF in one year follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Free of atrial fibrillation | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.32] |
Comparison 15. Comparision of sinus rhythm between CFAE+PV(A)I and PV(A)I.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sinus rhythm remaining | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
Comparison 16. Insidence of cardiac tamponade.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac tamponade | 3 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.15, 3.71] |
Comparison 17. Comparison of complication of superior PVs and four‐PV ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Asymptomatic PV stenosis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.69] |
Comparison 18. Comparison of complication of PV‐AJ ablation plus or not cavo‐tricuspid isthmus ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Asymptomatic PV stenosis | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.05, 12.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arentz 2007.
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: consecutive patients with highly symptomatic, drug‐refractory paroxysmal or persistent episodes of AF. 110 patients were included and assigned into two groups evenly. Age: 56+/‐10 years in small isolation area group, and 55+/‐10 years in large isolation area. % of male: 39/55 (70.9%) in small isolation area group and 44/55 (80%) in large isolation group. Follow‐up: 15+/‐4 months. Location: Herz‐Zentrum, Bad Krozingen, Germany. |
|
| Interventions | Small area of PV ablation compared with large area of PV ablation. | |
| Outcomes | Success rate, recurrence of AF, complications | |
| Notes | PV: Pulmonary vein. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | B‐Unclear. The authors did not depict adequate sequence generation. |
Calo 2006.
| Methods | Randomised by computer‐generated list | |
| Participants | 1) AF resistant to more than three attempts of pharmacological and/or electrical cardioversion; or 2) recurrent, persistent AF despite prophylaxis with a least three different antiarrhythmic drugs (class I and/or III). Age: 57.9+/‐8.9 years in biatrial ablation group, and 59.2+/‐9.1 years in left atrial ablation group. % of male: 26/39 (66.7%) in biatrial ablation group, and 26/41 (63.4%) in left atrial ablation group. Follow‐up: 14+/‐5 months (15+/‐5 months in the biatrial ablation group, and 13+/‐6 months in the left atrial ablation group). Location: Rome, Italy. |
|
| Interventions | 41 patients underwent circumferential ablation plus mitral and cavotricuspid isthmus ablation(left atrial ablation group), while 39 patients underwent biatrial ablation. | |
| Outcomes | Recurrence of AF, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Adequate. Sequence was generated by computer |
Deisenhofer 2009.
| Methods | Randomised controlled study | |
| Participants | 98 patients with age between 18 and 80 years, symptomatic paroxysmal AF with episodes lasting up to 7 days and with ≥4 AF episodes per month, and failed therapy with ≥1 class I or III antiarrhythmic drug were included. 62 patients had structural heart diseases. Age: 58+/‐10 years in PVI group, and 55+/‐10 years in PVI + CFAE group. % of male: 33/48 (69%) in PVI group, and 41/50 (82%) in PVI + CFAE group. Follow‐up: three months. In both groups, two patients were lost to long‐term (3 months) follow‐up. Location: Muenchen, Germany. |
|
| Interventions | PVI compared with PVI plus CFAE | |
| Outcomes | Symptoms improving, sinus rhythm remaining at three months and long‐term follow‐up. | |
| Notes | CFAE: complex fractionated atrial electrograms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | Two patients in both groups lost to follow up. But the incomplete outcome data were not addressed. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Dixit 2008.
| Methods | Randomised with 2*2 factorial table | |
| Participants | 105 patients with a mean age of 57+/‐9 years were included. 77 patients were paroxysmal AF. The median follow‐up duration was 20 months. 53 patients were arranged to the all‐vein group, 52 to arrhythmogenic vein group. Age: 57+/‐9 in all veins group, and 57 +/‐9 in arrhythmogenic veins group too. % of male: 40/53 (75%) in all veins group, and 36/52 (69%) in arrhythmogenic veins group. Follow‐up: one year. 103/105 patients (98%) completed the follow‐up. Location: Pennsylvania, USA. |
|
| Interventions | All four PV isolation compared with arrhythmogenic PV isolation | |
| Outcomes | Recurrence of AF, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A 2 * 2 factorial table. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | One patients died and one lost to follow up in all PV ablation group. The incomplete outcome data were not addressed. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Fassini 2005.
| Methods | Randomised controlled trial | |
| Participants | 187 patients presenting with drug‐refractory (at least 2 drugs, including amiodarone and Ic), recurrent paroxysmal, or persistent AF were enrolled. 92 patients were arranged to pulmonary vein disconnection group and 95 to pulmonary vein disconnection plus mitral isthmus line group. Age: 57+/‐8 years in PVD group (A group), and 54 +/‐10 years in PVD combined with MIL group (B group). % of male: 77/92 (83.7%) in A group, 73/95 (76.8%) in B group. Follow‐up: one year. Location: Milan, Italy. |
|
| Interventions | PVI compared to PVI plus mitral isthmus line ablation | |
| Outcomes | Complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Forleo 2009.
| Methods | Computer‐generated randomisation | |
| Participants | 70 patients with type 2 diabetes with a median age of about 64 years were included. 29 patients were paroxysmal AF, others were persistent AF, who were refractory to >=1 class 1‐3 antiarrhythmic drugs. 35 patients were assigned to ablation group and 35 patients were assigned to medicine group. Age:63.2+/‐8.6 years in ablation group, and 64.8+/‐6.5 years in ADT group. % of male:20/35 (57.1%) in ablation group, and 23/35 (65.7%) in ADT group. Follow‐up: one month. Location: Rome, Italy. |
|
| Interventions | PVI compared with medicines. In medicine group, class IC, sotalol, amiodarone, beta‐blocker, and calcium channel antagonist were applied alone or combined. | |
| Outcomes | Recurrence of AF, quality of life, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by computer program |
Haïssaguerre 2004.
| Methods | Randomised controlled trial | |
| Participants | 70 patients with drug‐refractory AF undergoing curative ablation were included. 35 patients were arranged to PV isolation group, and 30 to PV isolation plus mitral isthmus ablation group. Age: 53+/‐8 years in PVI group, and 53+/‐9 years in PVI + Mitral isthmus ablation group. % of male:26/35 ( 74.3%) in each group. Follow‐up: 12 months. Location: Bordeaux‐Pessac, France. |
|
| Interventions | PV isolation compared with PV isolation plus mitral isthmus ablation | |
| Outcomes | Recurrence of AF, sinus rhythm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Hocini 2005.
| Methods | Randomised controlled trial | |
| Participants | Patients referred for ablation of paroxysmal AF. 45 patients were arranged to PV isolation group, and 45 to isolation plus linear ablation group. Age: 55+/‐8 years in PVI group, and 54+/‐10 years in PVI+Roofline group. % of male: 34/45 (76%) in PVI group, and 37/45 (82%) in PVI+Roofline group. Follow‐up: 15+/‐4 months (14+/‐5 months in PVI+ roofline group, and 15+/‐4 months in PVI group). Location: Bordeaux, France. |
|
| Interventions | PVI compared to PVI plus roofline ablation. In both groups, the cavotricuspid isthmus, fragmented peri‐PV‐ostial electrograms and spontaneous non‐PV foci were ablated. Additional roofline ablation was applied in 45 patients. | |
| Outcomes | Sinus rhythm remaining, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Jaïs 2008.
| Methods | Randomised controlled trial | |
| Participants | 112 Patients aged more than 18 years and with symptomatic, documented paroxysmal AF over a span of >=6 months were included. 53 patients were arranged to ablation group and 59 patients to medicine group. Age: 49.7+/‐10.7 years in RF group, and 52.4+/‐11.4 years in AAD group. % of male: 45/53 (84.9%) in RF group, and 49/59 (83.1%) in AAD group. Follow‐up: 12 months. Location: Paris, France. |
|
| Interventions | PVI compared with medicines. Medicines included class 1, 2, 3, 4, and digoxin. | |
| Outcomes | Recurrence of AF, quality of life, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Karch 2005.
| Methods | Randomised by random code and concealed with sealed envelopes. | |
| Participants | 100 Patients with highly symptomatic, drug‐refractory AF episodes occurring at least twice a month were included. 50 patients were assigned to Circumferential pulmonary vein ablation group, and 50 to Segmental pulmonary vein ablation group. Age: 59 (52‐64) years in CPVA group, and 61 (54‐65) years in SPVA group. % of male: 28/50 (56%) in CPVA group, and 36/50 (72%) in SPVA group. Follow‐up: Six months. Location: Munich, Germany. |
|
| Interventions | CPVA compared to SPVA. | |
| Outcomes | Free of arrhythmia‐related symptoms, sinus rhythm restoring, recurrence of AT, complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Randomisation codes were concealed in the sealed envelopes. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by random code. |
Katritsis 2004.
| Methods | Randomised controlled trial | |
| Participants | 52 patients with drug‐refractory, symptomatic paroxysmal AF were included. | |
| Interventions | 27 in left superior pulmonary vein isolation added isolation of right superior pulmonary vein while 25 in the group with isolation of all four PVs followed by a repeat procedure. Age: 54 +/‐ 9 years in right superior pulmonary vein group (group A), and 50 +/‐ 10 years in all four PVs ablation group (group B). % of male: 22/27 (82%) in group A, and 21/25 (84%) in group B. Follow‐up: 12 months. Location: Athens, Greece. |
|
| Outcomes | Recurrence of AF, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Krittayaphong 2003.
| Methods | Randomised controlled trial. | |
| Participants | Included patients with: (1) 15‐75 years old; (2) symptomatic paroxysmal or persistent AF for more than 6 months; (3) refractory to at least 1 antiarrhythmic medication including class IA or IC agents, digitalis, beta‐blocker, or calcium channel blocker; (4) never been given amiodarone. 30 patients were enrolled. 15 patients were assigned to PVI and linear ablation of right atrium group, and 15 to medicine group. Age: 48.6+/‐15.4 years in amiodarone group, and 55.3+/‐10.5 years in RFCA group. % of male: 8/15 (53.3%) in amiodarone group, and 11/15 (73.3%) in RFCA group. Follow‐up: 12 months. Location: Bangkok, Thailand. |
|
| Interventions | Catheter ablation group: Pulmonary vein isolation and linear ablation of right atrium. Medicine group: amiodarone (1200mg per day for 1 week, 600mg per day for another 2 weeks, and the maintenance dose was 200mg per day). |
|
| Outcomes | Effects of treatment on symptoms and quality of life, effects of treatment on cardiac rhythm, adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Liu 2006.
| Methods | Computer‐generated randomisation | |
| Participants | 100 patiens with 20 to 80 years, symptomatic AF refractory to multiple antiarrhythmic drugs, NYHA functional class I or II, and at least 6 months follow‐up were included. 50 patients were assigned to A‐CPVA group and 50 to M‐CPVA group. Age: 55.4+/11.9 years in A‐CPVA group and 57.5+/‐11.3 years in M‐CPVA group. % of male: 35/50 (70%) in A‐CPVA group, and 34/50 (68%) in M‐CPVA group. Follow‐up: 18 months. Location: Beijing, China. |
|
| Interventions | A‐CPVA group: ipsilateral superior and inferior PVs were mapped carefully with one Lasso catheter sequentially during sinus rhythm (SR) or CS pacing. Supploementary ablations were applied along the CPVA lines close to the earliest ipsilateral PV spikes. An additional conduction gap was considered if the PV activation sequence changed after one conduction gap had been closed. M‐CPVA group: the sites with the earliest activation in each PV perimeter were targeted during SR or CS pacing. The ipsilateral superior and inferior veins were isolated separately in this group. |
|
| Outcomes | Recurrence of AT, AF, complications | |
| Notes | CPVA: circumferential pulmonary vein ablation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by computer program |
Liu02 2006.
| Methods | Computer‐generated randomisation. | |
| Participants | 110 Patients with 20‐80 years, refractory to multiple AADs, NYHA I or II, and at least 9 months follow‐up were included. 55 patients were arranged to stepwise SPVI, and 55 to CPVI. Age: 57.3+/9.6 years in CPVI group, and 58.0+/‐8.1 years in stepwise SPVI group. % of male: 38/55 (69%) in CPVI group, and 35/55 (64%) in SPVI group. Follow‐up: nine months. Location: Beijing, China. |
|
| Interventions | CPVI compared to SPVI. | |
| Outcomes | Recurrence of AT, AF, successful clinical outcome, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by computer program. |
Marrouche 2007.
| Methods | Randomised controlled trial | |
| Participants | 53 Symptomatic AF patients who were followed up for 3 months. 27 patients were assigned to PVAI using the MB monitoring approach, and 26 were using the irrigated tip catheter. Paroxysmal AF accounted for 61.5% and 63% in both group respectively. Age: 53+/‐8 years in OIT catheter group, and 54+/‐8 years in ICE guidance group. % of male: 19/26 (73%) in OIT catheter group, and 21/27 (78%) in ICE guidance group. Follow‐up: three months for paroxysmal AF patients, and six months for persistent AF patients. Location: Salt Lake City, Utah, USA. |
|
| Interventions | Group 1: Open irrigation ablation technology for PV isolation. Group 2: Pulmonary vein antrum isolation under ICE guidance. |
|
| Outcomes | Sinus rhythm restored, complications | |
| Notes | PVAI: pulmonary vein‐atrium isolation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Nilsson 2006.
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria were symptomatic paroxysmal or persistent AF for >=6 months, with at least 3 episodes in 3 months and refractory to >=2 antiarrhythmic drugs (class 1‐3). 100 patients were included. 54 patients were arranged to Ostial PV isolation group and 46 to extraostial PV isolation group. Age: 55+/‐10 years in ostial PVI group, and 57+/‐11 years in extraostial PVI group. % of male: 37/54 (69%) in ostial PVI group, and 34/46 (74%) in extraostial PVI group. Follow‐up: 12 months. Location: Copenbagen, Denmark. |
|
| Interventions | Circumferential extraostial PVI compared with segmental ostial PVI. | |
| Outcomes | Recurrence of AF, free of symptomatic AF, success rate, complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation sequence was administered by an independent clerk. Other detail was lacking. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Oral 2003.
| Methods | Randomised controlled trial | |
| Participants | 80 symptomatic paroxysmal AF patients were selected. Age: 51+/‐10 years in segmental ostial ablation group, and 54+/‐11 years in left atrial ablation group. % of male: 31/40 (78%) in segmental ostial ablation group and left atrial ablation group respectively. Follow‐up: mean follow‐up period was 164+/‐100 days. Location: Ann Arbor, Michigan. |
|
| Interventions | 40 patients were isolated by segmental ostial ablation, while 40 by left atrial ablation. | |
| Outcomes | Recurrence of AF, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Oral 2004.
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: inducible by atrial pacing in the baseline state who underwent LACA for symptomatic, drug‐resistant, paroxysmal AF. Age: 55+/‐11 years in posterior left atrium and mitral isthmus ablation group (group 2), and 56+/‐9 years in additional ablation lines along the left atrial septum, roof and/or anterior wall (group 3). % of male: 24/30 (80%) in group 2, and 26/30 (87%) in group 3. Follow‐up: 8+/‐2 months. Location: Ann Arbor, Michigan. |
|
| Interventions | 100 patients were included and received left atrium circumferential ablation (LACA). 40 patients were cured and the remaining 60 patients were randomly arranged into no further ablation group (group 2, n=30) and additional linear ablation group (group 3, n=30). | |
| Outcomes | Recurrence of AF, sinus rhythm remaining, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Oral 2006.
| Methods | Randomised controlled trial. | |
| Participants | Patients with chronic atrial fibrillation. A total of 146 patients were randomised. 77 assigned to CPVA, while 69 assigned to control group. Age: 58+/‐8 years in control group, and 55+/‐9 years in CPVA group. % of male: 62/69 (90%) in control group, and 67/77 (87%) in CPVA group. Follow‐up: 12 months. Location: Ann Arbor, Michigan. |
|
| Interventions | Circumferential pulmonary vein ablation. Control group: 200mg of amiodarone orally per day at least six weeks after randomisation before transthoracic cardioversion. |
|
| Outcomes | Freedom from AF and atrial flutter in 1 year. Incidence of complications, changes in the diameter of the left atrium and the LVEF, changes in the severity of symptoms. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Oral 2009.
| Methods | Randomised controlled trial | |
| Participants | 119 persistent AF patients aged 60 +/‐9 years were included and received APVI. AF were terminated in 19 patients, while the other 100 patients were evenly randomised to CFAE or not . Age: 58 +/‐10 years in APVI group, and 62+/‐8 years in APVI + CFAE group. % of male: 41/50 (82%) in both groups. Follow‐up: 10 +/‐ 3 months. Location: Ann Arbor, Michigan. |
|
| Interventions | APVI plus CFAE compared with APVI alone | |
| Outcomes | Recurrence of AT or AF, sinus rhythm remaining, complications | |
| Notes | APVI: atrium‐pulmonary vein isolation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Pappone 2004.
| Methods | Computer‐generated randomisation | |
| Participants | Inclusion criteria: 18 to 70 years old, symptomatic AF and NYHA functional class I or II. More than 61% patients were paroxysmal AF patients. 560 patients were included. 280 were randomised to CPVA group, and 280 to modified CPVA group. Age: 56.4+/‐6.5 years in CPVA group, and 56.6+/‐8.0 years in CPVA‐M group. % of male: 138/280 (49%) in CPVA group, and 153/280 (54.6%) in CPVA‐M group. Follow‐up: 12 months. Location: Milan, Italy. |
|
| Interventions | CPVA‐M: Circumferential pulmonary vein ablation plus 2 additional ablation lines in the posterior left atrium connecting the contralateral superior and inferior PVs and along the mitral isthmus between the inferior aspect of the left‐sided encircling ablation line and the mitral annulus. CPVA: Circumferential pulmonary vein ablation |
|
| Outcomes | Recurrence of arrhythmias | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by computer program |
Pappone 2006.
| Methods | Randomised controlled trial. | |
| Participants | Included patients with: Age >18 or<70 years; Creatinine concentration <1.5 mg/dl; AF history > 6 months; AF burden > 2 episodes/month in the last 6 months. 198 patients were included. 99 were arranged to CPVA group, and 99 to ADT group. Age: 55+/‐10 years in CPVA group, and 57+/‐10 years in ADT group. % of male: 69/99 (70%) in CPVA group, and 64/99 (65%) in ADT group. Follow‐up: 12 months. Location: Milan, Italy. |
|
| Interventions | Ablation group: Circumferential pulmonary vein ablation. ADT group: amiodarone, flecainide, or sotalol, either as single drugs or in combination, at the maximum tolerable doses. |
|
| Outcomes | Freedom from documented recurrent atrial tachycardia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Pontoppidan 2009.
| Methods | Block randomisation | |
| Participants | 149 paroxysmal and persistent AF patients aged 56+/‐8 years were enrolled. 73 were arranged to CPVA and CTIB group, and 76 patients were arranged to CPVA alone group. Age: 56 +/‐ 8 years in CTIB+ group, and 56 +/‐8 in CTIB‐ group. % of male: 74% in CTIB+ group, and 68% in CTIB‐ group. Follow‐up: 12 months. Location: Aarhus, Denmark. |
|
| Interventions | CPVA plus CTIB compared CPVA alone | |
| Outcomes | Recurrence of AF | |
| Notes | CTIB: cavotricuspid isthmus block. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | The study was not blinded. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Rajappan 2009.
| Methods | Randomised by random number concealed by closed envelopes | |
| Participants | 54 paroxysmal and persistent AF patients were enrolled. About 17 patients had structural heart disease. 27 patients were arranged in each group. Age: 57+/‐10 years in steering group, and 54+/‐10 years in fixed‐curve group. % of male: 19/27 (70%) in steering group, and 20/27 (74%) in fixed‐curve group. Follow‐up: six months. Location: London, UK. |
|
| Interventions | Ablation using fixed‐curve sheath compared with steerable sheath | |
| Outcomes | Recurrence of AT, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised number table. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Randomisation envelopes were used. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by randomised number table |
Sheikh 2006.
| Methods | Randomised controlled trial | |
| Participants | 100 consecutive patients with paroxysmal AF were included. 50 patients were arranged to PVI group, and 50 to PVI plus creation of two lines of ablation group. Age: 60+/12 years in lone PVI group, and 61+/‐10 years in PVI + linear lesion group. % of male: 34/50 (68%) in lone PVI group, and 29/50 (58%) in PVI + linear lesion group. Follow‐up: nine months. Location: Milwaukee, WI, USA. |
|
| Interventions | 50 patients was arranged to PV isolation group, and 50 patients to PV isolation plus creation of two lines of ablation, one from the left inferior PV to the mitral valve annulus and the other connecting the superior PVs. | |
| Outcomes | Sinus rhythm restoring, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Stabile 2006.
| Methods | Computer‐generated randomisation | |
| Participants | Included patients with paroxysmal or persistent atrial fibrillation and not‐tolerated drug regimens. 68 patients were arranged to ablation group, and 69 to drugs group. Age: 62.2+/‐9 years in ablation group, and 62.3+/‐10.7 years in control group. % of male: 37/68 (54%) in ablation group, and 44/69 (64%) in control group. Follow‐up: 12 months. One in each group lost of follow‐up. Location: Maddaloni (CE), Italy. |
|
| Interventions | Cavo‐tricuspid and left inferior pulmonary vein‐mitral isthmus ablation plus circumferential PV ablation. Amiodarone, flecainide, propafenone, sotalol were applied in both groups. |
|
| Outcomes | Recurrence of AF, sinus rhythm, complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | One patient in CPVA group and two in medicines group withdrew. But the incomplete outcome data were not addressed. |
| Adequate sequence generation | Low risk | Sequence was generated by computer program |
Verma 2007.
| Methods | Randomised controlled trial | |
| Participants | 60 consecutive patients with paroxysmal AF and 40 consecutive patients with persistent/permanent AF were included in both groups respectively. Age: 56+/‐9 years in adjuvant anterior LA ablation group (group I), and 57+/‐12 years in adjuvant ablation group (group II). % of male: 63% in both groups. Follow‐up: 12 months. Location: Cleveland, USA. |
|
| Interventions | CFAE in addition to PVAI compared to PVAI alone. Group I: standard PVAI plus adjuvant ablation under ICE guidance. Group II: first‐time PVAI without adjuvant ablation within the preceding three months. |
|
| Outcomes | Complications | |
| Notes | CFAE: complex fractionated atrial electrograms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Wang 2008.
| Methods | Computer‐generated randomisation | |
| Participants | 106 drug‐refractory paroxysmal AF patients aged 66 +/‐8.8 years were enrolled. 54 patients were assigned to CPVI group, and 52 to CPVI + SVCI group. Age: 66.6+/‐8.8 years in CPVI group, and 65.4+/‐8.9 years in CPVI+SVCI group. % of male: 28/54 (52%) in CPVI group, and 30/52 (58%) in CPVI+SVCI group. Follow‐up: 12 months. Location: Shanghai, China. |
|
| Interventions | CPVI plus SVCI compared with CPVI. | |
| Outcomes | AF‐free survival, recurrence of AT, complications. | |
| Notes | SVCI: superior vena cava isolation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by computer program |
Wazni 2003.
| Methods | Randomised controlled trial | |
| Participants | 108 patients with symptomatic AF/AFL were enrolled. 49 were arranged to PV‐LAJ‐CTI ablation group, and 59 to PV‐LAJ ablation group. Age: 54+/‐11 years in PV‐LAJ disconnection + CTI ablation group (group 1), and 55+/‐11 years in PV‐LAJ disconnection alone group (group 2). % of male: 41/49 (84%) in group 1, and 47/59 (80%) in group 2. Follow‐up: 12 months. Location: Rotondo, Italy. |
|
| Interventions | PV‐LAJ ablation group: ICE‐gruded mapping and ablation of all PV ostia was performed with the use of a 10F, 64‐element, phased‐array ultrasound‐imaging catheter (AcuNave, Acuson) introduced through an 11Fsheath through the left femoral vein. A decapolar Lasso catheter (Biosense) was used for circular mapping and isolation of all PVs. Ablation was extended to the PV antrum in front of the tube like portion of the PVs. Radiofrequency energy was delivered with the use of a cool‐tipped ablation catheter (EP Technologies). Energy delivery was titrated, with the operator watching for microbubble formation. PV‐LAJ+CTI ablation group: PV‐LAJ ablation sees PV‐LAJ ablation group. CTI block: Radiofrequency ablation was performed under anatomic and electrogram guidance. existence of double potentials along the ablation line was proved to be separated by >=100 ms during sinus rhythm. Bidirectional block by pacing from both sides of the ablation line was also assessed (coronary sinus ostium and lateral isthmus [7 o'clock position in 60° left anterior oblique]). |
|
| Outcomes | Sinus rhythm restored, recurrence of AF, AFL. Complications | |
| Notes | PV‐LAJ ablation: pulmonary vein‐left atrium junctional ablation; CTI: cavotricuspid isthmus isolation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Wazni 2005.
| Methods | Computer‐generated randomisation | |
| Participants | 70 patients experienced monthly symptomatic atrial fibrillation episodes for at least 3 months were enrolled. 33 were arranged to PVI group, and 37 to medicines group. Age: 55+/‐10 years in CPVA group and 57+/‐10 years in ADT group. % of male: 69/99 (70%) in CPVA group, and 64/99 (65%) in ADT group. Follow‐up: one years. Location: Rotondo, Italy. |
|
| Interventions | PVI group: Pulmonary vein isolation. Medicine group: The maximum tolerable doses of each antiarrhythmic drug were advised. Medicines included flecainide (100‐150mg) twice daily, propafenone (225‐300mg) 3 times daily, and sotalol (120‐160mg) twice daily. |
|
| Outcomes | Recurrence of atrial fibrillation. Hospitalisation rate during the 1 year, quality of life. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | A‐Yes. Sequence was generated by computer, which was better in allocation concealment. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | One patient in PVI group and two in medicines group lost to follow up. But the incomplete data were not addressed. |
| Adequate sequence generation | Low risk | Sequence was generated by computer program |
Willems 2006.
| Methods | Randomised by random number table | |
| Participants | Included patients with:1) at least 2 failed attempts of an antiarrhythmic drug therapy for symptomatic AF episodes; 2) persistent AF lasting for at least 1 month documented by daily trans‐telephonic transmitted ECG. 62 patients were enrolled. 32 were arranged to PVI plus SM group, and 30 to PVI alone group. Age: 58.3+/‐11.8 years in PVI+SM group, and 60.1+/‐9.3 years in PVI alone group. % of male: unknown. Follow‐up:487 days (range from 429 to 570 days). Location: Hamburg, Germany. |
|
| Interventions | Pulmonary vein isolation plus additional substrate modification compared to PVI alone. | |
| Outcomes | Complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Low risk | Sequence was generated by randomised number table. |
Zhang 2007.
| Methods | Randomised controlled trial | |
| Participants | Patients with paroxysmal AF were included. 63 patients were randomised. 30 patients were assigned to CPVA plus PVI group, and 33 to CPVA alone group. Age: 64+/‐10 years in CPVA plus PVI group, and 62+/‐14 years in CPVA alone group. % of male: 19/30 (63%) in CPVA plus PVI group, and 20/30 (67%) in CPVA alone group. Follow‐up: 11+/‐3 months. Location: Shanghai, China. |
|
| Interventions | CPVA plus PVI compared with CPVA alone. | |
| Outcomes | Sinus rhythm restored, recurrence of AF | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | B‐Unclear. The authors did not depict allocation concealment method. |
| Blinding | High risk | Blinding was not reported. |
| Incomplete outcome data addressed | High risk | There were no patients withdrawn or lost to follow up. |
| Adequate sequence generation | Unclear risk | It was not addressed. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bauer 2006 | The RCT was excluded as it did not use endpoint outcomes that the review required. |
| Brignole 1994 | The RCT included patients with chronic AF (lasting more than 3 months which was considered to be permanent AF). |
| Fiala 2008 | The patients were randomised by the date of birth. |
| Ito 2007 | The RCT did not included endpoint outcomes required by the review. |
| Kirkutis 2004 | The RCT included chronic AF patients and used only heart rate control as endpoint outcome. |
| Padeletti 2003 | The RCT was excluded as its endpoint outcomes were not required by the review. |
| Reddy 2007 | The outcomes reported by this study were different to those in this review. |
| Tse 2005 | The RCT did not observe clinical endpoint outcomes as required by the review. |
Differences between protocol and review
The comparison of CA versus medical therapies was added as an objective during the review stage.
Contributions of authors
Chen HS: Protocol draft, development of search strategy, study selection, quality assessment, data extraction, data analysis, development of final review and corresponding author. Wen JM: searching for trials, study selection, quality assessment, data extraction, data analysis, co development of final review. Wu SN: providing clinical perspectives, results explanation. Liu JP: Protocol revision, third party for study selection and quality assessment, providing methodological perspectives, co development of final review and revision.
Sources of support
Internal sources
Intensive Care Unit of Shenzhen People's Hospital, China.
Cardiovascular Department of Shenzhen People's Hospital, China.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Arentz 2007 {published data only}
- Thomas Arentz, Reinhold Weber, Gerd Bürkle, Claudia Herrera, Thomas Blum, Jochem Stockinger, et al. Small or Large Isolation Areas Around the Pulmonary Veins for the Treatment of Atrial Fibrillation? Results From a Prospective Randomized Study. Circulation 2007;115:3057‐3063. [DOI] [PubMed] [Google Scholar]
Calo 2006 {published data only}
- Leonardo Calò, Filippo Lamberti, Maria Luisa Loricchio, Ermenegildo De Ruvo, Furio Colivicchi, Leopoldo Bianconi, et al. Left Atrial Ablation Versus Biatrial Ablation for Persistent and Permanent Atrial Fibrillation: A Prospective and Randomized Study. Journal of the American College of Cardiology 2006;47(12):2504‐2512. [PUBMED: 16781381] [DOI] [PubMed] [Google Scholar]
Deisenhofer 2009 {published data only}
- Isabel Deisenhofer, Heidi Estner, Tilko Reents, Stephanie Fichtner, Axel Bauer, Jinjin Wu, et al. Does Electrogram Guided Substrate Ablation Add to the Success of Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation?A Prospective, Randomized Study. Journal of Cardiovascular Electrophysiology 2009;20(5):514‐521. [DOI] [PubMed] [Google Scholar]
Dixit 2008 {published data only}
- Sanjay Dixit, Edward P. Gerstenfeld, Sarah J. Ratcliffe, Joshua M. Cooper, Andrea M. Russo, Stephen E. Kimmel, et al. Single procedure efficacy of isolating all versus arrhythmogenic pulmonary veins on long‐term control of atrial fibrillation: A prospective randomized study. Heart rhythm 2008;5(2):174‐181. [DOI] [PubMed] [Google Scholar]
Fassini 2005 {published data only}
- Gaetano Fassini, Stefania Riva, Roberta Chiodelli, Nicola Trevisi, Marco Berti, Corrado Carbucicchio, et al. Left Mitral Isthmus Ablation Associated with PV Isolation: Long‐Term Results of a Prospective Randomized Study. J Cardiovasc Electrophysiol 2005;16(11):1150‐1156. [PUBMED: 16302895] [DOI] [PubMed] [Google Scholar]
Forleo 2009 {published data only}
- Giovannib Forleo, Massimo Mantica, Lucia De Luca, Roberto Leo, Luca Santini, Stefania Panigada, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. Journal of cardiovascular electrophysiology 2009;20(1):22‐28. [DOI] [PubMed] [Google Scholar]
Haïssaguerre 2004 {published data only}
- Michel Haïssaguerre, Prashanthan Sanders, Mélèze Hocini, Li‐Fern Hsu, Dipen C. Shah, Christophe Scavée, et al. Changes in Atrial Fibrillation Cycle Length and Inducibility During Catheter Ablation and Their Relation to Outcome. Circulation 2004;109(24):3007‐3013. [PUBMED: 15184286] [DOI] [PubMed] [Google Scholar]
Hocini 2005 {published data only}
- Mélèze Hocini, Pierre Jaïs, Prashanthan Sanders, Yoshihide Takahashi, Martin Rotter, Thomas Rostock, et al. Techniques, Evaluation, and Consequences of Linear Block at the Left Atrial Roof in Paroxysmal Atrial Fibrillation: A Prospective Randomized Study. Circulation 2005;112(24):3688‐3696. [PUBMED: 16344401] [DOI] [PubMed] [Google Scholar]
Jaïs 2008 {published data only}
- Pierre Jaïs, Bruno Cauchemez, Laurent Macle, Emile Daoud, Paul Khairy, Rajesh Subbiah, et al. Catheter Ablation Versus Antiarrhythmic Drugsfor Atrial Fibrillation: the A4 Study. Circulation 2008;118(24):2498‐2505. [DOI] [PubMed] [Google Scholar]
Karch 2005 {published data only}
- Axel Bauer, Isabel Deisenhofer, Raphael Schneider, Bernhard Zrenner, Petra Barthel, MD, * Martin Karch, et al. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm 2005;3:1428 –1435. [DOI] [PubMed] [Google Scholar]
- Martin R. Karch, Bernhard Zrenner, Isabel Deisenhofer, Jürgen Schreieck, Gjin Ndrepepa, Jun Dong, et al. Freedom From Atrial Tachyarrhythmias After Catheter Ablation of Atrial FibrillationA Randomized Comparison Between 2 Current Ablation Strategies. Circulation 2005;111(22):2875‐80. [PUBMED: 15927974] [DOI] [PubMed] [Google Scholar]
Katritsis 2004 {published data only}
- Katritsis DG, Ellenbogen KA, Panagiotakos DB, Giazitzoglou E, Karabinos I, Papadopoulos A, et al. ablation of superior pulmonary vein compared to ablation of all four pulmonary veins: a randomized clinical trial. J Cardiovasc Electrophysiol 2004;15(6):641‐645. [PUBMED: 15175057] [DOI] [PubMed] [Google Scholar]
Krittayaphong 2003 {published data only}
- Krittayaphong R. Raungrattanaamporn O. Bhuripanyo K. Sriratanasathavorn C. Pooranawattanakul S. Punlee K, et al. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. Journal of the Medical Association of Thailand 2003;86(Suppl 1):S8‐16. [PubMed] [Google Scholar]
Liu02 2006 {published data only}
- Xingpeng Liu, Jianzeng Dong, Hercules E. mavakis, Fuli Hu, Deyong Long, Dongping Fang, et al. Achievement of Pulmonary Vein Isolation in Patients Undergoing Circumferential Pulmonary Vein Ablation: A Randomized Comparison Between Two Different Isolation Approaches. J Cardiovasc Electrophysiol 2006;17(12):1263‐1270. [PUBMED: 17239094] [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Xingpeng Liu, Deyong Long, Jianzeng Dong, Fuli Hu, Ronghui Yu, Ribo Tang, et al. Is Circumferential Pulmonary Vein Isolation Preferable to Stepwise Segmental Pulmonary Vein Isolation for Patients With Paroxysmal Atrial Fibrillation? A Randomized Study. Circulation Journal 2006;70(11):1392‐1397. [PUBMED: 17062959] [DOI] [PubMed] [Google Scholar]
Marrouche 2007 {published data only}
- Nassir F. Marrouche, Jens Guenther, Nathan M. Segerson, Marcos Daccarett, Harald Rittger, Harald Marschang, et al. Randomized Comparison Between Open Irrigation Technology and Intracardiac‐Echo‐Guided Energy Delivery for Pulmonary Vein Antrum Isolation: Procedural Parameters, Outcomes, and the Effect on Esophageal Injury. J Cardiovasc Electrophysiol 2007;18(6):583‐588. [PUBMED: 17490437] [DOI] [PubMed] [Google Scholar]
Nilsson 2006 {published data only}
- Brian Nilsson, Xu Chen, Steen Pehrson, Lars Køber, Jørgen Hilden, Jesper H. Svendsen. Recurrence of pulmonary vein conduction and atrial fibrillation after pulmonary vein isolation for atrial fibrillation: A randomized trial of the ostial versus the extraostial ablation strategy. American Heart Journal 2006;152:537.e1‐e8. [DOI] [PubMed] [Google Scholar]
Oral 2003 {published data only}
- Hakan Oral, Christoph Scharf, Aman Chugh, Burr Hall, Peter Cheung, Eric Good, et al. Catheter Ablation for Paroxysmal Atrial Fibrillation Segmental Pulmonary Vein Ostial Ablation Versus Left Atrial Ablation. Circulation 2003;108(19):2355‐2360. [PUBMED: 14557355] [DOI] [PubMed] [Google Scholar]
Oral 2004 {published data only}
- Hakan Oral, Aman Chugh, Kristina Lemola, Peter Cheung, Burr Hall, Eric Good, et al. Noninducibility of Atrial Fibrillation as an End Point of Left Atrial Circumferential Ablation for Paroxysmal Atrial Fibrillation: A Randomized Study. Circulation 2004;110(18):2797‐2801. [PUBMED: 15505091 ] [DOI] [PubMed] [Google Scholar]
Oral 2006 {published data only}
- Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, et al. Circumferential Pulmonary‐Vein Ablation for Chronic Atrial Fibrillation. New England Journal of Medicine 2006;354(9):934‐41. [DOI] [PubMed] [Google Scholar]
Oral 2009 {published data only}
- Hakan Oral, Aman Chugh, Kentaro Yoshida, Jean F. Sarrazin, Michael Kuhne, Thomas Crawford, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long‐lasting persistent atrial fibrillation. Journal of the American College of Cardiology 2009;53(9):782‐789. [DOI] [PubMed] [Google Scholar]
Pappone 2004 {published data only}
- Carlo Pappone, Francesco Manguso, Gabriele Vicedomini, Filippo Gugliotta, Ornella Santinelli, Amedeo Ferro, et al. Prevention of Iatrogenic Atrial Tachycardia After Ablation of Atrial Fibrillation A Prospective Randomized Study Comparing Circumferential Pulmonary Vein Ablation With a Modified Approach. Circulation 2004;110(19):3036‐3042. [PUBMED: 15520310] [DOI] [PubMed] [Google Scholar]
Pappone 2006 {published data only}
- Pappone C. Augello G. Sala S. Gugliotta F. Vicedomini G. Gulletta S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. Journal of the American College of Cardiology 2006;48(11):2340‐7. [DOI] [PubMed] [Google Scholar]
Pontoppidan 2009 {published data only}
- Pontoppidan J, Nielsen JC, Poulsen SH, Jensen HK, Walfridsson H, Pedersen AK, et al. Prophylactic cavotricuspid isthmus block during atrial fibrillation ablation in patients without atrial flutter: a randomised controlled trial. Heart 2009;95(12):994‐999. [DOI] [PubMed] [Google Scholar]
Rajappan 2009 {published data only}
- Kim Rajappan, Victoria Baker, Laura Richmond, Peter M. Kistler, Glyn Thomas, Calum Redpath, et al. A randomized trial to compare atrial fibrillation ablation using a steerable vs. a non‐steerable sheath. Europace 2009;11(5):571‐575. [DOI] [PubMed] [Google Scholar]
Sheikh 2006 {published data only}
- Imran Sheikh, David Krum, Ryan Cooley, Anwer Dhala, Zalmen Blanck, Atul Bhatia, et al. Pulmonary vein isolation and linear lesions in atrial fibrillation ablation. J Interv Card Electrophysiol 2006;17(2):103‐109. [PUBMED: 17318445] [DOI] [PubMed] [Google Scholar]
Stabile 2006 {published data only}
- Stabile G. Bertaglia E. Senatore G. De Simone A. Zoppo F. Donnici G, et al. Catheter ablation treatment in patients with drug‐refractory atrial fibrillation: a prospective, multi‐centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). European Heart Journal 2006;27(2):216‐21. [DOI] [PubMed] [Google Scholar]
Verma 2007 {published data only}
- Atul Verma, Dimpi Patel, Tamer Famy, David O. Martin, J. David Burkhardt, S. Claude Elayi, et al. Efficacy of Adjuvant Anterior Left Atrial Ablation During Intracardiac Echocardiography‐Guided Pulmonary Vein Antrum Isolation for Atrial Fibrillation. J Cardiovasc Electrophysiol 2007;18(2):151‐156. [PUBMED: 17338763] [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang Xin‐Hua, Liu Xu, Sun Yu‐Min, Shi Hai‐Feng, Zhou Li, Gu Jia‐Ning. Pulmonary vein isolation combined with superior vena cava isolation for atrial fibrillation ablation: a prospective randomized study. Europace 2008;10(5):600‐605. [DOI] [PubMed] [Google Scholar]
Wazni 2003 {published data only}
- Oussama Wazni, Nassir F. Marrouche, David O. Martin, A. Marc Gillinov, Walid Saliba, Eduardo Saad, et al. Randomized Study Comparing Combined PulmonaryVein–Left Atrial Junction Disconnection and Cavotricuspid Isthmus Ablation Versus Pulmonary Vein–Left Atrial Junction Disconnection Alone in Patients Presenting With Typical Atrial Flutter and Atrial Fibrillation. Circulation 2003;108(20):2479‐2483. [DOI] [PubMed] [Google Scholar]
Wazni 2005 {published data only}
- Wazni OM. Marrouche NF. Martin DO. Verma A. Bhargava M. Saliba W, et al. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293(21):2634‐40. [PUBMED: 14610012] [DOI] [PubMed] [Google Scholar]
Willems 2006 {published data only}
- Stephan Willems, Hanno Klemm, Thomas Rostock, Benedikt Brandstrup, Rodolfo Ventura, Daniel Steven, et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. European Heart Journal 2006;27(23):2871‐2878. [PUBMED: 16782716] [DOI] [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang Jinlin, Xu Yawei, Xu Jiangang, Zhou Ke, Yu Xuejing. Necessity of pulmonary vein isolation as an end point for circumferential pulmonary vein ablation in patient with paroxysmal atrial fibrillation. Journal of Clinical Cardiology (China) 2007;23(7):516‐518. [Google Scholar]
References to studies excluded from this review
Bauer 2006 {published data only}
- Axel Bauer, Isabel Deisenhofer, Raphael Schneider, Bernhard Zrenner, Petra Barthel, Martin Karch, et al. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm 2006;3(12):1428‐1435. [PUBMED: 17161785 ] [DOI] [PubMed] [Google Scholar]
Brignole 1994 {published data only}
- Michele Brignole, Lorella Gianfranchi, Carlo Menozzi, Nicola Bottoni, Roberto Bollini, Gino Lolli, et al. Influence of Atrioventricular Junction Radiofrequency Ablation in Patients with Chronic Atrial Fibrillation and Flutter on Quality of life and Cardiac Performance. American Journal of Cardiology 1994;74(3):242‐246. [DOI] [PubMed] [Google Scholar]
Fiala 2008 {published data only}
- Martin Fiala, Jan Chovančík, Renáta Nevřalová, Radek Neuwirth, Otakar Jiravský, Igor Nykl, et al. Pulmonary vein isolation using segmental versus electroanatomical circumferential ablation for paroxysmal atrial fibrillation: over 3‐year results of a prospective randomized study. Journal of interventional cardiac electrophysiology 2008;22(1):13‐21. [DOI] [PubMed] [Google Scholar]
Ito 2007 {published data only}
- Sachiko Ito, Hiroshi Tada, Shigeto Naito, Yasunori Kutsumi, Isamu Miyamori, Akihiko Nogami, et al. Randomized Comparison of Bipolar vs Unipolar Plus Bipolar Recordings During Atrioventricular Junction Ablation: Importance and Efficacy of Unipolar Recording. Circ J 2007;71(6):874‐879. [PUBMED: 17526983] [DOI] [PubMed] [Google Scholar]
Kirkutis 2004 {published data only}
- Algimantas Kirkutis, Aušrius Poviliūnas, Pasaka Gricienė, Sotir Polena, Stephen Yang, Geeta Yalamanchi, et al. Cardiac Rate Normalization in Chronic Atrial Fibrillation: Comparison of Long‐term Efficacy of Treatment with Amiodarone versus AV Node Ablation and Permanent His‐bundle Pacing. Proc. West. Pharmacol. Soc 2004;47:69‐70. [PubMed] [Google Scholar]
Padeletti 2003 {published data only}
- Luigi Padeletti, Gianluca Botto, Andrea Spampinato, Antonio Michelucci, Andrea Colella, Maria Cristina Porciani, et al. Prevention of Paroxysmal Atrial Fibrillation in Patients with Sinus Bradycardia: Role of Right Atrial Linear Ablation and Pacing Site. Journal of Cardiovascular Electrophysiology 2003;14:733‐738. [DOI] [PubMed] [Google Scholar]
Reddy 2007 {published data only}
- Vivek Y. Reddy, Matthew R. Reynolds, Petr Neuzil, Allison W. Richardson, Milos Taborsky, Krit Jongnarangsin, et al. Prophylactic Catheter Ablation for the Prevention of Defibrillator Therapy. New England Journal of Medicine 2007;357(26):2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tse 2005 {published data only}
- Hung Fat Tse, Yok Lam Kwong, Chu Pak Lau. Transvenous cryoablation reduces platelet activation during pulmonary vein ablation compared with radiofrequency energy in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology 2005;16(10):1064‐1070. [DOI] [PubMed] [Google Scholar]
Additional references
Aguilar 2005
- MI Aguilar, R Hart. Oral anticoagulants for preventing stroke in patients with non‐valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001927.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrikopoulos 2009
- George Andrikopoulos, Stylianos Tzeis, Nikos Maniadakis, Hercules E. Mavrakis, Panos E. Vardas. Cost‐effectiveness of atrial fibrillation catheter ablation. Europace 2009;11:147‐151. [DOI] [PubMed] [Google Scholar]
Bechtel 2011
- Bechtel BF, Nunez TC, Lyon JA, Cotton BA, Barrett TW. Treatments for reversing warfarin anticoagulation in patients with acute intracranial hemorrhage: a structured literature review. Int J Emerg Med 2011;4(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernhardt 2006
- Bernhardt P, Schmidt H, Sommer T, Luderitz B, Omran H. Atrial fibrillation ‐ patients at high risk for cerebral embolism. Clinical Research in Cardiology 2006;95(3):148‐53. [DOI] [PubMed] [Google Scholar]
Bertaglia 2007
- Emanuele Bertaglia, Franco Zoppo, Claudio Tondo, Andrea Colella, Roberto Mantovan, Gaetano Senatore, et al. Early complications of pulmonary vein catheter ablation for atrial fibrillation: A multicenter prospective registry on procedural safety. Heart Rhythm 2007;4(10):1265–1271. [DOI] [PubMed] [Google Scholar]
Bonanno 2010
- Bonanno C, Paccanaro M, Vecchia LL, Ometto R, Fontanelli A. Efficacy and safety of catheter ablation versus antiarrhythmic drugs for atrial fibrillation: a meta‐analysis of randomized trials. Journal of Cardiovascular Medicine 2010;11(6):408‐418. [DOI] [PubMed] [Google Scholar]
Bordin 2003
- Bordin P, Mazzone C, Pandullo C, Goldstein D, Scardi S. Morbility and mortality in 229 elderly patients with nonrheumatic atrial fibrillation. A five year follow‐up. Italian Heart Journal 2003;4(8):537‐43. [PubMed] [Google Scholar]
Cappato 2009
- Riccardo Cappato, Hugh Calkins, Shih‐Ann Chen, Wyn Davies, Yoshito Iesaka, Jonathan Kalman, et al. Prevalence and causes of fatal outcomes in catheter ablation of atrial fibrillation. Journal of the American College of Cardiology 2009;53(19):1798‐1804. [DOI] [PubMed] [Google Scholar]
Cha 2004
- Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, et al. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation 2004;109(3):412‐418. [DOI] [PubMed] [Google Scholar]
Crandall 2009
- Crandall MA, Horne BD, Day JD, Anderson JL, Muhlestein JB, Crandall BG, et al. Atrial fibrillation significantly increases total mortality and stroke risk beyond that conveyed by the CHADS2 risk factors. Pacing Clin Electrophysiol 2009;32(8):981‐986. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garnier 2004
- Garnier LF, Rouesnel P, Espitalier F. Atrial fibrillation and anticoagulation. Arch Mal Coeur Vaiss 2004;97(10):1001‐1005. [PubMed] [Google Scholar]
Gelder 2002
- Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, et al. A Comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. New England Journal of Medicine 2002;347:1834‐40. [DOI] [PubMed] [Google Scholar]
Gluud 2001
- Gluud C. Alcoholic hepatitis: no glucocorticosteroids?. In: Leuschner OFW, Dancygier J, Dancygier H editor(s). Steatohepatitis : NASH and ASH proceedings of Falk Symposium 121. London: Kluwer Academic, 2001:322‐42. [Google Scholar]
Hashimoto 2007
- Hashimoto T, Tada H, Naito S, Miyaji K, Yamada M, Tadokoro K, et al. Prevalence and characteristics of left atrial tachycardia following left atrial catheter ablation. Pacing & Clinical Electrophysiology 2007;30(Suppl 1):S94‐7. [DOI] [PubMed] [Google Scholar]
Hassantash 2008
- Hassantash SA, Kalantarian S, Bikdeli B, Sadeghian M, Kasraii F, Haghdoost A. Surgical ablation for atrial fibrillation (Protocol). Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007318] [DOI] [Google Scholar]
Haïssaguerre 1998
- Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339(10):659‐666. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Hoppe 2006
- Hoppe UC, Casares JM, Eiskjaer H, Hagemann A, Cleland JG, Freemantle N, et al. Effect of cardiac resynchronization on the incidence of atrial fibrillation in patients with severe heart failure. Circulation 2006;114(1):18‐25. [DOI] [PubMed] [Google Scholar]
Kannel 1982
- Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. New England Journal of Medicine 1982;306:1018‐22. [DOI] [PubMed] [Google Scholar]
Karin 2004
- Humphries KH, Kerr CR, Steinbuch M, Dorian P. Limitations to antiarrhythmic drug use in patients with atrial fibrillation. Canadian Medical Association Journal 2004;171(7):741‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemola 2008
- Kristina Lemola, Denis Chartier, yung‐Hsin Yeh, Marc Dubuc, Raymond Cartier, Andrew Armour, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation, role of pulmonary veins versus autonomic ganglia. Circulation 2008;117:470‐477. [DOI] [PubMed] [Google Scholar]
Li 2008
- Li Yi‐gang, Wang Qun‐shan, Yu Jie‐fei, Sun Jian. Advances in catheter ablation of chronic atrial fibrillation. Journal of Shanghai Jiaotong University (Medical Science) 2008;10(28):1211‐1218. [Google Scholar]
Li 2011
- Li WJ, Bai YY, Zhang HY, Tang RB, Miao CL, Sang CH, et al. Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: a meta‐analysis. Circ Arrhythm Electrophysiol 2011;4(2):143‐148. [DOI] [PubMed] [Google Scholar]
Lip 2011
- Lip GY. The role of aspirin for stroke prevention in atrial fibrillation. Nat Rev Cardiol 2011;8(10):602‐606. [DOI: 10.1038/nrcardio.2011.112] [DOI] [PubMed] [Google Scholar]
Lu 2009
- Lu YM, Zhang Y, Wu XH, Li YG, Han B, Huang DM, et al. The impact of radiofrequency of catheter ablation on quality of life in the patients of persistent atrial fibrillation. J Clin Cardiol (China) 2009;25(1):39‐41. [Google Scholar]
Lutomsky 2008
- Lutomsky BA, Rostock T, Koops A, Steven D, Müllerleile K, Servatius H, et al. Catheter ablation of paroxysmal atrial fibrillation improves cardiac function: a prospective study on the impact of atrial fibrillation ablation on left ventricular function assessed by magnetic resonance imaging. Europace 2008;10(5):593‐599. [DOI] [PubMed] [Google Scholar]
Ma 2006
- Ma ChangSheng, Meng Xu, Zhu XueShi. Management of atrial fibrillation: surgical or internal catheter ablation, which one is better?. Chinese Journal of Cardiovascular Review 2006;4(11):801‐805. [Google Scholar]
McKenna 2009
- C McKenna, S palmer, M Rodgers, D Chambers, N Hawkins, S Golder, et al. Cost‐effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart 2009;95:542‐549. [DOI] [PubMed] [Google Scholar]
Mead 2005
- GE Mead, AT Elder, AD Flapan, A Kelman. Electrical cardioversion for atrial fibrillation and flutter. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002903.pub2] [DOI] [PubMed] [Google Scholar]
Meinertz 2011
- Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol 2011;100(10):897‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moe 1964
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. American Heart Journal 1964;67:200‐20. [DOI] [PubMed] [Google Scholar]
Morady 2005
- Fred Morady. Mechanisms and catheter ablation therapy of atrial fibrillation. Cardiac arrhythmias 2005;32(2):199‐201. [PMC free article] [PubMed] [Google Scholar]
Nademanee 2007
- Nademanee K. Trials and travails of electrogram‐guided ablation of chronic atrial fibrillation. Circulation 2007;115(20):2592‐2594. [DOI] [PubMed] [Google Scholar]
Nademanee 2008
- Nademanee K, Schwab MC, Kosar EM, Karwecki M, Moran MD, Visessook N, et al. Clinical outcomes of catheter substrate ablation for high‐risk patients with atrial fibrillation. Journal of the American College of Cardiology 2008;51(8):843‐849. [DOI] [PubMed] [Google Scholar]
Ohki 2005
- Ohki R, Yamamoto K, Ueno S, Mano H, Misawa Y, Fuse K, et al. Gene expression profiling of human atrial myocardium with a fibrillation by DNA microarray analysis. International Journal of Cardiology 2005;102(2005):233‐8. [DOI] [PubMed] [Google Scholar]
Oral 2002
- Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, Hassan S, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105(9):1077‐1081. [DOI] [PubMed] [Google Scholar]
Ouyang 2005
- Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111(2):127‐135. [DOI] [PubMed] [Google Scholar]
Padanilam 2006
- Padanilam BJ. Cerebral microembolism during AF ablation: an innocent bystander or an accessory to brain injury?. of Cardiovascular Electrophysiology 2006;17(5):502‐3. [DOI] [PubMed] [Google Scholar]
Pappone 2000
- Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation 2000;102(21):2619‐2628. [DOI] [PubMed] [Google Scholar]
Pappone 2001
- Pappone C, Santinelli V. Pulmonary vein isolation by circumferential radiofrequency lesions in atrial fibrillation. From substrate to clinical outcome. Annual Isternal Super Sanita 2001;37(3):401‐407. [PubMed] [Google Scholar]
Pappone 2007
- Pappone C, Augello G, Gugliotta F, Santinelli V. Robotic and magnetic navigation for atrial fibrillation ablation. How and why?. Expert Rev Med Devices 2007;4(6):885‐894. [DOI] [PubMed] [Google Scholar]
Pappone02 2006
- Pappone C, Vicedomini G, M anguso F, Gugliotta F, Mazzone P, Gulletta S, et al. Robotic magnetic navigation for atrial fibrillation ablation. J Am Coll Cardiol 2006;47(7):1390‐1400. [DOI] [PubMed] [Google Scholar]
Pontoppidan02 2009
- Pontoppidan J, Nielsen JC, Poulsen SH, Hansen PS. Symptomatic and asymptomatic atrial fibrillation after pulmonary vein ablation and the impact on quality of life. Pacing Clin Electrophysiol 2009;32(6):717‐726. [DOI] [PubMed] [Google Scholar]
Reynolds 2009
- Matthew R. Reynolds, Peter Zimetbaum, Mark E. Josephson, Ethan Ellis, Tatyana Danilov, David J. Cohen. Cost‐effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circulation Arrhythmia Electrophysiology 2009;2:362‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reynolds 2010
- Reynolds MR, Walczak J, White SA, Cohen DJ, Wilber DJ. Improvements in symptoms and quality of life in patients with paroxysmal atrial fibrillation treated with radiofrequency catheter ablation versus antiarrhythmic drugs. Circ Cardiovasc Qual Outcomes 2010;3(6):615‐623. [DOI] [PubMed] [Google Scholar]
Ryden 2001
- Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation). Circulation 2001;104(17):2118‐50. [PubMed] [Google Scholar]
Sacher 2007
- Sacher F, Jais P, Stephenson K, O'neill MD, Hocini M, Clementy J, et al. Phrenic nerve injury after catheter ablation of atrial fibrillation. Indian Pacing Electrophysiology Journal 2007;7(1):1‐6. [PMC free article] [PubMed] [Google Scholar]
Sawhney 2010
- Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3(3):243‐248. [DOI] [PubMed] [Google Scholar]
Singh 2007
- Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. New England Journal of Medicine 2007;357(10):987‐99. [DOI] [PubMed] [Google Scholar]
Snow 2003
- Snow V, Weiss KB, Lefevre M, McNamare R, Bass E, Green LA, et al. Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Annals of Internal Medicine 2003;139:1009‐17. [DOI] [PubMed] [Google Scholar]
STAF 2003
- Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S. Randomized trial of rate‐control versus rhythm‐control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. Journal of the American College of Cardiology 2003;41(10):1690‐6. [DOI] [PubMed] [Google Scholar]
Tang 2006
- Tang RB, Dong JZ, Liu XP, Fang DP, Long de Y, Liu XH. Safety and efficacy of catheter ablation of atrial fibrillation in patients with diabetes mellitus‐single center experience. Journal of Interventional Cardiac Electrophysiology 2006;17(1):41‐6. [DOI] [PubMed] [Google Scholar]
Taylor 2010
- Taylor CJ, Hodgkinson J, Hobbs F.D.R. Rhythm control agents and adverse events in patients with atrial fibrillation. Int J Clin Pract 2010;64(8):1069‐1075. [DOI] [PubMed] [Google Scholar]
Tracy 2006
- Tracy CM, Akhtar M, DiMarco JP, Packer DL, Weitz HH. American College of Cardiology/American Heart Association 2006 Update of the Clinical Competence Statement on Invasive Electrophysiology Studies, Catheter Ablation, and Cardioversion. A Report of the American College of Cardiology/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training Developed in Collaboration With the Heart Rhythm Society. Journal of the American College of Cardiology 2006;48(7):1503‐17. [DOI] [PubMed] [Google Scholar]
Verma 2008
- Verma A, Novak P, Macle L, Whaley B, Beardsall M, Wulffhart Z, et al. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm 2008;5(2):198‐205. [DOI] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19:159‐66. [DOI] [PubMed] [Google Scholar]
Wattigney 2003
- Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 2003;108(6):711‐6. [DOI] [PubMed] [Google Scholar]
Wijffels 1995
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92(7):1954‐68. [DOI] [PubMed] [Google Scholar]
Wu 2001
- Wu TJ, James JC, Chang CM, Doshi RN, Yashima M, Huang HLA, et al. Pulmonary veins and ligament of marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation 2001;103(8):1157‐63. [DOI] [PubMed] [Google Scholar]
Wyse 2002
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A Comparison of rate control and rhythm control in patients with atrial fibrillation. New England Journal of Medicine 2002;347(23):1825‐33. [DOI] [PubMed] [Google Scholar]
Yamashita 2007
- Yamashita E, Tada H, Tadokoro K, Hashimoto T, Kaseno K, Miyaji K, et al. Left atrial catheter ablation promotes vasoconstriction of the right coronary artery. Pacing & Clinical Electrophysiology 2007;30(Suppl 1):S98‐S102. [DOI] [PubMed] [Google Scholar]
Yamashita 2011
- Yamashita T. Frontiers of anticoagulation therapy for atrial fibrillation. J Cardiol 2011;58(1):1‐5. [DOI] [PubMed] [Google Scholar]


