Abstract
Background
Gastric cancer is difficult to cure once it progresses to an advanced or late stage. Although some chemotherapies or bio‐therapies have made progress in the remission of this disease, the mortality from gastric cancer remains high. A variety of Chinese medicinal herbs have been used to treat gastric cancer.
Objectives
To assess the effectiveness of Chinese medicinal herbs in the short‐term remission of advanced or late gastric cancer.
Search methods
We searched the The Cochrane Library, MEDLINE, EMBASE, AHMED (Allied and Complementary Medicine Database) and CBM (Chinese Biomedical Database) from the first year of the databases to June 2011. We handsearched a number of journals.
Selection criteria
All randomised clinical trials of Chinese herbs for advanced or late gastric cancer were included.
Data collection and analysis
Two authors independently extracted the data, which were analysed using RevMan 5.1 software (RevMan 2011). For dichotomous data, we estimated the relative risk. For continuous data, we calculated the weighted mean difference.
Main results
Eighty‐five trials with 6857 advanced or late gastric cancer patients were identified for inclusion, most were of low quality and used traditional Chinese medicinal herbs (TCMHs) plus chemotherapy compared with the same chemotherapy alone (65 trials). Apart from 23 trials of four different kinds of TCMHs, we could not pool the results because no more than two used the same intervention or outcomes.
TCMHs with or without chemotherapy, in 57 trials, showed statistically significant differences for the improvement of mortality in nine trials, quality of life in 16 trials, rate of remission in 11 trials, and leukopenia in five trials. The pooled results from the four injected TCMHs, Huachansu, Aidi, Fufangkushen, and Shenqifuzheng showed statistically significant differences for the improvement of leukopenia, but no significant difference in the rate of short‐term remission.
Authors' conclusions
This review did not provide assured evidence concerning the effectiveness of TCMHs in improving quality of life or rate of remission, alleviating the toxicity or side effects of chemotherapy, or reducing short‐term mortality. Limited, weak evidence showed that Huachansu, Aidi, Fufangkushen, and Shenqifuzheng improved leukopenia when used together with chemotherapy; and Huachansu, Aidi, and Fufangkushen were of benefit for adverse events in the digestive system caused by chemotherapy. These TCMHs did not improve the rate of short‐term remissions. Large, well designed clinical trials are required urgently before any definite conclusions can be drawn about the value of TCMHs for advanced or late stage gastric cancer.
Keywords: Humans; Antineoplastic Agents, Phytogenic; Antineoplastic Agents, Phytogenic/therapeutic use; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic; Remission Induction; Remission Induction/methods; Stomach Neoplasms; Stomach Neoplasms/drug therapy; Stomach Neoplasms/mortality; Stomach Neoplasms/pathology
Plain language summary
Traditional Chinese medicinal herbs for induction of remission in advanced or late gastric cancer
Gastric cancer, one of the malignant tumours in the gastrointestinal tract and with high morbidity among cancers, can easily lead to death once it progresses to an advanced or late stage. There are few interventions which can postpone or stop the malignant course of the illness. However, some kinds of traditional Chinese medicinal herbs (TCMHs) have been used as an alternative therapeutic measure to treat many gastric cancer patients in China, and might be effective as an auxiliary therapy for this illness in its advanced or late stages. Our primary investigation showed there was no assured evidence concerning the effectiveness of TCMHs in improving the quality of life or rate of remission, alleviating the toxic and side effects caused by the chemotherapy, or reducing short‐term mortality. Limited, weak evidence showed that four injections of the TCMHs Huachansu, Aidi, Fufangkushen, and Shenqifuzheng showed statistically significant differences for the improvement of leukopenia, and Huachansu, Aidi, and Fufangkushen for adverse events in the digestive system, but no significant differences in the rate of short‐term remission. Most of the included studies were of low quality and valid comparisons were scarce, meaning that more trials are needed for meta‐analysis to draw definite conclusions about their benefits.
Summary of findings
Summary of findings for the main comparison. Appraisal of the results of Huachansu in the short term for induction of remission in advanced or late gastric cancer.
| Appraisal of the results of Huachansu in the short term for induction of remission in advanced or late gastric cancer | ||||||
| Patient or population: patients with induction of remission in advanced or late gastric cancer Settings: Intervention: Appraisal of the results of Huachansu in the short term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Appraisal of the results of Huachansu in the short term | |||||
| the toxic and side effects in digestive system after chemotherapy (no special data in trial of Zhang 2006) Follow‐up: 6‐24 weeks | Study population | OR 0.43 (0.28 to 0.66) | 388 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 589 per 1000 | 381 per 1000 (286 to 486) | |||||
| Moderate | ||||||
| 523 per 1000 | 320 per 1000 (235 to 420) | |||||
| the rate of complete remission and partly remission (Copy) Follow‐up: 6‐24 weeks | Study population | OR 1.48 (1.01 to 2.17) | 448 (7 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 401 per 1000 | 498 per 1000 (403 to 592) | |||||
| Moderate | ||||||
| 367 per 1000 | 462 per 1000 (369 to 557) | |||||
| the toxic and side effects of leukopenia after chemotherapy(no special data in trial of Zhang 2006) Follow‐up: 6‐24 weeks | Study population | OR 0.32 (0.21 to 0.5) | 388 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 583 per 1000 | 309 per 1000 (227 to 412) | |||||
| Moderate | ||||||
| 539 per 1000 | 272 per 1000 (197 to 369) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The method of sequence generation was not offered by the authors in three studies,quasi‐randomised for three trials,and one simple randomisation study. 2 Allocation concealment and blinding of the method were not offered by the seven study authors. 3 total (cumulative) sample size is lower than the calculated optimal information size
Summary of findings 2. Appraisal of the results of Aidi in the short term for induction of remission in advanced or late gastric cancer.
| Appraisal of the results of Aidi in the short term for induction of remission in advanced or late gastric cancer | ||||||
| Patient or population: patients with induction of remission in advanced or late gastric cancer Settings: Intervention: Appraisal of the results of Aidi in the short term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Appraisal of the results of Aidi in the short term | |||||
| the rate of complete remission and partly remission(no special data in trial of Zhang 2009) Follow‐up: 6‐12 weeks | Study population | OR 1.51 (0.94 to 2.41) | 287 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 423 per 1000 | 525 per 1000 (408 to 638) | |||||
| Moderate | ||||||
| 409 per 1000 | 511 per 1000 (394 to 625) | |||||
| the toxic and side effects in digestive system after chemotherapy Follow‐up: 6‐12 weeks | Study population | OR 0.33 (0.2 to 0.54) | 354 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 575 per 1000 | 308 per 1000 (213 to 422) | |||||
| Moderate | ||||||
| 574 per 1000 | 308 per 1000 (212 to 421) | |||||
| the toxic and side effects of leukopenia after chemotherapy(no special data in trial of Jia 2003, Zhang 2009) Follow‐up: median 6‐12 weeks | Study population | OR 0.43 (0.23 to 0.8) | 242 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 675 per 1000 | 472 per 1000 (323 to 624) | |||||
| Moderate | ||||||
| 667 per 1000 | 463 per 1000 (315 to 616) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The method of sequence generation was not offered by the authors in all studies. 2 Allocation concealment and blinding of the method were not offered by the five study authors. 3 total (cumulative) sample size is lower than the calculated optimal information size
Summary of findings 3. Appraisal of the results of Fufangkushen in the short term for induction of remission in advanced or late gastric cancer.
| Appraisal of the results of Fufangkushen in the short term for induction of remission in advanced or late gastric cancer | ||||||
| Patient or population: patients with induction of remission in advanced or late gastric cancer Settings: Intervention: Appraisal of the results of Fufangkushen in the short term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Appraisal of the results of Fufangkushen in the short term | |||||
| the rate of complete remission and partly remission(no special data in trial of Fu 2011) Follow‐up: 3‐9 weeks | Study population | OR 1.22 (0.83 to 1.79) | 423 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 464 per 1000 | 514 per 1000 (418 to 608) | |||||
| Moderate | ||||||
| 454 per 1000 | 504 per 1000 (408 to 598) | |||||
| the toxic and side effects in digestive system after chemotherapy(no special data in trial of Fu 2011, Liu 2009a, Zhang 2010) Follow‐up: 3‐9 weeks | Study population | OR 0.42 (0.26 to 0.69) | 302 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 611 per 1000 | 397 per 1000 (290 to 520) | |||||
| Moderate | ||||||
| 628 per 1000 | 415 per 1000 (305 to 538) | |||||
| the toxic and side effects of leukopenia after chemotherapy Follow‐up: median 3‐9 weeks | Study population | OR 0.37 (0.25 to 0.56) | 503 (7 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 699 per 1000 | 462 per 1000 (367 to 565) | |||||
| Moderate | ||||||
| 719 per 1000 | 486 per 1000 (390 to 589) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The method of sequence generation was not offered by the authors in five studies,random number table for one trials, and the method of sequence generation was tossed of a coin in one study. 2 Allocation concealment and blinding of the method were not offered by the seven study authors. 3 total (cumulative) sample size is lower than the calculated optimal information size
Summary of findings 4. Appraisal of the results of Shenqifuzheng in the short term for induction of remission in advanced or late gastric cancer.
| Appraisal of the results of Shenqifuzheng in the short term for induction of remission in advanced or late gastric cancer | ||||||
| Patient or population: patients with induction of remission in advanced or late gastric cancer Settings: Intervention: Appraisal of the results of Shenqifuzheng in the short term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Appraisal of the results of Shenqifuzheng in the short term | |||||
| the rate of complete remission and partly remission Follow‐up: 4‐8 weeks | Study population | OR 1.48 (0.78 to 2.81) | 153 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 493 per 1000 | 590 per 1000 (432 to 732) | |||||
| Moderate | ||||||
| 500 per 1000 | 597 per 1000 (438 to 738) | |||||
| the toxic and side effects in digestive system after chemotherapy Follow‐up: 4‐8 weeks | Study population | OR 1.13 (0.18 to 7.24) | 153 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 413 per 1000 | 443 per 1000 (113 to 836) | |||||
| Moderate | ||||||
| 533 per 1000 | 563 per 1000 (170 to 892) | |||||
| the toxic and side effects of leukopenia after chemotherapy Follow‐up: 4‐8 weeks | Study population | OR 0.37 (0.18 to 0.74) | 153 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 467 per 1000 | 245 per 1000 (136 to 393) | |||||
| Moderate | ||||||
| 375 per 1000 | 182 per 1000 (97 to 307) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The method of sequence generation was not offered by the authors in all studies. 2 Allocation concealment and blinding of the method were not offered by the seven study authors. 3 total (cumulative) sample size is lower than the calculated optimal information size
Background
In general, therapeutic prescriptions of traditional Chinese medicinal herbs (TCMHs) for gastric cancer consist of a group of herbs (commonly seven to 15 kinds of herbs). Some, such as Rhizoma Curcumae, Herba Hedyotis Diffusae, Rhizoma Paridis, Astragali radix, Radix Clematidis, and Fructus Bruceae, are commonly used as direct anti‐tumour agents and others, such as Tangerine peel, Milk vetch root, Pilose asiabell root, Spatholobus stem, Chinese angelica root, Flos Carthami, and Red sage root, can be added to the main prescription as supporting treatments to decrease the side effects or toxicity of chemotherapy (Ning 1985) or to improve the curative effect. This is described as 'strengthening the body resistance, restoring normal functioning of the body to consolidate the constitution, relieving the depressed liver and soothing the stomach, invigorating qi and enriching the blood, removing the poisonous quality of any substance and resolving the stasis'. These are the terms used in traditional Chinese medical theory (Gu 1995). Unfortunately, there still seems to be no special herbs or recipes that have been found to have special effects on certain kinds of cancers, so these same TCMHs can be used for other malignant tumours such as oesophageal carcinoma, hepatocarcinoma, or pulmonary carcinoma. At present, TCMHs are not recommended to treat benign tumours, such as polyps, because such diseases can be cured effectively by surgery.
Medicines in complex prescriptions can be given by oral administration or intravenous drip, and there are many case reports showing that patients have been treated effectively with TCMHs administered either orally or intravenously, or by both methods (Duan 2002). The combination administration is based on the special diagnostic modes of Traditional Chinese Medicine (TCM), such as inspection, listening, smelling, inquiry, and palpation, which mainly depend on the experience of doctors and are very different from western diagnostic methods. Although many trials appear to demonstrate that TCMHs might have some effectiveness on cancer, there is no evidence showing that TCMHs could replace surgery or radio‐chemotherapy for cancer in its early stages. At present, TCMHs are mainly used as an auxiliary therapy and a palliative treatment with routine therapeutic methods for advanced or late cancer, including gastric cancer.
Description of the condition
Although many cancers can be cured in the early stages, once they progress to advanced or late stage (that is once widespread metastasis is confirmed by medical techniques such as X‐ray computed tomography (X‐CT), magnetic resonance imaging (MRI), or histologic examination) there are few interventions which can postpone or stop the malignant illness leading to death. Although bio‐therapies, such as gene therapy, immune therapy, bone‐marrow transplantation, etc, have made some progress in some kinds of advanced or late cancers, the mortality rate of most common late malignancy tumours (such as carcinomas derived from the digestive tract, gastric cancer, hepatocarcinoma) is still high. Both the morbidity and mortality of gastric cancer rank second of all malignant tumours (Tang 2004), varying from 30 per 10⁵ to 80 per 10⁵ and 15.9 per 10⁵ to 32.4 per 10⁵, respectively (Zheng 2001), in different countries and regions. According to the statistical data, China, Japan, and Chile are countries with high risk of morbidity and mortality, and the United States, Canada, and European countries are those with low risk (Tang 2004).
Description of the intervention
TCM is a common alternative therapy in China for late‐stage cancer, and all the herbs cited in this review can be found in the Traditional Chinese Medicine Dictionary. TCM has its own theories and systems for diagnostic and therapeutic methods for malignant tumours. It is thought that gastric cancer, called ye‐ge (similar to dysphagia) (Yang 1989), is caused mainly by overactive emotions (joy, anger, sorrow, anxiety, and fear) and eating or drinking too much, resulting in internal stasis of Yangqi and consumption of Yin fluid. Yin‐Yang theories of TCM, derived from Taoism, state that there are two substances, Yin and Yang, in the human body and that they should match each other to keep the balance, otherwise the body is at risk of all kinds of diseases. According to matched control research, it is shown that highly differentiated gastric adenocarcinoma (Wang 2000) is similar to insufficiency of the spleen (Yang), or lack of coordination between the liver and the spleen; and poorly differentiated gastric adenocarcinoma is similar to deficiency of both qi and blood, or stagnancy of qi and blood stasis. In TCM, qi means something similar to air. The theories of TCM believe there is a kind of air running throughout the entire human body, not only in the lungs but in every organ of the body, and some people can feel its existence through breathing exercises; though this viewpoint has not been proven by modern western science.
In traditional Chinese medicinal theory, therapeutic strategies aimed at late or advanced gastric cancer include three basic principles (Ji 1989):
replenishing and strengthening the vital‐qi;
reducing phlegm and resolving stasis;
clearing away heat and toxic material.
How the intervention might work
According to the principle that treatment of a disease should deal with both the symptoms and causes at the same time, some categories of traditional Chinese medicinal herbs (TCMHs) are used according to the Chinese medicinal typing of advanced or late gastric cancer (Guo 1997) as alternative interventions. It is generally acknowledged that in its early stages gastric cancer can be cured with surgery, so alternative interventions (including TCMHs) are unnecessary. Once metastasis develops (that is in advanced or late‐stage disease) and the opportunity for surgery is lost, the cancer can not be cured. Therefore alternative interventions, including TCMHs, are used either alone or as auxiliary therapies with radio‐chemotherapy or bio‐therapy (Zheng 2001).
Though the basic research on TCMHs is still weak, and most of the active ingredients are not extracted and confirmed at present, It is believed that some TCM herbs (including Astragulus membranaceus, dandelion herb, cassia twig, Poria, magnolia bark, chaenomeles fruit, costus root, barbat skullcap, lyrate nightshade, Chinese actinidia root, Coix seed, globethistle, hornet nest (Zhou 1999), and others such as bighead atractylodes rhizome, Oldenlandia diffusa Roxb (Wu 2001), Scutellaria baicalensis Georgi, Allium sativum L As2O3) could inhibit the proliferation of gastric tumour cells (Sun 2002; Zhao 2002). Some basic research showed that isoverbascoside (Chen 2001), found in Pedicularis strata, has the effect of cleaning up multifarious oxyradicals. This could transfer the growth signal in the gastric cancer cell thus inhibiting the proliferation of gastric cancer. Astragulus membranaceus, a commonly used herb (Shen 2007) can down‐regulate the expression of cyclooxygenase‐2 (COX‐2), vascular endothelial growth factor (VEGF), and polyethylene glycol (PEG)‐2 in the gastric cancer cells. Radix Astragali specifically inhibits the growth of gastric cancer cells in vitro, but it is mainly cytostatic and not cytotoxic and does not induce apoptosis (Lin 2003). Another result from a pilot study suggests that a polysaccharide isolated from Echinacea purpurea herba cell cultures might be effective in reducing chemotherapy‐induced leukopenia (Melchart 2002); and the extract from Radix Curcumae, obtained by steam distillation, has a chemopreventive effect on gastric cancer induced by N‐methyl‐N'‐nitro‐N‐nitrosoguanidine (MNNG) in rats (Lu 2008). Furthermore, the alkaloid Matrine can inhibit cell proliferation and induce apoptosis of SGC‐7901 cells in vitro; the apoptosis induction appears to be through up‐regulating Fas/FasL expression and activating caspase‐3 enzyme (Dai 2009). Aidi injection, a commonly used TCM recipe in China, appears to have the effect of inhibiting the proliferation of cancer cells, including gastric cancer cells, but the effect is uncertain and needs to be assessed more thoroughly (Sa 2003). Other TCMHs can improve immunity (Bu 2001b; Lu 1996), for example Fuzhenghuayu Recipe can improve the function of T‐cells and inhibit metastasis after surgery in patients with gastric cancer. Some herbal recipes are believed to reduce the incidence of atypical hyperplasia in the gastric mucosa (Qiu 1993), and many TCMHs (including Sijunzi decoction, As2O3, Radix Astragali seu Hedysari, Bulbus Alliican) lead to apoptosis of gastric cancer cells (Wu 2001) by inducing expression of gene P53, P21. Huoxuehuayu recipe has the same effect, by inducing over‐expression of Bcl‐2 and inhibiting the expression of epidermoid growth factor receptors (EGFR).
Why it is important to do this review
TCMHs have been used widely and for many years to treat gastric cancer. Much clinical experience has been summarised and the first randomised controlled trial (RCT) appeared in 1986 (Zeng 1986). Most of the literature about TCMH for gastric cancer, especially the RCTs (Huang 2005; Liu 2006a; Xie 2006), have concluded that the TCMHs have positive effects on quality of life, prolonging the life span, and alleviating adverse events caused by routine chemotherapy, but the effectiveness and adverse effects of TCMHs have not been assessed systematically. The objective of this review was to assess the effectiveness of TCMHs for eradicating gastric cancerous cells and to determine whether TCMHs can improve the patient's general condition and prolong the average life span compared with routine clinical therapy for late or advanced gastric cancer, such as chemotherapy and radiotherapy.
Objectives
1. To appraise the improvement of and remission in patients by comparing the intervention group (TCMHs) with the control group (no TCMHs), including:
(i) studies which compared TCMH to placebo (these may be either with or without concomitant treatment); or
(ii) studies which compared TCMH to other treatments.
The efficacy parameters included mortality and median survival time, time to progression, quality of life.
2. To determine adverse events associated with TCMH treatment in patients with advanced or late gastric cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) which compared TCMHs with either placebo or other drugs.
Types of participants
Patients of any age whose final diagnosis was T (tumour) 1 to 4, N (lymph nodes) 1 to 3, M (metastasis) 1, confirmed by the new tumour, node, metastasis (TNM) descriptive stage (UICC 1997) and for whom surgery was not an option. This descriptive stage means that metastasis exists and the cancer has gone into advanced or late stage, that is, III or IV.
Patients who have confirmed recurrence of gastric cancer accompanied by distant metastasis after operation.
Types of interventions
RCTs of TCMH (oral or intravenous administration, or both) used for treatment of patients with advanced or late‐stage gastric cancer. This included TCMH treatment studies and clinical trials in which TCMHs were added to the other treatments for patients in advanced or late‐stage gastric cancer.
Types of outcome measures
Endoscopic, radiographic, clinical, or histological remission as defined by the primary studies and expressed as a percentage of the number of patients randomised (intention‐to‐treat analysis) was the outcome measure of interest. Since definitions of advanced or late‐stage disease can vary from trial to trial, we used the individual definitions from each study. The number of patients with clinical improvement or remission of advanced or late gastric cancer was recorded. The exact definition of improvement and remission also varied from study to study, making exact comparisons across studies difficult or impossible. However, for the purpose of this analysis, we used the definition of improvement or remission as used in each study for extraction of data from the individual studies.
Other outcomes of interest included life span, drug adverse effects, withdrawals for toxicity or adverse events, and the effects of drug interactions.
Primary outcomes
Mortality
Secondary outcomes
Quality of life (QOL): the QOL index was assessed by the Karnofsky score, if the score increased to over 10 at the end of the therapeutic period it was defined as improvement of QOL.
Rate of remission (short‐term and long‐term): following the standards of the International Union Against Cancer (UICC), the rate of remission included complete remission (the tumour disappeared in a period of at least three months) and part remission (half of the tumour disappeared over at least three months).
Median survival time (MST): MST is the median value for patient survival time.
Time to progression (TTP): the time from the stage of remission to the stage of advancing cancer, i.e., the time for tumour relapse.
Adverse events:
life threatening;
toxic response;
resulting in the discontinuation of treatment.
The side effects were those caused by either Chinese medicinal herbs or the comparator, or both.
Search methods for identification of studies
See: Cochrane Upper Gastrointestinal and Pancreatic Diseases Group methods used in reviews.
We conducted a search to identify all published and unpublished RCTs.
Electronic searches
We searched the following electronic databases:
The Cochrane Library (Issue 3, 2011) (Appendix 1),
MEDLINE (from 1950 to June 2011) (Appendix 2),
EMBASE (from 1980 to June 2011) (Appendix 3),
AHMED (Allied and Complementary Medicine Database), and
CBM (Chinese Biomedical Database) (from 1974 to June 2011) (Appendix 4).
The search strategy for the review was constructed by using a combination of MeSH subject headings and text words relating to the use of TCMHs in the treatment of advanced or inoperable gastric cancer.
Searching other resources
We handsearched reference lists from trials selected by electronic searching to identify further relevant trials. We contacted authors of identified studies to request any further published or unpublished work.
We handsearched the following journals:
Acta Medicinae Sinica,
Cancer Research on Prevention and Treatment,
China Journal of Chinese Materia Medica,
China Oncology,
Chinese Journal of Cancer Research,
Chinese Journal of Clinical Oncology and Rehabilitation,
Chinese Journal of Integrated Traditional and Western Medicine on Digestion,
Chinese Journal of Oncology,
Chinese Journal of Radiation Oncology,
Henan Journal of Traditional Chinese Medicine,
Jiangshu Journal of Tradition Chinese Medicine,
Journal of Beijing of Tradition Chinese Medicine,
Journal of Fujian of Traditional Chinese Medicine,
Journal of Jilin of Traditional Chinese Medicine,
Journal of Practical Oncology,
Journal of Nanjing University of Traditional Chinese Medicine,
Journal of Sichuang of Traditional Chinese Medicine,
JTCM (Journal of Traditional Chinese Medicine),
Traditional Chinese Medicinal Research.
In addition, we contacted the World Health Organization, experts in the field, and medicinal herb manufacturers to request details of outstanding clinical trials or any relevant unpublished materials.
Data collection and analysis
Where appropriate, we combined the extracted data (Parmar 1998) from the various trials by calculating a pooled estimate of the odds ratio using the method of Mantel‐Haenszel, the relative risk and risk difference, and the 95% confidence intervals for dichotomous data. We used both fixed‐effect and random‐effects models. Where outcomes were measured as continuous data in a standard way across studies, we calculated the weighted mean difference and 95% confidence interval using a random‐effects model. Dropouts were analysed according to the principle of inefficiency in the intervention group and efficiency in the control group, and these conservative results were recorded.
Selection of studies
Three authors reviewed potentially relevant studies to determine their eligibility based on the criteria (and mortality, MST, TTP, QOL outcomes) described above.
Data extraction and management
Three review authors independently appraised each study and recorded the methodological criteria and the results of each study on standard data forms. For crossover studies, only data from the first portion of the study would have been incorporated in order to avoid possible carryover effects of medications into the second part of the study, and to make these studies more comparable to those studies not of crossover design. We determined all results on an intention‐to‐treat basis.
Assessment of risk of bias in included studies
The criteria for assessment of risk of bias included the specific methods of randomisation and allocation concealment, the blinding method, and reporting of dropouts or withdrawal of patients according to the Cochrane Handbook for Systematic Reviews of Interventions, Table 8.5.c (criteria for judging risk of bias in the 'risk of bias' assessment tool) (Higgins 2008).
Measures of treatment effect
If the heterogeneity across the included trials was low the treatment effects were pooled in a meta‐analysis, or a descriptive method was used.
Unit of analysis issues
There was a unit of analysis issue for one study. General information about the included studies is provided in the 'Characteristics of included studies' table.
Dealing with missing data
Analyses were performed on an intention‐to‐treat basis if data were missing. For dichotomous data, patients in the treatment group with incomplete or missing data were regarded as treatment failures and those in the control group were regarded as treatment successes. According to this principle, a 'worst‐best case' scenario analysis would be carried out.
Assessment of heterogeneity
The heterogeneity of the included studies mainly resulted from the different recipes of TCHM used, assessed in the Results section.
Assessment of reporting biases
No
Data synthesis
The dichotomous data were presented as relative risk (RR), and continuous outcomes by weighted mean difference (WMD), if possible, both with 95% confidence intervals (CI).
Subgroup analysis and investigation of heterogeneity
No
Sensitivity analysis
Where meta‐analysis was performed, we also carried out a sensitivity analysis.
Results
Description of studies
Results of the search
Our completed searches (June 2011) identified 179 articles: 172 from the electronic searches and seven from handsearching. After reading titles, abstracts, and the content of the articles we excluded 99 because they had study objectives that were different from those for this review, the reasons for exclusion are listed under Characteristics of excluded studies. The remaining 80 articles were selected for further assessment.
Included studies
Design
All of the included studies had a parallel design and no crossover design was used.
According to the intervention measures, the 80 articles were subdivided into four types:
TCMHs plus western therapeutic methods in the intervention group versus the same western therapeutic methods in the control group (type I in Table 5, 65 articles),
TCMHs plus western therapeutic methods in the intervention group versus the same TCMHs in the control group (type II in Table 6, six articles),
TCMHs in the intervention group versus other TCMHs in the control group (type III in Table 7, two articles),
TCMHs in the intervention group versus western therapeutic methods in the control group (type IV in Table 8, seven articles).
1. Table of administration of Chinese medicinal herbs (TCMHs + medicine versus medicine).
| NUMBER OF TRIALS | HERBS IN REGIMEN | ROUTE OF ADMIN | PERIOD OF ADMIN | RCT AND BLIND METHOD | FOLLOW TIME |
| Cao 1992 | Radix Codonopsis Pilosulae, Radix Astragali. No specific dosage of the herbs. | intravenous drip | 4 to 5 weeks | RCT without BLIND | No |
| Cao 1997 | Emulsion of Lanxiangxi, No specific dosage of the herbs. | intravenous drip | 6 to 8 weeks | RCT without BLIND | No |
| Chen 1997 | Radix Curcumae, Alumen, Natrii Sulphas, Faeces Trogopterorum, Radix Achyranthis Bidentatae, Semen Strychni Pulveratum, Hebra Agrimoniae, No specific dosage of the herbs. | oral administration | 2 months | RCT without BLIND | No |
| Chen 2005 | Rhizoma Curcumae, Pseudobulbus Cremastrae Seu Pleiones, Fructus Bruceae, Semen Strychni Pulveratum, Nidus Vespae, Radix Astragali, Calculus Bovis. No specific dosage of the herbs. | oral administration | 3 to 4 weeks | RCT without BLIND | 4 to 22 months |
| Chen 2008 | Aidi injecta (Radix Ginseng, Astragalus Mongholicus, Radix Acanthopanacis Senticosi, Chinese Cantharides). No specific dosage of the herbs. | intravenous drip | 6 weeks | RCT without BLIND | No |
| Chen 2009 | Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 6 weeks | RCT without BLIND | No |
| Deng 2001 | Emulsion of Lanxiangxi. No specific dosage of the herbs. | intravenous drip | 3 weeks | RCT without BLIND | No |
| Deng 2011 | Shenfu injecta (Radix Ginseng, Radix aconiti lateralis preparata). No specific dosage of the herbs. | intravenous drip | 4 weeks | RCT without BLIND | No |
| Du 2010 | TCMH fomula (Astragalus Mongholicus 30g, Rhizoma Polygonati 20g, Rhizoma atractylodis macrocephala 10g, Poria 10g, Radix Glycyrrhizae 6g, Fructus Ligustri Lucidi 10g, Rhizoma Sanguisorbae 20g, Caulis Spatholobi 30g, Colla Corii Asini 6g, Pericarpium Citri Reticulatae 10g, Rhizoma Pinelliae 6g, Radix Actinidiae Chinensis 20g) | oral administration | 6 weeks | RCT without BLIND | No |
| Fu 2011 | Compound matrine injection (Sophora flavescens, Poria Alba). No specific dosage of the herbs. | intravenous drip | 6 weeks | RCT without BLIND | No |
| Gao 2008 | Mojisankeli (Astragalus Mongholicus, Radix Codonopsitis, Semen Coicis, Fructus Amomi, Ventriculi Galli Mucosa, Sophora Flavescens, Hedyotis Diffusa, Spica Prunellae Vulgaris, Rhizoma Pinelliae, Rhizoma Arisaematis, Kelp, Rhizoma Curcumae Aeruginosae, Rhizoma Sparganii, Radix Curcumae Longae, Scolopendra). No specific dosage of the herbs. | oral administration | 3 months | RCT without BLIND | No |
| Gong 2006 | Radix ginseng, Radix Astragali, Radix Acanthopanacis Senticosi, Chinese Cantharides. No specific dosage of the herbs. | intravenous drip | 12 weeks | RCT without BLIND | 42 months |
| Guan 2001 | Emulsion of Lanxiangxi. No specific dosage of the herbs. | intravenous drip | 3 weeks | RCT without BLIND | No |
| Guo 1989 | Radix Codonopsis Pilosulae, Largehead Atractylodes, Rhizome Cuscuta Japonica, fructus psoraleae, Fructus Ligustri Lucidi, Fructus Lycii. No specific dosage of the herbs. | oral administration | 4 to 6 weeks | RCT without BLIND | No |
| Hu 2011 | Fuzhengxiao'ai I formula (Poria 10g, Radix angelicae seu hemsley 10g, Radix Saposhnikoviae 10g, Rhizoma atractylodis macrocephala 10g, Rhizoma Rehmanniae 10g, Rhizoma Chuanxiong 10g, Cortex Moutan 10g, Radix Actinidiae Chinensis 10g, Radix Pseudoxtellariae 15g, Astragalus Mongholicus 15g, Radix Ophiopogonis 15g, Pseudobulbus Cremastrae Seu Pleiones 15g, Hedyotis Diffusa 30g, Radix Glycyrrhizae 6g, Scolopendra 2) | oral administration | 3 to 6 weeks | RCT without BLIND | No |
| Hua 1999 | radix ginseng 20g, Radix Astragali 15g, Largehead Atractylodes Rhizome 15g, Prepared Resina Olibani 10g, Prepared Myrrha 10g, Herba Hedyotis Diffusae 30g, Hebra Agrimoniae 30g, Rhizoma Curcumae 15g, Radix Trichosanthis 20g, Venenum Bufonis 0.3g | oral administration | 9 to 12 weeks | RCT without BLIND | No |
| Huang 2002 | Injecta of Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 1 months | RCT without BLIND | No |
| Huang 2005 | Emulsion of Lanxiangxi. No specific dosage of the herbs. | intravenous drip | 9 weeks | RCT without BLIND | No |
| Jia 2003 | Radix Ginseng, Radix Astragali, Radix Acanthopanacis Senticosi. Chinese Cantharides. No specific dosage of the herbs. | intravenous drip | 6 weeks | RCT without BLIND | Not clear |
| Jia 2009 | Shenqifuzheng injecta (Radix Codonopsitis, Astragalus Mongholicus). No specific dosage of the herbs. | intravenous drip | 8 weeks | RCT without BLIND | No |
| Li 2002 | Radix Astragali 40g, Radix Codonopsis Pilosulae 30g, Radix Salviae Miltiorrhizae 30g, Radix Paeoniae Rubra 30g, Radix Rubiae 30g, Rhizoma Sparganii 30g, Ochra Haematitum 30g, Poria 15g, Largehead Atractylodes Rhizome 10g, Radix Glycyrrhizae 10g, Rhizome of Oldworld Arrowhead 10g, Flos Inulae 6g | oral administration | Not clear | RCT without BLIND | Not clear |
| Lin 2011 | Compound matrine injection (Sophora flavescens, Poria Alba). No specific dosage of the herbs. | intravenous drip | 9 weeks | RCT without BLIND | No |
| Liu 2006 | Radix Astragali 30g, Radix Codonopsis Pilosulae 20g, Largehead Atractylodes Rhizome 10g, Poria10g, Radix Paeoniae Alba 15g, Radix Angelicae Sinensis 12g, Prepared Radix Rehmanniae 10g, Rhizoma Ligustici Chuanxiong 10g, Prepared Rhizoma Pinelliae 9g, Percarpiu Citri Reticulatae 6g, Carapax Trionycis 30g, Squama Manitis 15g, Caulis Spathoobi 30g, Panax Notoginseng 6g, Radix Glycyrrhizae 5g | oral administration | 9 weeks | RCT without BLIND | No |
| Liu 2006a | Gekko Japonicus Dumeril et Bibron 4g, Herba Rabdosiae 30g, Rhizoma Smilacls Chinensis 30g, Radix Actinidiae Chinensis 30g, Radix Ginseng 30g, Radix Astragali 30g, Poria 20g, Dried Semen Coicis 30g, Fructus Crataegi 15g, Rhizoma Curcumae15g, Akebia Trifoliata Koidz 30g | oral administration | 6 weeks | RCT without BLIND | No |
| Liu 2009 | Aidi injecta (Radix Ginseng, Astragalus Mongholicus, Radix Acanthopanacis Senticosi, Chinese Cantharides). No specific dosage of the herbs. | intravenous drip | 8 weeks | RCT without BLIND | No |
| Liu 2009a | Compound matrine injection (Sophora flavescens, Poria Alba). No specific dosage of the herbs. | intravenous drip | 6 weeks | RCT without BLIND | No |
| Luo 2011 | Shenqifuzheng injecta (Radix Codonopsitis, Astragalus Mongholicus). No specific dosage of the herbs. | intravenous drip | 5˜6 weeks | RCT without BLIND | No |
| Lv 1999 | Decoction of Taohongsiwu, Decoction of Maimendong. No specific dosage of the herbs. | oral administration | 3 to 4 weeks | RCT without BLIND | No |
| Niu 2006 | Percarpiu Citri Reticulatae, Cortex Magnoliae Officinalis 12g, Ramulus Cinnamomi, Largehead Atractylodes Rhizome, Rhizoma Alismatis10g, Rhizoma Atractylodis 15g, Poria 15g, Umbellate Pore Fungus 9g, Scorpio 5 to 10g, Scolopendra Subspinipes Mutilans L.koch 1 to 3, Fructus Ziziphi Jujubae 10, Radix Glycyrrhizae 6g, Hebra Agastachis, Semen Coicis, Radix Paeoniae Rubra, Radix Codonopsis Pilosulae, Fructus Hordei Germinatus, Fructus Oryzae Germinatus, Faeces Trogopterorum, Pollen Typhae, Rhizoma Polygonati Odorati, Radix Adenophorae, Cortex Cinnamomi, Fructus Evodiae, Panax Notoginseng, Radix Rubiae. No specific dosage of the partly herbs. | oral administration | Not clear | RCT without BLIND | Not clear |
| Peng 2006 | Radix Astragali 40g, Radix Paeoniae Alba 24g, Rhizoma Corydalis 15g, Fructus Tsaoko 15g, Os Spepiella Seu Sepiae 15g, Poria 15g, PreparedRadix Glycyrrhizae 15g, Baked Concha Arcae 12g, Radix Panacis Quinquefolii 10g, Faeces Trogopterorum 10g, Myrrha 10g, Radix Angelicae Sinensis 10g, Largehead Atractylodes Rhizome 10g, Endothelium Corneum Gigeriae Galli 10g, Panax Notoginseng 15g | oral administration | 3 months | RCT without BLIND | No |
| Rao 1994 | Radix Astragali 30g, Radix Pseudostellariae 30g, Caulis Spathoobi 30g, Largehead Atractylodes Rhizome 10g, Poria10g, Fructus Lycii15g, Fructus Ligustri Lucidi 15g, Cuscuta Jjaponica 15g | oral administration | 3 to 6 months | RCT without BLIND | No |
| Si 2004 | Percarpiu Citri Reticulatae, Prepared Rhizoma Pinelliae, Poria, Radix Glycyrrhizae, Radix Aucklandiae, Semen Sinapis Albae, Radix Codonopsis Pilosulae, Largehead Atractylodes Rhizome, Radix Adenophorae, Radix Ophiopogonis, Rhizoma Polygonati Odorati, Dried Radix Rehmanniae, Prepared Radix Rehmanniae, Radix Angelicae Sinensis, Fructus Meliae Toosendan, Radix Bupleuri, Rhizoma Cyperi, Pericarpium Citri Reticulatae, Percarpiu Citri Reticulatae, Fructus Citri Sarcodactylis, Radix Angelicae Sinensis, Semen Persicae, Radix Paeoniae Alba, Radix Salviae Miltiorrhizae. No specific dosage of the herbs. | oral administration | Not clear | RCT without BLIND | Not clear |
| Sun 1999 | Yangweikangliuchongji. No specific dosage of the herbs. | oral administration | Not clear | RCT without BLIND | 12 to 36 months |
| Tian 1999 | Emulsion of Lanxiangxi. No specific dosage of the herbs. | intravenous drip | 4 weeks | RCT without BLIND | Not clear |
| Wang 1993 | Radix Codonopsis Pilosulae 15g, Radix Astragali 15g, Largehead Atractylodes Rhizome 15g, Poria 10g, Percarpiu Citri Reticulatae 6g, Rhizoma Pinelliae 6g, Caulis Spathoobi 30g, Fructus Lycii 15g, Fructus Ligustri Lucidi 15g, Radix Paeoniae Alba 15g, Radix Ophiopogonis 12g, Herba Hedyotis Diffusae 15g | oral administration | 8 weeks | RCT without BLIND | No |
| Wang 2002 | Arisaemacum Bile, Rhizoma Pinelliae, Percarpiu Citri Reticulatae, Fructus Aurantii Immaturus, Bulbus Fritillariae Cirrhosae, Semen Sinapis Albae, Scorpio, Endothelium Corneum Gigeriae Galli, Radix Glycyrrhizae. No specific dosage of the herb. | oral administration | 6 months | RCT without BLIND | No |
| Wang 2004 | Fructus Bruceae. No specific dosage of the herbs. | intravenous drip | 1 to 3 months | RCT without BLIND | Not clear |
| Wang 2004a | Radix Astragali 20g, Radix Codonopsis Pilosulae 15g, Largehead Atractylodes Rhizome 12g, Rhizoma Dioscoreae 12g, Semen Coicis 30g, Percarpiu Citri Reticulatae 6g, Radix Salviae Miltiorrhizae 10g, Rhizoma Curcumae 15g, Herba Salviae Chinensis 10g, Herba Solani Nigri 15g | oral administration | 2 to 3 months | RCT without BLIND | No |
| Wang 2009 | Aidi injecta (Radix Ginseng, Astragalus Mongholicus, Radix Acanthopanacis Senticosi, Chinese Cantharides). No specific dosage of the herbs. | intravenous drip | 12 weeks | RCT without BLIND | No |
| Wang 2009a | Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 8 weeks | RCT without BLIND | No |
| Wang 2010 | Shenqifuzheng injecta (Radix Codonopsitis, Astragalus Mongholicus). No specific dosage of the herbs. | intravenous drip | 31 days | RCT without BLIND | No |
| Wang 2010a | Compound matrine injection (Sophora flavescens, Poria Alba). No specific dosage of the herbs. | intravenous drip | 6 weeks | RCT without BLIND | No |
| Wang 2010b | Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 16 weeks | RCT without BLIND | No |
| Wu 1999 | Radix Bupleuri 10g, Radix Curcumae 10g, Fructus Aurantii 6g, Rhizoma Cyperi 10g, Radix Codonopsis Pilosulae 20g, Poria 10g, Prepared Radix Rehmanniae 10g, Aspongopus 10g, Nidus Vespae 10g, Herba Scutellariae Barbatae 30g, Rhizoma Zingiberis 6g, Largehead Atractylodes Rhizome 10g, Rhizoma Pinelliae 10g, Chinese Buckeye Seed 10g, Percarpium Citri Reticulatae 6g, Radix Astragali 30g, Radix Paeoniae Alba 10g, Radix Angelicae Sinensis 10g, Pollen Typhae 10g, Faeces Trogopterorum 10g, Rhizoma Ligustici Chuanxiong 6g, Hebra Agrimoniae 30g, Rhizoma Polygonati Odorati 10g, Semen Persicae 10g | oral administration | Not clear | RCT without BLIND | 60 months |
| Wu 2000 | Radix Astragali 300g, Rhizoma Curcumae 150g, Herba Hedyotis Diffusae 150g, Dried Semen Coicis 150g, Herba Salviae Chinensis 150g, Radix Clematidis 100g, Powder of Shark Cartilage 150g | oral administration | 6 to 8 weeks | RCT without BLIND | 6 to 24 months |
| Wu 2000a | Emulsion of Lanxiangxi. No specific dosage of the herbs. | intravenous drip | 6 to 8 weeks | RCT without BLIND | Not clear |
| Xie 2006 | Radix Sophorae Subprostratae, Herba Hedyotis Diffusae, Radix Astragali, Pseudobulbus Cremastrae Seu Pleiones, Radix Curcumae, Radix Semiaquilegiae, Spica Prunellae. No specific dosage of the herbs. | oral administration | 3 months | RCT without BLIND | No |
| Xiong 2008 | Compound matrine injection (Sophora flavescens, Poria Alba). No specific dosage of the herbs. | intravenous drip | 9 weeks | RCT without BLIND | No |
| Xu 1993 | Mesona Chinensis Benth, DriedPrepared Radix Rehmanniae, Radix Astragali, Radix Codonopsis Pilosulae, Panax Notoginseng, Radix Salviae Miltiorrhizae, Calculus Bovis, Moschus, Hebra Euphorbiae Lunulatae, Herba Solani Nigri, Herba Scutellariae Barbatae £¨No specific dosage of the herbs. | oral administration | 1 months | RCT without BLIND | 60 months |
| Xu 1999 | Dried Radix Astragali, Radix Scrophulariae, Fructus Ligustri Lucidi, Umbellate Pore Fungus, Semen Coicis, Hebra Agrimoniae, Caulis Spathoobi, Herba Solani Lyrati, Herba Hedyotis Diffusae. No specific dosage of the herbs. | oral administration | 2 months | RCT without BLIND | No |
| Yang 2005 | Radix Ginseng Rubra, Radix Aconiti Praeparata. No specific dosage of the herbs. | intravenous drip | 10 days | RCT without BLIND | No |
| Zhang 1997 | Prepared Radix Rehmanniae 15g, Radix Paeoniae Alba 12g, Rhizoma Ligustici Chuanxiong 15g, Radix Angelicae Sinensis 12g, Radix Codonopsis Pilosulae 15g, Radix Astragali 20g | oral administration | 3 to 4 weeks | RCT without BLIND | 4 to 22 months |
| Zhang 2001 | Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 6 weeks | RCT without BLIND | No |
| Zhang 2004 | Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 3 weeks | RCT without BLIND | 6 to 24 months |
| Zhang 2005 | Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 3 weeks | RCT without BLIND | 6 to 24 months |
| Zhang 2005a | Capsule of Jinlong | oral administration | 6 weeks | RCT without BLIND | No |
| Zhang 2006 | Venenum Bufonis. No specific dosage of the herbs. | intravenous drip | 3 weeks | RCT without BLIND | No |
| Zhang 2008 | Shenlingbaizhusanjiawei (Radix Codonopsitis 20g, Astragalus Mongholicus 30g, Poria 15g, Rhizoma atractylodis macrocephala 15g, Semen Coicis 30g, Dolichos Lablab 10g, Radix Saposhnikoviae 15g, Semen Nelumbinis 10g, Fructus Amomi 9g, Halloysitum Rubrum 20g, Rhizoma Pinelliae 10g, Radix Platycodi 10g,Radix Glycyrrhizae 6g, Red Dates 5) | oral administration | 2 weeks | RCT without BLIND | No |
| Zhang 2009 | Aidi injecta (Radix Ginseng, Astragalus Mongholicus, Radix Acanthopanacis Senticosi, Chinese Cantharides). No specific dosage of the herbs. | intravenous drip | 9 weeks | RCT without BLIND | No |
| Zhang 2010 | Compound matrine injection (Sophora flavescens, Poria Alba). No specific dosage of the herbs. | intravenous drip | 3 weeks | RCT without BLIND | 3 years |
| Zhang 2010a | Kanglaite injecta (Semen Coicis). No specific dosage of the herbs. | intravenous drip | 8 weeks | RCT without BLIND | No |
| Zhang 2010b | Compound matrine injection (Sophora flavescens, Poria Alba). No specific dosage of the herbs. | intravenous drip | 6 to 8 weeks | RCT without BLIND | No |
| Zheng 1999 | Radix Astragali 30g, Radix Salviae Miltiorrhizae 20g, Largehead Atractylodes Rhizome 15g, Prepared Radix Rehmanniae 20g, Placenta Hominis 10g, Fructus Lycii 15g, Caulis Spathoobi 30g, Radix Morindae Officinalis 12g, Colla Plastri Testudinis and Colla Cornus Cervi 20g, Radix Polygoni Multiflori Preparata 20g, Colla Corii Asini 10g, Radix Astragali 30g, Radix Salviae Miltiorrhizae 20g, Largehead Atractylodes Rhizome 15g, Prepared Radix Rehmanniae 20g, Placenta Hominis 10g, Fructus Lycii 15g, Caulis Spathoobi 30g, Radix Morindae Officinalis 12g, Colla Plastri Testudinis and Colla Cornus Cervi 20g, Radix Polygoni Multiflori Preparata 20g, Colla Corii Asini 10g | oral administration | 3 to 4 weeks | RCT without BLIND | Not clear |
| Zhu 2005 | Semen Crotonis Pulveratum(º¬10 % oil), Bulbus Fritillariae, Radix Platycodi, Prepared Radix Glycyrrhizae, Eupolyphaga Seu Steleophaga, Rhizoma Zedoariae, Moschus, Dried Panax Notoginseng, Dried Semen Coicis, Radix Bupleuri, Radix Aucklandiae, Scorpio, Nidus Vespae, Parched Radix Paeoniae Alba, Radix Codonopsis Pilosulae. No specific dosage of the herbs. | oral administration | 4 weeks | RCT without BLIND | No |
| Zhu 2006 | Radix Codonopsis Pilosulae 15g, Largehead Atractylodes Rhizome 20g, Radix Astragali 30g, Semen Coicis 30g, Herba Solani Lyrati20g, Rhizoma Paridis 30g, Herba Hedyotis Diffusae 30g, fructus psoraleae 10g, Herba Salviae Chinensis 30g, PreparedRadix Glycyrrhizae 5g | oral administration | 2 months | RCT without BLIND | No |
2. Table of administration of Chinese medicinal herbs (TCMHs + medicine versus TCMHs).
| NUMBER OF TRIALS | HERBS IN REGIMEN | ROUTE OF ADMIN | PERIOD OF ADMIN | RCT AND BLIND METHOD | FOLLOW TIME |
| Chen 1997a | Decoction of Maimendo, Xiaoyao San, Shixiao San, Decoction of Lizhong ‐ No specific dosage of the herbs. | oral administration | 40 days | RCT without BLIND | Not clear |
| Liu 2002 | DriedPollen Typhae 10g, Faeces Trogopterorum 10g, Herba Solani Nigri 30g, Dried Leaf of Cycasrevoluta 30g, Radix Actinidiae Chinensis 30g, Hebra Agrimoniae 30g, Herba Taraxaci 30g, Rhizoma Corydalis 10g, Radix Paeoniae Rubra 10g, Semen Persicae 10g, Rhizoma PolygonatiOdorati 20g Hebra Chelidonii 20g Nodus Nelumbinis Rhizomatis 20g, Percarpiu Citri Reticulatae 10g, Rhizoma Pinelliae 10g, Radix Curcumae 10g, Sargassum 10g, Thallus Eckloniae 10g, Bulbus Fritillariae 10g, Poria 15g, Full Fructus Trichosanthis 30g, Dried Concha Ostreae 30g, Radix Glycyrrhizae 6g, Radix Codonopsis Pilosulae 20g, Largehead Atractylodes Rhizome 10g, Radix Aconiti Praeparata 10g, Semen Alpiniae Katsumadai 6g, Rhizoma Zingiberis 6g, Umbellate Pore Fungus 15g, fructus psoraleae 15g, Radix Astragali 30g, Radix Angelicae Sinensis 15g, Rhizoma Ligustici Chuanxiong 10g, Radix Paeoniae Alba 10g, Fructus Aurantii 10g, Prepared Radix Rehmanniae 10g, Cortex Cinnamomi 6g, cuscuta japonica 12g, Fructus Lycii 12g | oral administration | 8 to 12 weeks | RCT without BLIND | 4 to 22 months |
| Wang 1998 | Radix Codonopsis Pilosulae, Dried Radix Astragali, Dried Largehead Atractylodes Rhizome, Fructus Psoraleae, Herba Salviae Chinensis, Herba Hedyotis Diffusae, Rhizoma Paridis, Dried Semen Coicis ‐ No specific dosage of the herbs | oral administration | 2 to 3 months | RCT without BLIND | No |
| Xu 1989 | Radix Ginseng, Cuscuta Japonica, Fructus Psoraleae, Colla Corii Asini, Fructus Lycii, Radix Polygoni Multiflori Preparata, Largehead Atractylodes Rhizome, Fructus Ligustri Lucidi, Radix Paeoniae Alba, Radix Paeoniae Alba, Caulis Spatholobi, Massa Medicata Fermentata, Fructus Hordei Germinatus, Fructus Crataegi, Endothelium Corneum Gigeriae Galli, Fructus Aurantii Immaturus, Pericarpium Citri Reticulatae, Rhizoma Pinelliae, Caulis Bambusae In Taeniam, Radix Astragali, Herba Scutellariae Barbatae, Herba Hedyotis Diffusae ‐ No specific dosage of the herbs | oral administration | 4 to 6 weeks | RCT without BLIND | 60 months |
| You 2005 | Radix Codonopsis Pilosulae 540g, Umbellate Pore Fungus 540g, ParchedLargehead Atractylodes Rhizome 180g, Poria 180g, Folium Eriobotryae 180g, Prepared Rhizoma Pinelliae 108g, Semen Coicis 540g, Fructus Hordei Germinatus 180g, DriedRadix Glycyrrhizae 54g | oral administration | 8 weeks | RCT without BLIND | No |
| Zhao 2005 | Radix ginseng, Radix Ophiopogonis, Fructus Schisandrae Chinensis ‐ No specific dosage of the herbs | intravenous drip | 2 weeks | RCT without BLIND | No |
3. Table of administration of Chinese medicinal herbs (TCMHs versus TCMHs).
| NUMBER OF TRIALS | HERBS IN REGIMEN | ROUTE OF ADMIN | PERIOD OF ADMIN | RCT AND BLIND METHOD | FOLLOW TIME |
| Shao 1998 | Lysimachia Pentapetala Bunge, Ramulus Euonymi Alatae, Hirudo, Semen Coicis, Radix Sophorae Flavescentis, Dried Lacquer, Faeces Trogopterorum, Radix Curcumae, Alumen, Hebra Agrimoniae, Potassium Nitrate, Prepared Semen Strychni Pulveratum ‐ No specific dosage of the herbs. | oral administration | 3 months | RCT without Blind | Not clear |
| Shi 2004 | Venenum Bufonis ‐ No specific dosage of the herbs. | intravenous drip | 2 months | RCT without Blind | Not clear |
4. Table of administration of Chinese medicinal herbs (TCMHs versus medicine).
| NUMBER OF TRIALS | HERBS IN REGIMEN | ROUTE OF ADMIN | PERIOD OF ADMIN | RCT AND BLIND METHOD | FOLLOW TIME |
| Jiang 1994 | Radix Codonopsis Pilosulae 15g, Poria 15g, Rhizoma Atractylodis Macrocephalae 12g, Radix Astragali sue Hedysari 20g, Rhizoma Zedoariae 10g, Radix Salviae Miltiorrhizae 30g, Rhizoma Cyperi 12g, Rhizoma Pinellinae Praeparata 10g, Herba Scutellariae Barbatae 30g, Herba Hedyotis Diffusae 30g, Paris Polyphylla Smith 30g, Herba Salviae Chinensis 50g, Radix Glycyrrhizae 6g | oral administration | 2 months | RCT without BLIND | 1 to 5 years |
| Li 2001 | Arisaemacum Bile, Rhizoma Pinelliae, Percarpiu Citri Reticulatae, Fructus Aurantii Immaturus, Bulbus Fritillariae Cirrhosae, Semen Sinapis Albae, Scorpio, Endothelium Corneum Gigeriae Galli, Radix Glycyrrhizae ‐ No specific dosage of the herbs | oral administration | 6 months | RCT without BLIND | No |
| Ye 2009 | Shenqifuzheng injecta (Radix Codonopsitis, Astragalus Mongholicus). No specific dosage of the herbs | intravenous drip | 3 weeks | RCT without BLIND | No |
| Yang 2006 | Radix Codonopsis Pilosulae, Umbellate Pore Fungus, Largehead Atractylodes Rhizome, Rhizoma Dioscoreae, Semen Coicis, Rhizoma Pinelliae, Rhizoma Zingiberis, Endothelium Corneum Gigeriae Galli, Massa Fermentata Medicinalis, Fructus Crataegi, Fructus Hordei Germinatus, Semen Raphani, Prepared Radix Rehmanniae ‐ No specific dosage of the herbs | oral administration | 2 to 3 months | RCT without BLIND | 18 months |
| Yang 2010 | Shenqifuzheng injecta (Radix Codonopsitis, Astragalus Mongholicus). No specific dosage of the herbs | intravenous drip | 3 weeks | RCT without BLIND | No |
| You 2000 | Umbellate Pore Fungus 30g, Radix Codonopsis Pilosulae 10g, ParchedLargehead Atractylodes Rhizome 10g, Endothelium Corneum Gigeriae Galli 10g, ParchedFructus Oryzae Germinatus 15g, ParchedFructus Hordei Germinatus 15g, Caulis Perillae 10g, Radix Cynanchi Paniculati 15g, Fructus Aurantii Immaturus 10g, Hydrocotyle Sibthorpioides Lam 10g, Dried Radix Rehmanniae 15g, Prepared Radix Rehmanniae 15g, Radix Polygoni Multiflori Preparata 10g, Fructus Corni 10g, Cortex Moutan Radicis 10g, Rhizoma Alismatis 10g, Rhizoma Anemarrhenae 10g, Cortex Phellodendri 10g, Herba Epimedii 15g, Radix Astragali 30g, Rhizoma Polygonati 30g, Radix Ginseng 10g, Fructus Ligustri Lucidi 10g, Fructus Schisandrae Chinensis 10g, Fructus Gardeniae 10g, Radix Bupleuri 6g, Radix Angelicae Sinensis 6g, Radix Salviae Miltiorrhizae 30g, Radix Paeoniae Rubra 30g, Radix Paeoniae Alba 30g, Rhizoma Sparganii 15g, Rhizoma Curcumae 15g | oral administration | 4 months | RCT without BLIND | Not clear |
| Zhou 2000 | Herba Hedyotis Diffusae, Frucutus Xanthii, Herba Taraxaci ‐ No specific dosage of the herbs | intravenous drip | 2 months | RCT without BLIND | Not clear |
All the specific herbs used in the articles are listed in Tables 1 to 4 (Additional tables). None of the trials implemented blinding methods. We found no RCTs comparing a single herb with another single herb or herbal compounds. No placebo controlled trials were identified. We excluded another 99 articles because they did not meet our inclusion criteria. The reasons for exclusion, mainly because the selected patients did not have a TNM stage or the study was not a RCT, are listed under Characteristics of included studies.
Type I (TCMHs plus western therapeutic methods versus the same western therapeutic methods)
The 65 articles in type I included four kinds of injected TCMHs (a total of 23 trials for meta‐analysis) and reported random allocation of 5483 patients with advanced or late gastric cancer (ALGC) to TCMHs plus western therapeutic methods versus the same western therapeutic methods. Treatment was with non‐patented TCMHs in 24 trials and with patented TCMHs in 41 trials. The commonly used herbs were:
Huachansu, in seven trials (Chen 2009; Wang 2009a; Wang 2010b; Zhang 2001; Zhang 2004; Zhang 2005; Zhang 2006),
Injections of Fufangkushen, in seven trials (Fu 2011; Lin 2011; Liu 2009a; Wang 2010a; Xiong 2008; Zhang 2010; Zhang 2010b),
Injections of Aidi, in six trials (Chen 2008; Gong 2006; Jia 2003; Liu 2009; Wang 2009; Zhang 2009), and
Injections of Shenqifuzheng, in three trials (Jia 2009; Luo 2011; Wang 2010).
An emulsion of Lanxiangxi was used in five trials (Cao 1997; Deng 2001; Guan 2001; Tian 1999; Wu 2000a), and others in 17 trials.
In the 65 trials the western therapeutic interventions were:
regimen of MFV (mitomycin C 4 mg intravenously (iv) drop factor (gtt) once daily (qd) X 1 day + fluorouracilum 0.5 to 1.0 iv gtt qd X 1 to 5 days + vincristine sulphate 2 mg iv qd X 1 to 2 days per week X 4 to 6) as comparator in 7 trials,
regimen of ELF (etoposide 100 mg/m² iv gtt qd X 1 to 5 days + lencovorin 100 mg/m² iv gtt qd X 1 to 5 days + fluorouracilum 500 mg/m² iv gtt qd X 1 to 5 days per week X 2) as comparator in 18 trials,
regimen of FAM (fluorouracilum 100 mg/m² iv gtt qd X 1 to 5 days + adriamycinum 30 to 40 mg/m² iv qd X 1 day + mitomycin C 8 to 10 mg/m² iv qd X 1 day per week X 3 to 4) as comparator in 6 trials,
regimen of EAP (etoposide 100 mg/m² iv gtt qd X 4 to 6 days + adriamycinum 30 mg/m² iv qd X 1, 7 days + cisplatinum 40 mg/m² iv gtt qd X 2, 8 days per period X 2) as comparator in 3 trials,
regimen of OFL (oxaliplatin 70 mg/m² iv gtt day 1 + calcium folinate 400 mg/m² iv gtt day 1 + fluorouracilum 500 mg/m² iv gtt day 1 to 5 per week X 3 to 4) in 4 trials,
regimen of FOLFOX4 (oxaliplatin 100mg/m² iv gtt day 1 + calcium folinate 200 mg/m² iv gtt day 1 to 5 + fluorouracilum 500 mg/m² iv gtt day 1 to 5 per week X 6) in 6 trials,
regimen of TPF (paclitaxel 175 mg/m² iv gtt day 1 + cisplatinum 200 mg/m² iv gtt day 1 to 5 + fluorouracilum 600 mg/m² iv gtt day 1 to 5 per week X 6) in 3 trials, and
others in 19 trials.
The relevant contents are described in the 'Additional tables' (Table 5: administration of Chinese medical herbs (TCMHs + medicine versus medicine)).
In type I, seven trials (Chen 2009; Wang 2009a; Wang 2010b; Zhang 2001; Zhang 2004; Zhang 2005; Zhang 2006) used the same TCMH (Huachansu) with a similar dosage and therapeutic period. In the seven identified trials, the age ranged from 25 to 82 years in the intervention group and from 23 to 75 years in the control group; the number of cases varied from 20 to 43 in the intervention group and from 23 to 43 in the control group. All five trials except two (Wang 2010b; Zhang 2001), which only contained patients in stage IV, contained patients from both stages III and IV. The Huachansu was given by iv gtt 10 to 30 ml qd X 10 to 28 days in the intervention group during one therapeutic period, so the included participants, interventions, and outcomes were similar enough to allow combination of the data for meta‐analysis. The number of patients in each trial was too small to carry out subgroup analyses. In each trial, the same chemotherapeutic regimen and period were used for patients in the intervention group and the control group:
in the first trial (Chen 2009), the regimen was TPF (paclitaxel 175 mg/m² iv gtt day 1 + cisplatinum 200 mg/m² iv gtt day 1 to 5 + fluorouracilum 600 mg/m² iv gtt day 1 to 5 per week X 6);
in the second trial (Wang 2009a), the regimen was FOLFOX4 (oxaliplatin 85 mg/m² iv gtt day 1 + calcium folinate 100 mg/m² iv gtt day 1 to 2 + fluorouracilum 400 mg/m² iv day 1 to 2 + fluorouracilum 600 mg/m² iv gtt day 1 to 2 per week X 8);
in the third trial (Wang 2010b), the regimen was FOLFOX4 (oxaliplatin (85 to 100 mg/m² iv gtt day 1 + calcium folinate 200 mg/m² iv gtt day 1 to 2 + fluorouracilum 400 mg/m² iv day 1 to 2 + fluorouracilum 600 mg/m² iv gtt day 1 to 2 per week X 16);
in the fourth trial (Zhang 2001), the regimen was ELF (etoposide 100 mg/m² iv gtt qd X 1 to 5 days + lencovorin 100 mg/m² iv gtt qd X 1 to 5 days + fluorouracilum 500 mg/m² iv gtt qd X 1 to 5 days per week X 2);
in the fifth trial (Zhang 2004), the regimen was HLF (10‐hydroxycamptothecine 7 mg/m² iv gtt qd X 1 to 5 days + lencovorin 200 mg/m² iv gtt qd X 1 to 5 days + fluorouracilum 500 mg/m² iv gtt qd X 1 to 5 days per week X 3);
in the sixth trial (Zhang 2005), the regimen was FLO (oxaliplatin 130 mg/m² iv gtt qd X 1 days + lencovorin 200 mg/m² iv gtt qd X 1 to 3 days + fluorouracilum 500 mg/m² iv gtt qd X 1 to 3 days per week X 3); and
in the seventh trial (Zhang 2006), the regimen was HCPT (10‐hydroxycamptothecine 5 mg iv gtt qd X 1 to 5 days per week X 3).
None of the seven trials except one (Chen 2009) explained the specific method of randomisation (drew a lot) except to simply mention that randomisation was used.
In type I, six trials (Chen 2008; Gong 2006; Jia 2003; Liu 2009; Wang 2009; Zhang 2009) used the same TCMH (Aidi) with a similar dosage and therapeutic period. In the six identified trials, the age ranged from 30 to 78 years in the intervention group and from 35 to 85 years in the control group; the number of cases varied from 23 to 35 in the intervention group and from 22 to 34 in the control group. All six trials contained patients in both stage III and IV. The AIdi was given by iv gtt 50 ml qd X 10 to 42 (mostly 10 to 21) days in the intervention group during the therapeutic period, so the included participants, interventions, and outcomes were similar enough to allow the combination of data for meta‐analysis. The number of patients in each trial was too small to carry out subgroup analyses. In each trial, the same chemotherapeutic regimen and period were used for patients in the intervention group and the control group:
in the first trial (Chen 2008), the regimen was FOLFOX4 (oxaliplatin 85 mg/m² iv gtt day 1+ calcium folinate 200 mg/m² iv gtt day 1 to 2 + fluorouracilum 400 mg/m² iv gtt day 1 + fluorouracilum 600 mg/m² iv gtt day 2 per week X 6);
in the second trial (Gong 2006), the regimen was TCF (paclitaxel (Taxol) 135 mg/m² iv, day 1 + fluorouracilum 500 mg/m² iv (4 h) day 1 to 5 + calcium folinate 100 mg/m² iv day 1 to 5 + cisplatinum 30 mg/m² Iv gtt day 1 to 3 per week X 12);
in the third trial (Jia 2003), the regimen was CF (fluorouracilum 500 mg/m² iv gtt day 1 to 5 + cisplatinum 50 mg iv gtt day 1 to 3 per week X 6);
in the fourth trial (Liu 2009), the regimen was TPF (paclitaxel (Taxol) 175 mg/m² iv gtt day 1+ cisplatinum 20 mg/m² iv gtt day 1 to 5 + fluorouracilum 600 mg/m² iv gtt day 1 to 5 per week X 8);
in the fifth trial (Wang 2009), the regimen was FAM (fluorouracilum 0.5 iv day 1 + adriamycinum 20 mg iv day 1 + mitomycin C 20mg iv day 1 X 12 weeks);
in the sixth trial (Zhang 2009), the regimen was FOLFOX4 (L‐OXA 100 mg/m² iv gtt day 1+ LV 200 mg/m² iv gtt day 1 to 2 + 5‐FU 400 mg/m² iv day 1 to 2 + 5‐FU 600mg/m² ) iv day 1 to 2 per week X 9).
None of the six trials except one (Liu 2009) explained the specific method of randomisation (drew a lot) except to mention simply that randomisation was used.
In type I, seven trials (Fu 2011; Lin 2011; Liu 2009a; Wang 2010a; Xiong 2008; Zhang 2010; Zhang 2010b) used the same TCMH (Fufangkushen) with a similar dosage and therapeutic period. In the seven identified trials, the age ranged from 30 to 73 years in the intervention group and from 32 to 75 years in the control group; the number of cases varied from 25 to 48 in the intervention group and from 25 to 48 in the control group. All seven trials except Xiong 2008, which contained patients in stage IV, contained patients in both stage III and IV. The Fufangkushen was given by iv gtt 20 ml qd X 10 to 28 days in the intervention group during the therapeutic period, so the included participants, interventions, and outcomes were similar enough to allow combination of the data for meta‐analysis. The number of patients in each trial was too small to carry out subgroup analyses. In each trial, the same chemotherapeutic regimen and period were given to the patients in the intervention group and the control group:
in the first trial (Fu 2011), the regimen was FLO (oxaliplatin 85 mg/m² iv gtt day 1 + calcium folinate 200 mg/m² iv gtt day 1 + fluorouracilum 2600 mg/m² iv gtt day 1 per week X 4);
in the second trial (Lin 2011), the regimen was FDO (oxaliplatin 130 mg/m² iv gtt day 1 + docetaxe 75 mg/m² iv gtt day 1 + fluorouracilum 1500 mg/m² iv gtt day 1, 8 per week X 9);
in the third trial (Liu 2009a), the regimen was FOT (oxaliplatin 130 mg/m² iv gtt day 1 + calcium folinate 100 mg/m² iv gtt day 1 to 5 + tegafur 1000 mg/m² iv gtt day 1 to 5 per week X 6);
in the fourth trial (Wang 2010a), the regimen was DCF (docetaxel 30 mg/m² iv gtt day 1, 8 + cisplatinum 20 mg/m² iv gtt day 1 to 5 + fluorouracilum 750 mg/m² iv gtt day 1 to 5 per week X 6);
in the fifth trial (Xiong 2008), the regimen was TO (paclitaxel (Taxol) 130 mg/m² iv gtt day 1 + oxaliplatin 135 mg/m² iv gtt day 2 per week X 9);
in the sixth trial (Zhang 2010) the regimen was ECF (epirubicin 50 mg/m² iv gtt day 1 + cisplatinum 60 mg/m² iv gtt day 1 + fluorouracilum 600 mg/m² iv day 1 to 5 per week X 3);
in the seventh trial (Zhang 2010b), the regimen was FLP (cisplatinum 20 mg/m² iv gtt day 1 to 5 + calcium folinate 200 mg/m² iv gtt day 1 to 5 + fluorouracilum 500 mg/m² iv gtt day 1 to 5 per week X 6 to 8).
None of the seven trials except two trials (Wang 2010a; Zhang 2010) explained the specific method of randomisation (drew a lot and random number table, respectively) except to mention simply that randomisation was used.
In type I, three trials (Jia 2009; Luo 2011; Wang 2010), used the same TCMH (Shenqifuzheng) with a similar dosage and therapeutic period. In the three identified trials, the age ranged from 26 to 75 years in the intervention group and from 33 to 75 years in the control group; the number of cases varied from 22 to 32 in the intervention group and from 21 to 30 in the control group. All three trials except (Luo 2011), which only contained patients in stage IV, contained patients in both stage III and IV. The Shenqifuzheng was given by iv gtt 250 ml once daily (qd) X 10 to 31 days in the intervention group during the therapeutic period, so the included participants, interventions, and outcomes were similar enough to allow combination of the data for meta‐analysis. The number of patients in each trial was too small to carry out subgroup analysis. In each trial, the same chemotherapeutic regimen and period were used for patients in the intervention group and the control group:
in the first trial (Jia 2009), the regimen was FOLFOX4 (oxaliplatin 85 mg/m² iv gtt day 1+ calcium folinate 200 mg/m² iv gtt day 1 to 2 + fluorouracilum 600 mg/m² iv + fluorouracilum 400 mg/m² iv gtt day 1 to 2 per week X 8);
in the second trial (Luo 2011), the regimen was FLO (oxaliplatin 130 mg/m² iv gtt day 1+ calcium folinate 100 mg/m² iv gtt day 1 to 5 + fluorouracilum 200 mg/m² iv gtt day 1 to 5 per week X 6);
in the third trial (Wang 2010), the regimen was LF regimen (day 1 to 4, day 28 to 31, no specific dosage).
None of the three trials explained the specific method of randomisation except to simply mention that randomisation was used.
Type II (TCMHs plus western therapeutic methods versus the same TCMHs)
The six articles (Chen 1997a; Liu 2002; Wang 1998; Xu 1989; You 2005; Zhao 2005) in type II reported random allocation of 587 patients with advanced or late gastic cancer to TCMHs plus western therapeutic methods versus control (treatment with non‐patented TCMHs in five trials, the commonly used herbs listed). The relevant contents are described in the 'Additional tables' (Table 6: administration of Chinese medicinal Herbs (TCMHs plus medicine versus TCMHs)).
Type III (TCMHs versus other TCMHs)
The two articles (Shao 1998; Shi 2004) in type III reported random allocation of 194 patients with advanced or late gastric cancer to TCMHs versus control (treatment with non‐patented TCMHs in one trial, the commonly used herbs listed; treatment with patented TCMHs, compound oral fluid Zhenjian in one trial (Shao 1998)). The relevant contents are described in the 'Additional tables' (Table 7: administration of Chinese medicinal herbs (TCMHs versus other TCMHs)).
Type IV (TCMHs versus western therapeutic methods)
The seven articles (Jiang 1994; Li 2001; Yang 2006; Yang 2010; Ye 2009; You 2000; Zhou 2000) in type IV reported random allocation of 593 patients with advanced or late gastric cancer to TCMHs versus control (treatment with non‐patented TCMHs in four trials, the commonly used herbs). The relevant contents are described in the 'Additional tables' (Table 8: administration of Chinese medicinal herbs (TCMHs versus medicines)).
Sample sizes
None of the trials reported a sample size calculation. In type I, the sample sizes varied from 36 to 249 cases, with a mean value of 84. In type II, the sample sizes varied from 41 to 246 cases, with a mean value of 144. In type III, the sample sizes varied from 51 to 143 cases, with a mean value of 97. In type IV, the sample sizes varied from 60 to 176 cases, with a mean value of 85.
Setting
Both inpatients and outpatients were Included. In type I, only 33 trials included inpatients and no trial included only outpatients. Three trials included both inpatients and outpatients; the other 29 trials did not specify the status of the patients. In type II, four trials included inpatients and the other trials did not specify the status of the patients. In type III, one trial included inpatients and the other trial did not specify the status of the patients. In type IV, three trials included inpatients and the other trials did not specify the status of the patients. No special medicine was given to patients as primary care.
Participants
All the patients in the 80 trials were adults with gastric cancer in stage III or IV (that is T 1‐4, N 1‐3, M1). None of the trials reported the specific ratio of gender for all participants, and the general ratio of male to female was close to 2:1. In type I, the age of the participants varied from 22 to 85 years in 46 trials, another 19 trials did not specify the age range, and the mean age was 54 years. In type II, the age of the participants varied from 28 years to 72 years in six trials, and the mean age was 62 years. In type III, the age of the participants varied from 30 years to 79 years in two trials, and the mean age was 54 years. In type IV, the age of the participants varied from 22 years to 85 years in five trials, and the mean age was 62 years. The other two trials did not give data on the participants' age.
Interventions
The intervention (TCMHs) consisted of patented herbal medicine and self‐produced herbal compounds. The method of administration was by the oral route in 40 trials, and by intravenous administration in the other 40 trials. The period of administration varied from two weeks to one year, with a median period of one to three months. The specific dosage (range 10 g to 50 g) of the herbs and the regimens, which commonly consisted of seven to 15 herbs, are listed in Table 5; Table 6; Table 7; Table 8, but some authors did not specify the dosage of herbs because of commercial or technological secrecy. In the 80 trials, there were more than 200 categories of herbs used for treating gastric cancer in the advanced or late stage. The frequency (≥ 20%) of the most commonly used TCMHs in the 80 trials was: Radix Astragali seu Hedysari 50.0% (40/80), Rhizoma Atractylodis Macrocephalae 30.0% (24/80), Poria 30.0% (24/80), Radix Codonopsis Pilosulae 30.0% (24/80), Rhizoma Zedoariae 20.0% (16/80), Semen Coicis 20.0% (16/80).
Due to the lack of a reliable and recognised standards, it should be emphasised that the definition of TCMHs is non‐specific and the associated concepts are diverse.
Outcomes
The commonly reported outcomes were mortality, improvements in quality of life (QOL), rate of remission (short‐term and long‐term), median survival time (MST), time to progression (TTP), as well as adverse effects, such as life threatening and toxic responses, resulting in the discontinuation of treatment.
Excluded studies
Most of the excluded studies had no clear TNM stage and the illness of some patients was not in the late or advanced stage. A few of the studies were not related to TCMH or TCMH for gastric cancer, so they were excluded for this reason.
Risk of bias in included studies
The methodological quality of the 80 included studies was very poor (Figure 1). Other than mentioning that the studies were randomised, none of the trials gave any information that would allow a formal assessment of quality. Most of the articles described the method of randomisation that was used, but none of the trials described double blinding or the methods used for blinding. Only five trials provided a description of withdrawals or dropouts (Gao 2008; Xie 2006; Xu 1989; Xu 1999; Zhang 1997). None of the studies mentioned allocation concealment. In China, the results of clinical trials are given more importance than the methodology, especially if the methodology is known within the academic circle; so the methodology is not described in detail. We contacted the authors of the included studies by letter to request further data, but we received no response.
1.
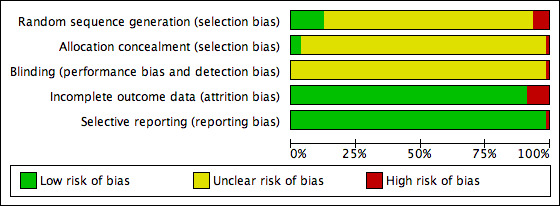
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
1.1 A total of 448 patients were enrolled in the seven trials of Huachansu for gastric cancer: 226 were randomised to the TCMH Huachansu and 222 to the control intervention. After three to four periods of treatment, 113 of 226 patients responded clinically to the Huachansu compared to 89 of 222 who responded to the control intervention. The pooled odds ratio (OR) for clinical complete and partial remission was 1.48 (95% CI 1.01 to 2.17; P = 0.05) using a fixed‐effect model and the result was the same using a random‐effects model. Toxic and side effects on the digestive system were noted in 78/196 patients in the intervention group compared to 113/192 in the control group. The pooled OR of toxic and side effects on the digestive system was 0.43 (95% CI 0.28 to 0.66; P = 0.0001) using a fixed‐effect model and 0.43 (95% CI 0.22 to 0.84; P = 0.01) using a random‐effects model. The toxic and side effects of leukopenia were noted in 67/196 patients in the intervention group compared to 112/192 in the control group. The pooled OR for toxic and side effects of leukopenia was 0.32 (95% CI 0.21 to 0.50; P < 0.00001) using a fixed‐effect model and 0.32 (95% CI 0.21 to 0.51; P < 0.00001) using a random‐effects model.
Sensitivity analyses showed that the result for toxic and side effects on the digestive system was sensitive to change with the random‐effects model (risk ratio (RR) 0.82, 95% CI 0.67 to 0.99; P = 0.04) and when the selected patients were in IV stage, the trials included patients with median age > 50 years (RR 0.69, 95% CI 0.49 to 0.97; P = 0.03) using a fixed‐effect model, the trials with samples > 60 cases (RR 0.81, 95% CI 0.66 to 0.98; P = 0.03), and the intervention with dosage of Huachansu equal to 20 ml iv gtt qd (RR 0.67, 95% CI 0.53 to 0.84; P = 0.0007) using a random‐effects model. Sensitivity analyses showed that the toxic and side effects of leukopenia were sensitive to change with the fixed‐effect model (RR 0.62, 95% CI 0.45 to 0.86; P = 0.004) when the selected patients were in stage IV, the trials included patients with median age > 50 years (RR 0.50, 95% CI 0.37 to 0.69; P = 0.0001 using a fixed‐effect model; RR 0.52, 95% CI 0.39 to 0.71; P = 0.0001 using a random‐effects model), the trials with samples > 60 cases (RR 0.63, 95% CI 0.50 to 0.78; P = 0.001 using a fixed‐effect model; RR 0.64, 95% CI 0.49 to 0.83; P = 0.0009 using a random‐effects model), and the intervention with dosage of Huachansu equal to 20 ml iv gtt qd (RR 0.67, 95% CI 0.53 to 0.84; P = 0.0005 using a fixed‐effect model; RR 0.67, 95% CI 0.50 to 0.89; P = 0.0005 using a random‐effects model) (Table 9). However, due to lack of specific data (Table 9), it was not possible to analyse changes in the inclusion criteria. Because only the general results were presented in the trials, the results for patients in stage III and stage IV were not available separately.
5. Summary table of sensitivity analyses for Huachansu.
| Fixed effect model of RR (M‐H, Fixed, (95% CI) | Random effects model of RR (M‐H, Random, (95% CI) | Only for patients in IV stage, RR (M‐H, Random, Fixed, 95% CI) | Only for trials with patients in IV stage, RR (M‐H, Random, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Fixed, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Random, 95% CI) | Only for trials with samples >60, RR (M‐H, Fixed, 95% CI) | Only for trials with samples >60, RR (M‐H, Random, 95% CI) | Only for trials with dosage of injection Huachansu = 20ml IV gtt Qd, RR (M‐H, Fixed, 95% CI) | Only for trials with dosage of injection Huachansu = 20ml IV gtt Qd, RR (M‐H, Random, 95% CI) | |
| Rate of complete remission and partly remission | 1.48 [1.01 to 2.13] | 1.48 [1.01 to 2.13] | 1.15[0.83 to 1.58] | 1.15[0.83 to 1.58] | 1.28[1.01 to1.62] | 1.29 [1.02 to1.62] | 1.23 [0.99 to 1.54] | 1.25 [1.01 to 1.56] | 1.24 [0.98 to 1.57] | 1.26 [1.00 to 1.59] |
| Toxic and side effects in digestive system | 0.43 [0.28 to 0.66] | 0.43 [0.22 to 0.84] | 0.84 [0.68 to 1.05] | 0.82 [0.67 to 0.99] | 0.69 [0.49 to 0.97] | 0.72 [0.40 to 1.30] | 0.62 [0.50 to 0.77] | 0.59 [0.35 to 0.98] | 0.67 [0.53 to 0.84] | 0.62 [0.32 to 1.20] |
| Toxic and side effects of leukopenia | 0.32 [0.21 to 0.50] | 0.32 [0.21 to 0.51] | 0.62 [0.45 to 0.86] | 0.52 [0.24 to 1.12] | 0.50 [0.37 to 0.69] | 0.52 [0.39 to 0.71] | 0.63 [0.50 to 0.78] | 0.64 [0.49 to 0.83] | 0.67 [0.53 to 0.84] | 0.67 [0.50 to 0.89] |
1.2. The authors of the seven trials did not report on side effects. Four trials (Chen 2009; Wang 2010b; Zhang 2004; Zhang 2005) followed up the patients for 0.5 to two years. Except for Chen 2009, the other trials concluded that there was no statistical difference in life expectancy (MST) between the intervention group and the control group. Zhang 2006 concluded that the patients in the intervention group had improved their QOL and alleviated the cancerous ache following treatment in the short term compared with patients in the control group. Other measures, such as mortality and TTP, were not provided in full so could not be analysed.
2.1. A total of 287 patients were enrolled in the six trials of Aidi for gastric cancer: 145 were randomised to the TCMH Aidi and 142 to the control intervention. After three to four periods of treatment, 76 of 145 patients responded clinically to Aidi compared to 60 of 142 who responded to the control intervention. The pooled OR for clinical complete and partial remission was 1.51 (95% CI 0.94 to 2.41; P = 0.09) using a fixed‐effect model and 1.50 (95% CI 0.93 to 2.42; P = 0.09) using a random‐effects model. The toxic and side effects on the digestive system were noted in 62/180 patients in the intervention group compared to 100/174 in the control group. The pooled OR of toxic and side effects on the digestive system was 0.33 (95% CI 0.20 to 0.54; P < 0.00001) using a fixed‐effect model and 0.33 (95% CI 0.20 to 0.55; P < 0.00001) using a random‐effects model. The toxic and side effects of leukopenia were noted in 62/122 patients in the intervention group compared to 81/120 in the control group. The pooled OR for toxic and side effects of leukopenia was 0.43 (95% CI 0.23 to 0.80; P = 0.008) using a fixed‐effect model and the same result using a random‐effects model.
Sensitivity analyses could not be done for toxic and side effects on the digestive system of patients in stage IV, because no trials only included that kind of patients, but showed that the result for toxic and side effects on the digestive system was sensitive to the change with a random‐effects model (RR 0.34, 95% CI 0.20 to 0.58; P < 0.0001) and fixed‐effect model (RR 0.33, 95% CI 0.20 to 0.57; P < 0.0001) when the included patients' median age was > 50 years, the trials with samples > 60 cases (RR 0.29, 95% CI 0.15 to 0.57; P = 0.0003) using a random‐effects model and the same results using a fixed‐effect model, and the intervention with dosage of Aidi equal to 50 ml iv gtt qd (RR 0.37, 95% CI 0.20 to 0.66; P = 0.0008 using a random‐effects model; RR 0.36, 95% CI 0.20 to 0.65; P = 0.0006 using a fixed‐effect model). Sensitivity analyses could not give results for toxic and side effects of leukopenia in stage IV because no trials only included that kind of patient, but showed that the result for toxic and side effects of leukopenia was sensitive to change with a random effects model (RR 0.46, 95% CI 0.22 to 0.97; P = 0.04) and fixed‐effect model (RR 0.46, 95% CI 0.22 to 0.96; P = 0.04) when the included patients' median age was > 50 years, for the trials with samples > 60 cases (RR 0.37, 95% CI 0.17 to 0.83; P = 0.02 using a random‐effects model; and the same result using a fixed‐effect model), and the intervention with dosage of Aidi equal to 50 ml IV gtt qd (RR 0.46, 95% CI 0.22 to 0.97; P = 0.04 using a random‐effects model; and the same result using a fixed‐effect model) (Table 10). However, due to lack of specific data (Table 10), it was not possible to analyse changes in the inclusion criteria. Because only the general results were presented in the trials, the results for patients in stage III stage and stage IV were not available separately.
6. Summary table of sensitivity analyses for Aidi.
| Fixed effect model of RR (M‐H, Fixed, (95% CI) | Random effects model of RR (M‐H, Random, (95% CI) | Only for patients in IV stage, RR (M‐H, Random, Fixed, 95% CI) | Only for trials with patients in IV stage, RR (M‐H, Random, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Fixed, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Random, 95% CI) | Only for trials with samples >60, RR (M‐H, Fixed, 95% CI) | Only for trials with samples >60, RR (M‐H, Random, 95% CI) | Only for trials with dosage of injection Aidi = 50ml IV gtt Qd, RR (M‐H, Fixed, 95% CI) | Only for trials with dosage of injection Aidi = 50ml IV gtt Qd, RR (M‐H, Random, 95% CI) | |
| Rate of complete remission and partly remission | 1.51[0.94 to 2.41] | 1.50[0.93 to 2.42] | No specific data just for patients in IV stage | No specific data just for patients in IV stage | 1.69[0.99 to 2.87] | 1.68[0.98 to 2.88] | 1.23[0.61 to 2.48] | 1.23[0.61 to 2.49] | 1.88[1.03 to 3.42] | 1.88[1.03 to 3.43] |
| Toxic and side effects in digestive system | 0.33[0.20 to 0.54] | 0.33[0.20 to 0.55] | No specific data just for patients in IV stage | No specific data just for patients in IV stage | 0.33[0.20 to 0.57] | 0.34[0.20 to 0.58] | 0.29[0.15 to 0.57] | 0.29[0.15 to 0.57] | 0.36[0.20 to 0.65] | 0.37[0.20 to 0.66] |
| Toxic and side effects of leukopenia | 0.43[0.23 to 0.80] | 0.43[0.23 to 0.80] | No specific data just for patients in IV stage | No specific data just for patients in IV stage | 0.46[0.22 to 0.96] | 0.46[0.22 to 0.97] | 0.37[0.17 to 0.83] | 0.37[0.17 to 0.83] | 0.46[0.22 to 0.96] | 0.46[0.22 to 0.97] |
2.2. The authors of the six trials did not report on side effects. Only one trial (Gong 2006) followed up the patients for 42 months, and the author concluded that there was no statistical difference in life expectancy (MST) between the intervention group and control group. Chen 2008 and Zhang 2009 concluded that the patients in the intervention group had improved their QOL and alleviated the cancerous ache following treatment in the short term compared with patients in the control group. Other measures, such as mortality and TTP, were not provided and could not be analysed.
3.1. A total of 503 patients were enrolled in the seven trials of Fufangkushen for gastric cancer: 254 were randomised to the TCMH Fufangkushen and 249 to the control intervention. After three to four periods of treatment, 110 of 214 patients responded clinically to Fufangkushen compared to 97 of 209 who responded to the control intervention. The pooled OR for clinical complete and partial remission was 1.22 (95% CI 0.83 to 1.79; P = 0.31) using a fixed‐effect model and the result was the same using a random‐effects model. The toxic and side effects on the digestive system were noted in 63/153 patients in the intervention group compared to 91/149 in the control group. The pooled OR for toxic and side effects on the digestive system was 0.42 (95% CI 0.26 to 0.69; P = 0.0005) using a fixed‐effect model and 0.43 (95% CI 0.26 to 0.69; P = 0.0005) using a random‐effects model. The toxic and side effects of leukopenia were noted in 128/254 patients in the intervention group compared to 174/249 treated in the control group. The pooled OR for toxic and side effects of leukopenia was 0.37 (95% CI 0.25 to 0.56; P < 0.0001) using a fixed‐effect model and the same results using a random‐effects model.
Sensitivity analyses showed that the result for toxic and side effects on the digestive system was sensitive to change from a fixed‐effect model to random‐effects model (RR 0.35, 95% CI 0.12 to 0.98; P = 0.05) when the selected patients were in IV stage, for the trials with samples > 60 cases (RR 0.42, 95% CI 0.25 to 0.71; P = 0.001), and the intervention with a dosage of Fufangkushen equal to 20 ml iv gtt qd (day 10 to 14) (RR 0.42, 95% CI 0.25 to 0.71; P = 0.001). Sensitivity analyses showed that the result for toxic and side effects of leukopenia was sensitive to change from fixed‐effect model to random‐effects model (RR 0.36, 95% CI 0.13 to 0.99; P = 0.05) when the selected patients were in IV stage, for the trials with samples > 60 cases (RR 0.37, 95% CI 0.23 to 0.59; P < 0.001), and the intervention with a dosage of Fufangkushen equal to 20 ml (day 10 to 14) iv gtt qd (RR 0.36, 95% CI 0.24 to 0.56; P < 0.0001) (Table 11). However, due to lack of specific data (Table 11), it was not possible to analyse changes in the inclusion criteria. Because only the general results were presented in the trials, the results for patients in stage III and stage IV were not available separately.
7. Summary table of sensitivity analyses for Fufangkushen.
| Fixed effect model of RR (M‐H, Fixed, (95% CI) | Random effects model of RR (M‐H, Random, (95% CI) | Only for patients in IV stage, RR (M‐H, Random, Fixed, 95% CI) | Only for trials with patients in IV stage, RR (M‐H, Random, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Fixed, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Random, 95% CI) | Only for trials with samples >60, RR (M‐H, Fixed, 95% CI) | Only for trials with samples >60, RR (M‐H, Random, 95% CI) | Only for trials with dosage of injection Fufangkushen = 20ml IV gtt Qd, RR (M‐H, Fixed, 95% CI) | Only for trials with dosage of injection Fufangkeshen = 20ml IV gtt Qd, RR (M‐H, Random, 95% CI) | |
| Rate of complete remission and partly remission | 1.22[0.83 to 1.79] | 1.22[0.83 to 1.79] | 1.06[0.42 to 2.68] | 1.06[0.42 to 2.68] | 1.22[0.83 to 1.79] | 1.22[0.83 to 1.79] | 1.16[0.75 to 1.81] | 1.16[0.75 to 1.81] | 1.30[0.86 to 1.97] | 1.30[0.86 to 1.97] |
| Toxic and side effects in digestive system | 0.42[0.26 to 0.69] | 0.43[0.26 to 0.69] | 0.35[0.12 to 0.98] | 0.35[0.12 to 0.98] | 0.42[0.26 to 0.69] | 0.43[0.26 to 0.69] | 0.42[0.25 to 0.71] | 0.42[0.25 to 0.71] | 0.42[0.25 to 0.71] | 0.42[0.25 to 0.71] |
| Toxic and side effects of leukopenia | 0.37[0.25 to 0.56] | 0.37[0.25 to 0.56] | 0.36[0.13 to 0.99] | 0.36[0.13 to 0.99] | 0.37[0.25 to 0.56] | 0.37[0.25 to 0.56] | 0.37[0.23 to 0.59] | 0.37[0.23 to 0.59] | 0.36[0.24 to 0.56] | 0.36[0.24 to 0.56] |
3.2. The authors of the seven trials did not report on side effects. Only one trial (Zhang 2010) followed up the patients for 0.5 to two years, and they concluded that there was no statistical difference in life expectancy (MST) between the intervention group and control group. Lin 2011 and Zhang 2006 concluded that the patients in the intervention group had improved their QOL and alleviated the cancerous ache following treatment in the short term compared with patients in the control group. Other measures such as mortality and TTP were not provided and could not be analysed.
4.1. A total of 153 patients were enrolled in the three trials of Shenqifuzheng for gastric cancer: 78 were randomised to the TCMH Shenqifuzheng and 75 to the control intervention. After three to four periods of treatment, 46 of 78 patients responded clinically to Shenqifuzheng compared to 37 of 75 who responded to the control intervention. The pooled OR for clinical complete and partial remission was 1.48 (95% CI 0.78 to 2.81; P = 0.23) using a fixed‐effect model and the result was the same using a random‐effects model. The toxic and side effects on the digestive system were noted in 30/78 patients in the intervention group compared to 31/75 in the control group. The pooled OR for toxic and side effects on the digestive system was 0.90 (95% CI 0.48 to 1.67; P = 0.74) using a fixed‐effect model and the same using a random‐effects model. The toxic and side effects of leukopenia were noted in 20/78 patients in the intervention group compared to 35/75 in the control group. The pooled OR for toxic and side effects of leukopenia was 0.37 (95% CI 0.18 to 0.74; P = 0.005) using a fixed‐effect model and the same using a random‐effects model.
Sensitivity analyses showed that the result for toxic and side effects on the digestive system was sensitive to the change in the fixed‐effect model and random‐effects model (RR 11.40, 95% CI 2.12 to 61.25; P = 0.005) when the selected patients were in IV stage, for the trials with included patients' median age > 50 years (RR 1.63, 95% CI 0.72 to 3.69; P = 0.24 using a fixed‐effect model; and RR 2.26, 95% CI 0.11 to 48.60; P = 0.60 using a random‐effects model), the trials with samples > 60 cases (RR 0.34, 95% CI 0.12 to 0.98; P = 0.05 using a fixed‐effect model and a random‐effects model), and the intervention with dosage of Shenqifuzheng equal to 250 ml (day 10 to 14) iv gtt qd (RR 1.63, 95% CI 0.72 to 3.69; P = 0.24 using a fixed‐effect model; and RR 2.26, 95% CI 0.11 to 48.60; P = 0.60 using a random‐effects model). Sensitivity analyses showed that the result for toxic and side effects of leukopenia was sensitive to change in the fixed‐effect model and random‐effects model (RR 0.32, 95% CI 0.07 to 1.44; P = 0.14) when the selected patients were in IV stage, for the trials with included patients' median age > 50 years (RR 0.38, 95% CI 0.14 to 1.02; P = 0.05), the trials with samples > 60 cases (RR 0.35, 95% CI 0.12 to 0.97; P = 0.04), and the intervention with dosage of Shenqifuzheng equal to 250 ml (day 10 to 14) iv gtt qd (RR 0.38, 95% CI 0.14 to 1.02; P = 0.05) (Table 12). However, due to lack of specific data (Table 12), it was not possible to analyse changes in the inclusion criteria. Because only the general results were presented in the trials, the results for patients in stage III stage and stage IV were not available separately.
8. Summary table of sensitivity analyses for Shenqifuzheng.
| Fixed effect model of RR (M‐H, Fixed, (95% CI) | Random effects model of RR (M‐H, Random, (95% CI) | Only for patients in IV stage, RR (M‐H, Random, Fixed, 95% CI) | Only for trials with patients in IV stage, RR (M‐H, Random, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Fixed, 95% CI) | Only for trials with patients' median age>50 years old, RR (M‐H, Random, 95% CI) | Only for trials with samples >60, RR (M‐H, Fixed, 95% CI) | Only for trials with samples >60, RR (M‐H, Random, 95% CI) | Only for trials with dosage of injection Shenqifuzheng = 250ml IV gtt Qd, RR (M‐H, Fixed, 95% CI) | Only for trials with dosage of injection Shenqifuzheng = 250ml IV gtt Qd, RR (M‐H, Random, 95% CI) | |
| Rate of complete remission and partly remission | 1.48[0.78 to 2.81] | 1.48[0.78 to 2.81] | 1.31[0.39 to 4.39] | 1.31[0.39 to 4.39] | 1.49[0.65 to 3.42] | 1.49[0.65 to 3.42] | 1.47[0.54 to 4.00] | 1.47[0.54 to 4.00] | 1.49[0.65 to 3.42] | 1.49[0.65 to 3.42] |
| Toxic and side effects in digestive system | 0.90[0.48 to 1.67] | 1.13[0.18 to 7.24] | 11.40[2.12 to 61.25] | 11.40[2.12 to 61.25] | 1.63[0.72 to 3.69] | 2.26[0.11 to 48.60] | 0.34[0.12 to 0.98] | 0.34[0.12 to 0.98] | 1.63[0.72 to 3.69] | 2.26[0.11 to 48.60] |
| Toxic and side effects of leukopenia | 0.37[0.18 to 0.74] | 0.37[0.18 to 0.74] | 0.12[0.07 to 1.44] | 0.12[0.07 to 1.44] | 0.38[0.14 to 1.02] | 0.38[0.14 to 1.02] | 0.35[0.14 to 0.97] | 0.35[0.14 to 0.97] | 0.38[0.14 to 1.02] | 0.38[0.14 to 1.02] |
4.2. The authors of the three trials did not report on side effects. No trials followed up the patients, and no trials concluded that there was a statistical difference in life expectancy (MST) between the intervention group and control group. Two trials (Luo 2011; Wang 2010) concluded that the patients in the intervention group had improved their QOL and alleviated the cancerous ache following treatment in the short term compared with patients in the control group. Other measures such as mortality and TTP were not provided and could not be analysed.
In the other 57 trials, the different intervention measures prevented pooling of data in a meta‐analysis, and subgroup analysis on the herbs could not be performed. There were four types of clinical trials included: formulas of TCMHs with western medicine versus the same western medicine in the intervention group (type I group, 42 trials), western medicine with formulas of TCMHs versus the same formulas of TCMHs (type II group, six trials), formulas of TCMHs versus another formula of TCMHs (type III group, two trials), formulas of TCMHs versus western medicine (type IV group, seven trials).
Type I (TCMHs with western medicine versus the same western medicine)
Mortality
In the 42 trials, which could not be pooled for meta‐analysis, three trials showed a significant difference in mortality at one year between the intervention group and the control group, and the comparison result was the following:13 patients had died in the intervention group (30 patients) and 20 patients in the control group (28 patients) (Chen 1997; Analysis 5.1) (RR 0.61, 95% CI 0.38 to 0.97); 10 patients had died in the intervention group (77 patients) and 26 patients in the control group (46 patients) (Sun 1999; Analysis 5.4) (RR 0.23, 95% CI 0.12 to 0.43); 67 patients had died in the intervention group (90 patients) and 20 patients in the control group (40 patients) (Wang 2002; Analysis 5.5) (RR 1.49, 95% CI 1.07 to 2.08).
5.1. Analysis.
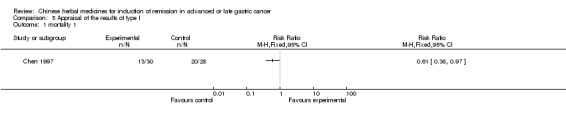
Comparison 5 Appraisal of the results of type I, Outcome 1 mortality 1.
5.4. Analysis.
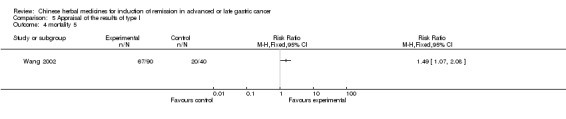
Comparison 5 Appraisal of the results of type I, Outcome 4 mortality 5.
5.5. Analysis.
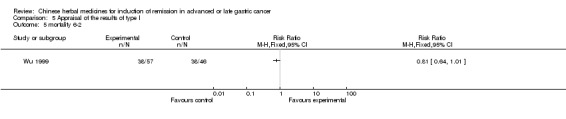
Comparison 5 Appraisal of the results of type I, Outcome 5 mortality 6‐2.
Three trials showed a significant difference in mortality at two years between the intervention group and the control group, and the comparison result was the following:13 patients had died in the intervention group (57 patients) and 20 patients in the control group (46 patients) (Wu 1999; Analysis 5.6) (RR 0.52, 95% CI 0.29 to 0.94). There was no significant difference in mortality at three years and five years, the comparison result was the following, respectively: 38 patients had died in the intervention group (57 patients) and 38 patients in the control group (46 patients) (Wu 1999; Analysis 5.7) (RR 0.81, 95% CI 0.64 to 1.01); 50 patients had died in the intervention group (57 patients) and 43 patients in the control group (46 patients) (Wu 1999; Analysis 5.8) (RR 0.94, 95% CI 0.83 to 1.07).
5.6. Analysis.
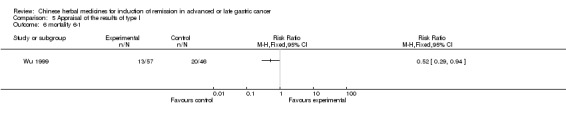
Comparison 5 Appraisal of the results of type I, Outcome 6 mortality 6‐1.
5.7. Analysis.
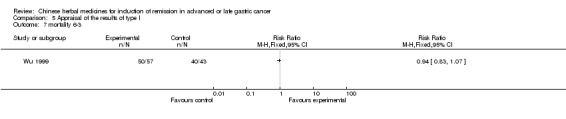
Comparison 5 Appraisal of the results of type I, Outcome 7 mortality 6‐3.
5.8. Analysis.
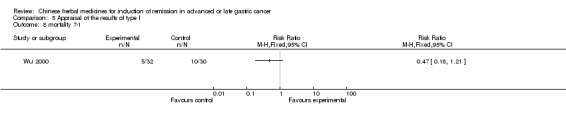
Comparison 5 Appraisal of the results of type I, Outcome 8 mortality 7‐1.
One trial showed no significant difference in mortality at one year between the intervention group and the control group, and the comparison result was the following: 26 patients had died in the intervention group (50 patients) and 23 patients in the control group (40 patients) (Guo 1989; Analysis 5.3) (RR 0.90, 95% CI 0.62 to 1.32).
5.3. Analysis.
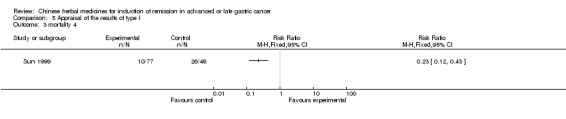
Comparison 5 Appraisal of the results of type I, Outcome 3 mortality 4.
Three trials showed no significant difference in mortality at six months between the intervention group and the control group, and the comparison result was the following: five patients had died in the intervention group (32 patients) and 10 patients in the control group (30 patients) (Wu 2000; Analysis 5.9) (RR 0.47, 95% CI 0.18 to 1.21). There was a significant difference in mortality at one year and two years, the comparison results were the following, respectively: 10 patients had died in the intervention group (32 patients) and 21 patients in the control group (30 patients) (Wu 2000; Analysis 5.10) (RR 0.45, 95% CI 0.25 to 0.79); 21 patients had died in the intervention group (32 patients) and 27 patients in the control group (30 patients) (Wu 2000; Analysis 5.11) (RR 0.73, 95% CI 0.55 to 0.96).
5.9. Analysis.
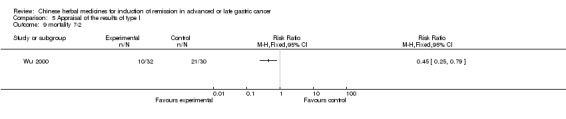
Comparison 5 Appraisal of the results of type I, Outcome 9 mortality 7‐2.
5.10. Analysis.
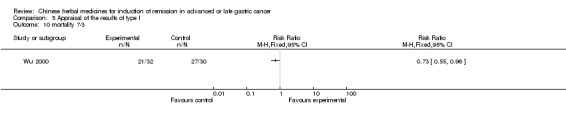
Comparison 5 Appraisal of the results of type I, Outcome 10 mortality 7‐3.
5.11. Analysis.
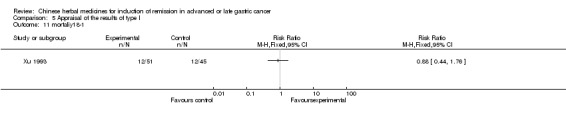
Comparison 5 Appraisal of the results of type I, Outcome 11 mortaliyt 8‐1.
Another trial showed no significant difference in mortality at six months and one year between the intervention group and the control group, and the comparison result was the following, respectively: 12 patients had died in the intervention group (51 patients) and 12 patients in the control group (45 patients) (Xu 1993; Analysis 5.12) (RR 0.88, 95% CI 0.44 to 1.76); 19 patients had died in the intervention group (51 patients) and 22 patients in the control group (45 patients) (Xu 1993; Analysis 5.13) (RR 0.76, 95% CI 0.48 to 1.21). There was a significant difference in mortality at two years, the comparison result was the following: 25 patients had died in the intervention group (51 patients) and 32 patients in the control group (45 patients) (Xu 1993; Analysis 5.14) (RR 0.69, 95% CI 0.49 to 0.96).
5.12. Analysis.
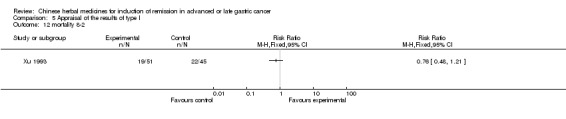
Comparison 5 Appraisal of the results of type I, Outcome 12 mortality 8‐2.
5.13. Analysis.
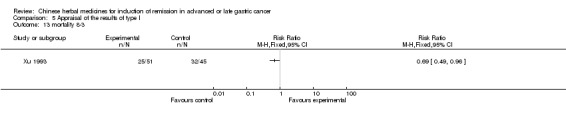
Comparison 5 Appraisal of the results of type I, Outcome 13 mortality 8‐3.
5.14. Analysis.
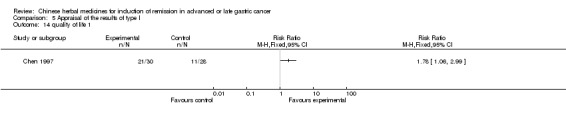
Comparison 5 Appraisal of the results of type I, Outcome 14 quality of life 1.
Quality of life (QOL) (< six months)
Of the 42 individual trials, 11 trials showed a significant difference in Karnofsky score (> 60 points) between the intervention group and the control group after the trial, and the comparison result was the following: 21 patients kept their score > 60 points in the intervention group (30 patients) compared with 11 patients in the control group (28 patients) at two months (Chen 1997; Analysis 5.15) (RR 1.78, 95% CI 1.06 to 2.99). Twenty‐seven patients kept their score > 60 points in the intervention group (31 patients) compared with 16 patients in the control group (28 patients) at one month (Huang 2002; Analysis 5.19) (RR 1.52, 95% CI 1.08 to 2.16). Nineteen patients had increased their score (an increase > 10 points) in the intervention group (35 patients) compared with 7 patients in the control group (33 patients) at six weeks (Li 2002; Analysis 5.20) (RR 2.56, 95% CI 1.24 to 5.28). Twelve patients had increased their score (an increase > 10 points) in the intervention group (38 patients) compared with three patients in the control group (36 patients) at nine weeks (Liu 2006; Analysis 5.21) (RR 3.79, 95% CI 1.16 to 12.33). Twenty‐seven patients had increased their score (an increase > 10 points) in the intervention group (48 patients) compared with seven patients in the control group (34 patients) at nine weeks (Lv 1999; Analysis 5.23) (RR 2.73, 95% CI 1.35 to 5.53). Twenty‐five patients had increased their score (an increase > 10 points) in the intervention group (31 patients) compared with 10 patients in the control group at three months (31 patients) (Si 2004; Analysis 5.24) (RR 2.50, 95% CI 1.46 to 4.28). Twenty‐eight patients had increased their score (an increase > 10 points) in the intervention group (38 patients) compared with 13 patients in the control group (30 patients) at three months (Wang 2004; Analysis 5.27) (RR 1.70, 95% CI 1.08 to 2.67). Twenty‐four patients had increased their score (an increase > 10 points) in the intervention group (32 patients) compared with 18 patients in the control group (36 patients) at six to eight weeks (Wu 2000a; Analysis 5.29) (RR 1.50, 95% CI 1.02 to 2.20). Twenty‐four patients had increased their score (an increase > 10 points) in the intervention group (30 patients) compared with 16 patients in the control group (30 patients) four weeks after the therapeutic period (Zhu 2005; Analysis 5.32) (RR 1.50, 95% CI 1.03 to 2.19). The Karnofsky score was 83.33 ± 6.18 (30 patients) in the intervention group and 77.94 ± 6.14 months (30 patients) in the control group at six to nine weeks (Hu 2011) (WMD 5.39, 95% CI 2.27 to 8.51); the Karnofsky score was 85.26 ± 4.21 (36 patients) in the intervention group and 71.19 ± 4.38 (36 patients) in the control group at 15 days (Zhang 2008) (WMD 14.07, 95% CI 12.09 to 16.05).
5.15. Analysis.
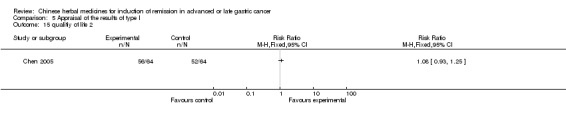
Comparison 5 Appraisal of the results of type I, Outcome 15 qualitiy of life 2.
5.19. Analysis.
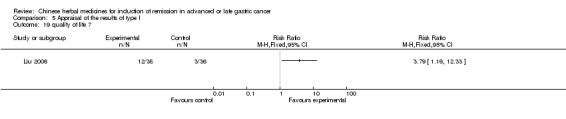
Comparison 5 Appraisal of the results of type I, Outcome 19 quality of life 7.
5.20. Analysis.
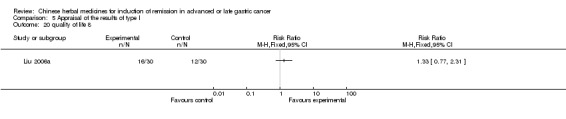
Comparison 5 Appraisal of the results of type I, Outcome 20 quality of life 8.
5.21. Analysis.
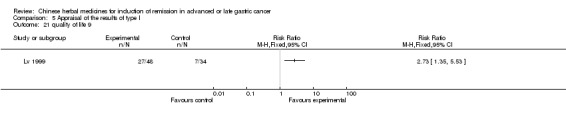
Comparison 5 Appraisal of the results of type I, Outcome 21 quality of life 9.
5.23. Analysis.
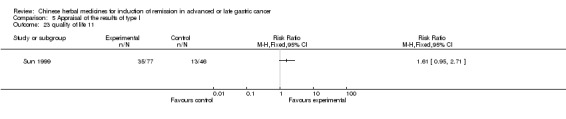
Comparison 5 Appraisal of the results of type I, Outcome 23 quality of life 11.
5.24. Analysis.
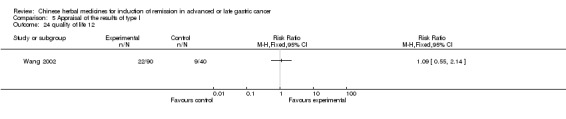
Comparison 5 Appraisal of the results of type I, Outcome 24 quality of life 12.
5.27. Analysis.
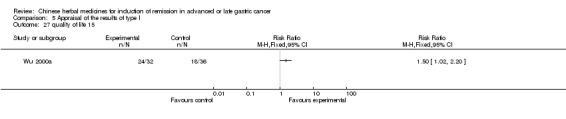
Comparison 5 Appraisal of the results of type I, Outcome 27 quality of life 15.
5.29. Analysis.
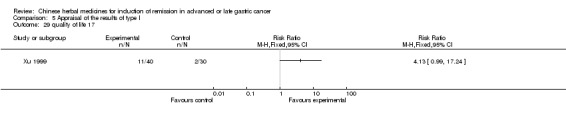
Comparison 5 Appraisal of the results of type I, Outcome 29 quality of life 17.
5.32. Analysis.
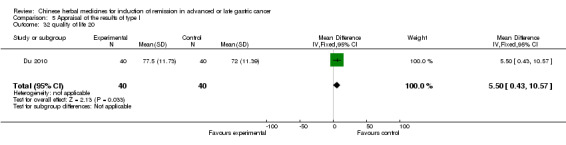
Comparison 5 Appraisal of the results of type I, Outcome 32 quality of life 20.
Eleven trials showed no significant difference in Karnofsky score (> 60 points) between the intervention group and the control group, and the comparison result was the following:
56 patients had kept their score > 60 points in the intervention group (64 patients) and 52 patients in the control group (64 patients) at three months (Chen 2005; Analysis 5.16) (RR 1.08, 95% CI 0.93 to 1.25);
30 patients had kept their score > 60 points in the intervention group (61 patients) and 8 patients in the control group (30 patients) at three months (Hua 1999; Analysis 5.18) (RR 1.84, 95% CI 0.97 to 3.52);
16 patients had increased their score (an increase > 10 points) in the intervention group (30 patients) and 12 patients in the control group (30 patients) at six weeks (Liu 2006a; Analysis 5.22) (RR 1.33, 95% CI 0.77 to 2.31);
35 patients had increased their score (an increase > 10 points) in the intervention group (77 patients) and 13 patients in the control group (46 patients) at three months (Sun 1999; Analysis 5.25) (RR 1.61, 95% CI 0.95 to 2.71);
22 patients had increased their score (an increase > 10 points) in the intervention group (90 patients) and 9 patients in the control group (40 patients) at 9 to 12 weeks (Wang 2002; Analysis 5.26) (RR 1.09, 95% CI 0.55 to 2.14);
18 patients had increased their score (an increase > 10 points) in the intervention group (32 patients) and 12 patients in the control group (30 patients) at six to eight weeks (Wu 2000; Analysis 5.28) (RR 1.41, 95% CI 0.82 to 2.40);
78 patients had increased their score (an increase > 10 points) in the intervention group (90 patients) and 65 patients in the control group (82 patients) at 12 weeks (Xie 2006; Analysis 5.30) (RR 1.09, 95% CI 0.95 to 1.25);
11 patients had increased their score (an increase > 10 points) in the intervention group (40 patients) and 2 patients in the control group (30 patients) at two months (Xu 1999; Analysis 5.31) (RR 4.13, 95% CI 0.99 to 17.24);
20 patients had increased their score (an increase > 10 points) in the intervention group (40 patients) and 14 patients in the control group (40 patients) at four weeks (Zhu 2006; Analysis 5.33) (RR 1.25, 95% CI 0.77 to 2.04).
The Karnofsky score was 77.50 ± 11. 73 (40 patients) in the intervention group and 72.00 ± 11.39 months (40 patients) in the control group at six weeks (Du 2010; Analysis 5.20) (MD 5.50, 95% CI 0.43 to 10.57);
15 patients had increased their score (an increase > 10 points) in the intervention group (24 patients) and 12 patients in the control group (23 patients) at three months (Gao 2008; Analysis 5.3) (RR 1.53, 95% CI 0.48 to 4.89).
5.16. Analysis.
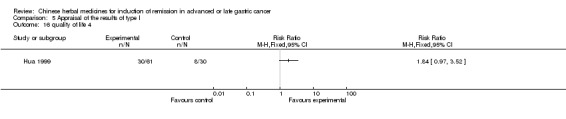
Comparison 5 Appraisal of the results of type I, Outcome 16 quality of life 4.
5.18. Analysis.
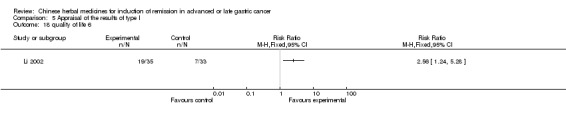
Comparison 5 Appraisal of the results of type I, Outcome 18 quality of life 6.
5.22. Analysis.
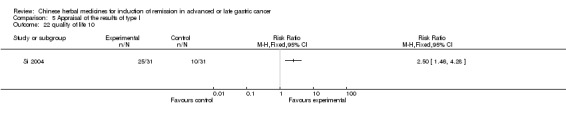
Comparison 5 Appraisal of the results of type I, Outcome 22 quality of life 10.
5.25. Analysis.
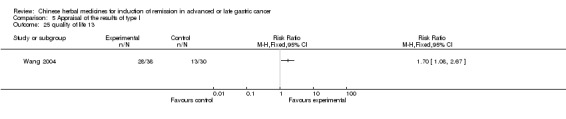
Comparison 5 Appraisal of the results of type I, Outcome 25 quality of life 13.
5.26. Analysis.
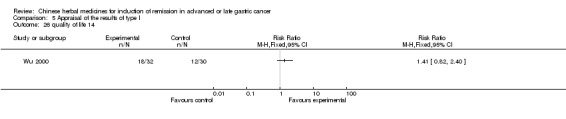
Comparison 5 Appraisal of the results of type I, Outcome 26 quality of life 14.
5.28. Analysis.
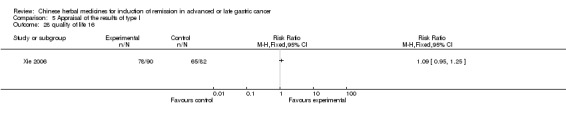
Comparison 5 Appraisal of the results of type I, Outcome 28 quality of life 16.
5.30. Analysis.
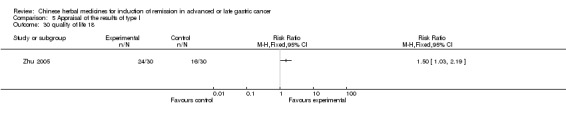
Comparison 5 Appraisal of the results of type I, Outcome 30 quality of life 18.
5.31. Analysis.
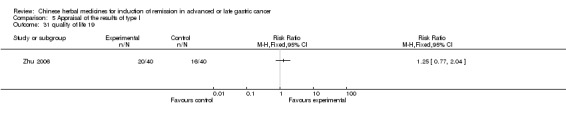
Comparison 5 Appraisal of the results of type I, Outcome 31 quality of life 19.
5.33. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 33 quality of life 21.
Rate of short‐term remission
Of the 37 individual trials, nine trials showed a significant difference in the rate of short‐term remission between the intervention group and the control group after the trial, and the comparison result was the following:
18 patients in the intervention group (30 patients) and 9 patients in the control group (28 patients) at six weeks (Chen 1997; Analysis 5.34) (RR 1.87, 95% CI 1.01 to 3.44);
26 patients in the intervention group (38 patients) and 15 patients in the control group (36 patients) at nine weeks (Liu 2006; Analysis 5.41) (RR 1.64, 95% CI 1.05 to 2.56);
45 patients in the intervention group (60 patients) and 31 patients in the control group (60 patients) at 40 days (Niu 2006; Analysis 5.44) (RR 1.45, 95% CI 1.09 to 1.93);
24 patients in the intervention group (31 patients) and 15 patients in the control group (31 patients) at nine weeks (Si 2004; Analysis 5.45) (RR 1.60, 95% CI 1.06 to 2.41);
17 patients in the intervention group (30 patients) and 6 patients in the control (30 patients) group at 45 days (Wang 1993; Analysis 5.47) (RR 2.83, 95% CI 1.30 to 6.19);
23 patients in the intervention group (40 patients) and 11 patients in the control (40 patients) group at eight weeks (Yang 2005; Analysis 5.55) (RR 2.09, 95% CI 1.18 to 3.69);
30 patients in the intervention group (35 patients) and 17 patients in the control group (35 patients) at four to six weeks (Zhang 1997; Analysis 5.56) (RR 1.76, 95% CI 1.12 to 2.55);
21 patients in the intervention group (28 patients) and 10 patients in the control group (22 patients) at six to eight weeks (Zheng 1999; Analysis 5.58) (RR 1.65, 95% CI 1.00 to 2.73).
5.34. Analysis.
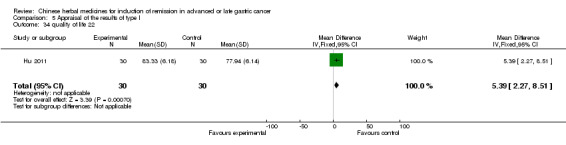
Comparison 5 Appraisal of the results of type I, Outcome 34 quality of life 22.
5.41. Analysis.
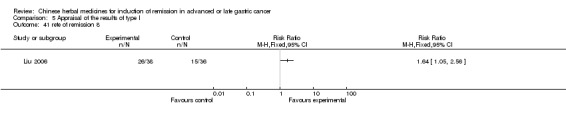
Comparison 5 Appraisal of the results of type I, Outcome 41 rete of remission 8.
5.44. Analysis.
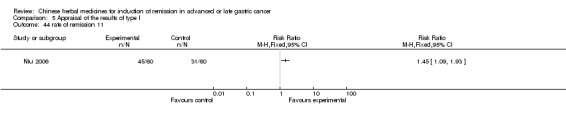
Comparison 5 Appraisal of the results of type I, Outcome 44 rate of remission 11.
5.45. Analysis.
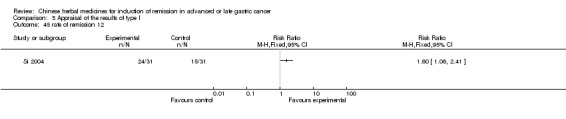
Comparison 5 Appraisal of the results of type I, Outcome 45 rate of remission 12.
5.47. Analysis.
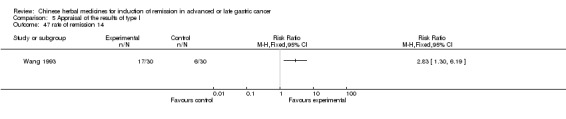
Comparison 5 Appraisal of the results of type I, Outcome 47 rate of remission 14.
5.55. Analysis.
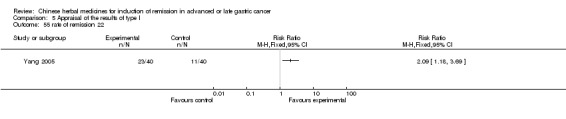
Comparison 5 Appraisal of the results of type I, Outcome 55 rate of remission 22.
5.56. Analysis.
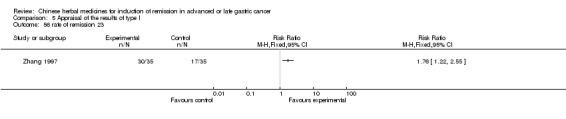
Comparison 5 Appraisal of the results of type I, Outcome 56 rate of remission 23.
5.58. Analysis.
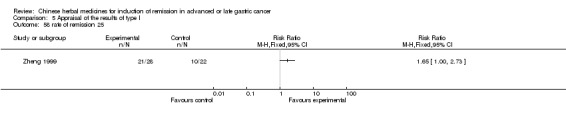
Comparison 5 Appraisal of the results of type I, Outcome 58 rate of remission 25.
Twenty trials showed no significant difference in the rate of short‐term remission between the intervention group and the control group after the trial, and the comparison result was the following:
42 patients in the intervention group (64 patients) compared with 34 patients in the control group (64 patients) at three months (Chen 2005; Analysis 5.35) (RR 1.24, 95% CI 0.92 to 1.65);
20 patients in the intervention group (61 patients) compared with 4 patients in the control group (30 patients) at three months (Hua 1999; Analysis 5.37) (RR 2.46, 95% CI 0.92 to 6.56);
9 patients in the intervention group (31 patients) compared with 5 patients in the control group (28 patients) at four weeks (Huang 2002; Analysis 5.38) (RR 1.63, 95% CI 0.62 to 4.27);
15 patients in the intervention group (34 patients) compared with 10 patients in the control group (34 patients) at nine weeks (Huang 2005; Analysis 5.39) (RR 1.50, 95% CI 0.79 to 2.86);
14 patients in the intervention group (30 patients) compared with 11 patients in the control group (30 patients) at six weeks (Liu 2006a; Analysis 5.42) (RR 1.27, 95% CI 0.69 to 2.33);
20 patients in the intervention group (48 patients) compared with 13 patients in the control group (34 patients) (treatment period unspecified) (Lv 1999; Analysis 5.43) (RR 1.09, 95% CI 0.63 to 1.88);
20 patients in the intervention group (77 patients) compared with 29 patients in the control group (49 patients) (treatment period unspecified) (Sun 1999; Analysis 5.46) (RR 0.44, 95% CI 0.28 to 0.68);
42 patients in the intervention group (90 patients) compared with 15 patients in the control group (40 patients) at three months (Wang 2002; Analysis 5.48) (RR 1.24, 95% CI 0.79 to 1.97);
22 patients in the intervention group (38 patients) compared with 10 patients in the control group (30 patients) at 30 to 90 days (Wang 2004; Analysis 5.49) (RR 1.74, 95% CI 0.98 to 3.08);
11 patients in the intervention group (24 patients) compared with 7 patients in the control group (22 patients) at 8 to 12 weeks (Wang 2004a; Analysis 5.50) (RR 1.44, 95% CI 0.68 to 3.05);
16 patients in the intervention group (32 patients) compared with 12 patients in the control group (30 patients) at six to eight weeks (Wu 2000; Analysis 5.51) (RR 1.25, 95% CI 0.71 to 2.19);
12 patients in the intervention group (32 patients) compared with 16 patients in the control group (36 patients) at six to eight weeks (Wu 2000a; Analysis 5.50) (RR 0.84, 95% CI 0.47 to 1.50);
52 patients in the intervention group (90 patients) compared with 40 patients in the control group (82 patients) at six weeks (Xie 2006; Analysis 5.51) (RR 1.19, 95% CI 0.89 to 1.57);
28 patients in the intervention group (40 patients) compared with 14 patients in the control group (30 patients) at six weeks (Xu 1999; Analysis 5.52) (RR 1.50, 95% CI 0.97 to 2.31);
28 patients in the intervention group (40 patients) compared with 14 patients in the control group (40 patients) at six weeks (Zhang 2005a; Analysis 5.53) (RR 1.23, 95% CI 0.75 to 2.00);
7 patients in the intervention group (40 patients) compared with 6 patients in the control group (40 patients) at six weeks (Du 2010) (RR 1.20, 95% CI 0.37 to 3.95);
13 patients in the intervention group (24 patients) compared with 11 patients in the control group (23 patients) at three months (Gao 2008) (RR 1.29, 95% CI 0.41 to 4.06);
16 patients in the intervention group (30 patients) compared with 13 patients in the control group (30 patients) at six to nine weeks (Hu 2011) (RR 1.31, 95% CI 0.47 to 3.61);
10 patients in the intervention group (22 patients) compared with 8 patients in the control group (23 patients) at eight weeks (Zhang 2010a) (RR 1.56, 95% CI 0.47 to 5.19);
25 patients in the intervention group (40 patients) compared with 23 patients in the control group (40 patients) at four weeks (Deng 2011; Analysis 5.57) (RR 1.23, 95% CI 0.50 to 3.02).
5.35. Analysis.
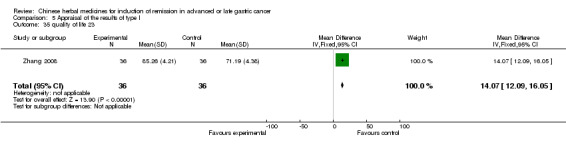
Comparison 5 Appraisal of the results of type I, Outcome 35 quality of life 23.
5.37. Analysis.
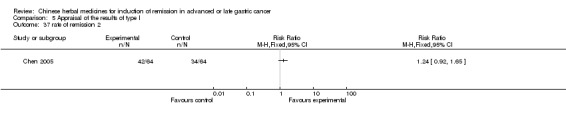
Comparison 5 Appraisal of the results of type I, Outcome 37 rate of remission 2.
5.38. Analysis.
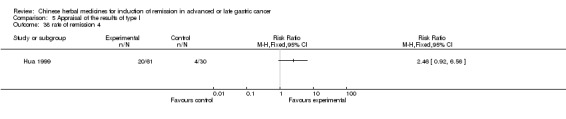
Comparison 5 Appraisal of the results of type I, Outcome 38 rate of remission 4.
5.39. Analysis.
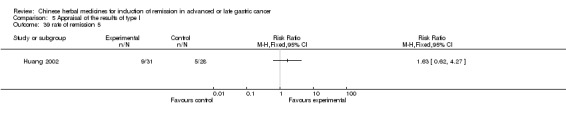
Comparison 5 Appraisal of the results of type I, Outcome 39 rate of remission 5.
5.42. Analysis.
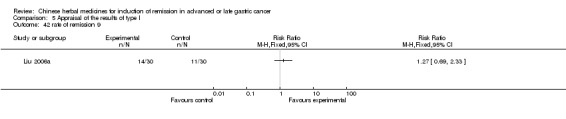
Comparison 5 Appraisal of the results of type I, Outcome 42 rate of remission 9.
5.43. Analysis.
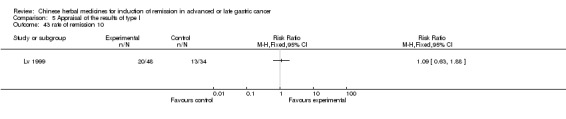
Comparison 5 Appraisal of the results of type I, Outcome 43 rate of remission 10.
5.46. Analysis.
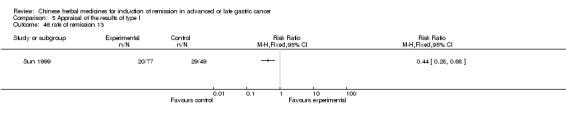
Comparison 5 Appraisal of the results of type I, Outcome 46 rate of remission 13.
5.48. Analysis.
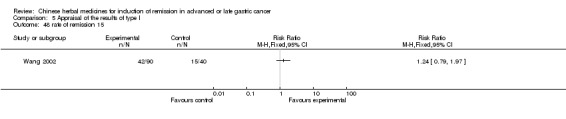
Comparison 5 Appraisal of the results of type I, Outcome 48 rate of remission 15.
5.49. Analysis.
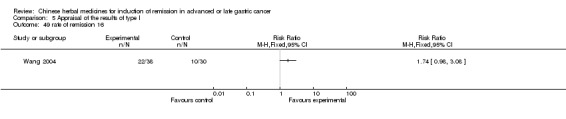
Comparison 5 Appraisal of the results of type I, Outcome 49 rate of remission 16.
5.50. Analysis.
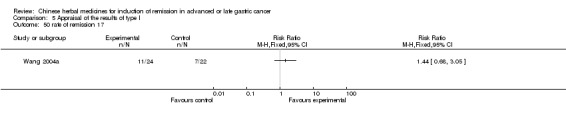
Comparison 5 Appraisal of the results of type I, Outcome 50 rate of remission 17.
5.51. Analysis.
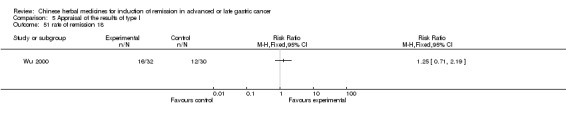
Comparison 5 Appraisal of the results of type I, Outcome 51 rate of remission 18.
5.52. Analysis.
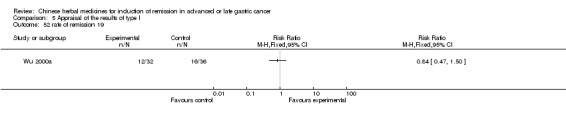
Comparison 5 Appraisal of the results of type I, Outcome 52 rate of remission 19.
5.53. Analysis.
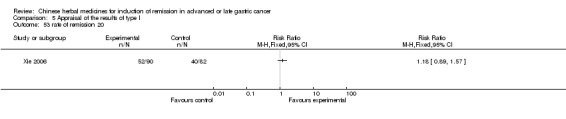
Comparison 5 Appraisal of the results of type I, Outcome 53 rate of remission 20.
5.57. Analysis.
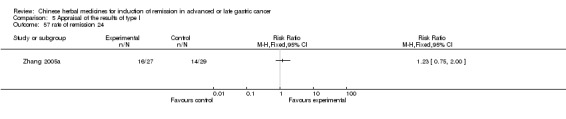
Comparison 5 Appraisal of the results of type I, Outcome 57 rate of remission 24.
Median survival time (MST)
Of the 42 individual trials, only one trial presented the entire data, which showed a significant difference in the MST between the intervention group and the control group. The comparison results were the following: the MST was 24.9 ± 1.36 months (40 patients) in the intervention group and 13.7 ± 0.72 months (40 patients) in the control group (Zhu 2006; Analysis 5.59) (WMD 11.20, 95% CI 10.72 to 11.68).
5.59. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 59 rate of remission 26.
Time to progression (TTP)
Of the 42 individual trials, no trial presented specific data on TTP so it could not be appraised.
Adverse events
Life threatening
No trial reported adverse events which were life threatening and no patients died of adverse events.
Toxic response
Of the 42 individual trials, the common toxic responses included gastrointestinal side effects (such as nausea, vomiting, abdominal pain, diarrhoea, etc), leukopenia, thrombopenia caused by arrest of bone marrow, damage of liver or kidney function, phlebitis, etc. Eighteen trials reported severe adverse events, including leukopenia in 11 trials, thrombopenia in nine trials, diarrhoea in three trials, decrease of haemoglobin in five trials, nausea and vomiting in 10 trials, damage to liver or kidney function in six trials.
Leukopenia
Five trials with specific data showed a significant difference in leukopenia between the intervention group and the control group, and the comparison result was the following: 3 patients in the intervention group (38 patients) compared with 11 patients in the control group (30 patients) (Wang 2004; Analysis 5.61) (RR 0.29; 95% CI 0.10 to 0.85). The average score for leukopenia in the intervention group (24 patients) was 3.85 ± 0.57 compared with 3.37 ± 0.47 in the control group (23 patients) (Gao 2008) (RR 0.48, 95% CI 0.18 to 0.78); 8 patients in the intervention group (30 patients) compared with 16 patients in the control group (30 patients) at six to nine weeks (Hu 2011) (RR 0.32, 95% CI 0.11 to 0.94); 8 patients in the intervention group (22 patients) compared with 18 patients in the control group (23 patients) at eight weeks (Zhang 2010a) (RR 0.16, 95% CI 0.04 to 0.59). The leukocyte score was 5.39 ± 1.07 (40 patients) in the intervention group and 3.39 ± 1.08 months (40 patients) in the control group at four weeks (Deng 2011) (WMD 2.00, 95% CI 1.53 to 2.47).
5.61. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 61 rate of remission 28.
Three trials with specific data showed no significant difference in leukopenia between the intervention group and the control group, and the comparison result was the following:
4 patients in the intervention group (51 patients) compared with 12 patients in the control group (45 patients) (Xu 1993; Analysis 5.62) (RR 0.31, 95% CI 0.10 to 1.01);
5 patients in the intervention group (35 patients) compared with 18 patients in the control group (35 patients) (Zhang 1997; Analysis 5.63) (RR 0.28, 95% CI 0.12 to 0.67).
5.62. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 62 rate of remission 29.
5.63. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 63 rate of remission 30.
Nausea or vomiting
One trial with specific data showed a significant difference in nausea and vomiting between the intervention group and the control group, and the comparison result was the following: 0 patients in the intervention group (31 patients) compared with 12 patients in the control group (28 patients) (Huang 2002; Analysis 5.66) (RR 0.04, 95% CI 0.00 to 0.59).
5.66. Analysis.
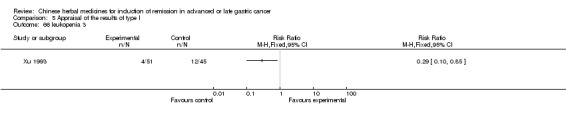
Comparison 5 Appraisal of the results of type I, Outcome 66 leukopenia 3.
Four trials with specific data showed no significant difference in nausea and vomiting between the intervention group and the control group, and the comparison result was the following:
6 patients in the intervention group (61 patients) compared with 7 patients in the control group (30 patients) (Hua 1999; Analysis 5.65) (RR 0.42, 95% CI 0.16 to 1.14);
1 patient in the intervention group (35 patients) compared with 4 patients in the control group (33 patients) (Li 2002; Analysis 5.67) (RR 0.24, 95% CI 0.03 to 2.00);
4 patients in the intervention group (51 patients) compared with 9 patients in the control group (45 patients) (Xu 1993; Analysis 5.68) (RR 0.39, 95% CI 0.13 to 1.19);
30 patients in the intervention group (36 patients) compared with 34 patients in the control group (36 patients) (Zhang 2008) (RR 0.29, 95% CI 0.06 to 1.57);
5 patients in the intervention group (30 patients) compared with 12 patients in the control group (30 patients) (Fu 2011) (RR 0.30, 95% CI 0.09 to 1.00);
12 patients in the intervention group (22 patients) compared with 19 patients in the control group (23 patients) (Zhang 2010a) (RR 0.25, 95% CI 0.06 to 0.99).
5.65. Analysis.
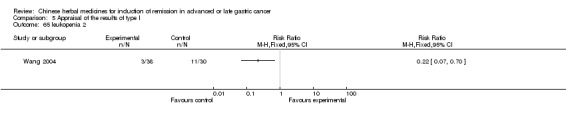
Comparison 5 Appraisal of the results of type I, Outcome 65 leukopenia 2.
5.67. Analysis.
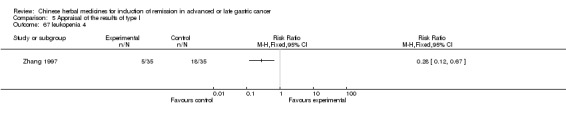
Comparison 5 Appraisal of the results of type I, Outcome 67 leukopenia 4.
5.68. Analysis.
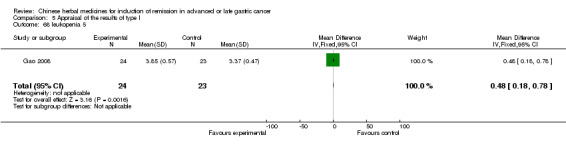
Comparison 5 Appraisal of the results of type I, Outcome 68 leukopenia 5.
Thrombopenia
One trial with specific data showed no significant difference in thrombopenia between the intervention group and the control group, and the comparison result was the following: 2 patients in the intervention group (30 patients) compared with 1 patient in the control group (30 patients) (Wang 1993; Figure 74) (RR 2.00, 95% CI 0.19 to 20.90).
Four trials with specific data showed a significant difference in thrombopenia between the intervention group and the control group, and the comparison result was the following: 3 patients in the intervention group (30 patients) compared with 10 patients in the control group (30 patients) (Hu 2011) (RR 0.22, 95% CI 0.05 to 0.91); the platelet score was 3.89 ± 0.47 (24 patients) in the intervention group and 3.74 ± 0.54 (23 patients) in the control group (Gao 2008) (MD 0.15, 95% CI ‐0.14 to 0.44); the platelet score was 132.85 ± 22.45 (40 patients) in the intervention group and 119.58 ± 30.52 (40 patients) in the control group (Deng 2011; Analysis 5.69) (MD 13.27, 95% CI 1.53 to 25.01); 4 patients in the intervention group (22 patients) compared with 11 patients in the control group (23 patients) (Zhang 2010a; Analysis 5.70) (RR 2.00, 95% CI 0.19 to 20.90).
5.69. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 69 leukopenia 6.
5.70. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 70 leukopenia 7.
Diarrhoea
One trial with specific data showed no significant difference in diarrhoea between the intervention group and the control group, and the comparison result was the following:
25 patients in the intervention group (36 patients) compared with 31 patients in the control group (36 patients) (Zhang 2008) (RR 0.37, 95% CI 0.11 to 1.19).
Decrease of haemoglobin
One trial with specific data showed no significant difference in the decrease of haemoglobin between the intervention group and the control group, and the comparison result was the following:
4 patients in the intervention group (30 patients) compared with 3 patients in the control group (30 patients) (Wang 1993; Analysis 5.74) (RR 1.33, 95% CI 0.33 to 5.45).
5.74. Analysis.
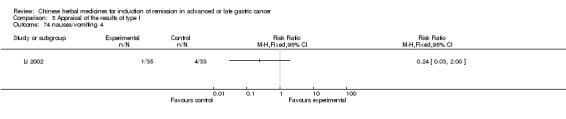
Comparison 5 Appraisal of the results of type I, Outcome 74 nausea/vomiting 4.
Two trials with specific data showed a significant difference in the decrease of haemoglobin between the intervention group and the control group, and the comparison result was the following:
haemoglobin score was 114.25 ± 30.42 (40 patients) in the intervention group and 98.38 ± 26.35 (40 patients) in the control group (Deng 2011) (MD 15.87, 95% CI 3.40 to 28.34);
haemoglobin score was 2.45 ± 0.51 (24 patients) in the intervention group and 1.95 ± 0.42 (23 patients) in the control group (Gao 2008) (MD 0.50, 95% CI 0.23 to 0.77).
Damage to liver or kidney function, or both
Four trials with specific data showed no significant difference in damage to the liver or kidney function between the intervention group and the control group. The comparison result was the following: 3 patients in the intervention group (90 patients) compared with 7 patients in the control group (82 patients) (Xie 2006; Analysis 5.75) (RR 0.39, 95% CI 0.10 to 1.46); 2 patients in the intervention group (30 patients) compared with 6 patients in the control group (30 patients) (Hu 2011) (RR 0.29, 95% CI 0.05 to 1.55); 2 patients in the intervention group (22 patients) compared with 8 patients in the control group (23 patients) (Zhang 2010a) (RR 0.19, 95% CI 0.03 to 1.01); 7 patients in the intervention group (22 patients) compared with 15 patients in the control group (23 patients) (Deng 2011) (RR 0.35, 95% CI 0.13 to 1.00).
5.75. Analysis.
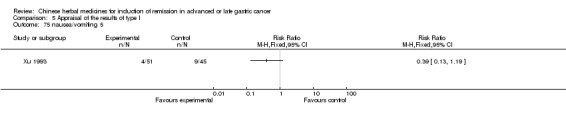
Comparison 5 Appraisal of the results of type I, Outcome 75 nausea/vomiting 5.
Discontinuation of treatment
Of the 42 individual trials, two trials showed a significant difference in discontinuation due to an adverse event during the therapeutic period between the intervention group and the control group, and the comparison result was the following:
3 patients in the intervention group (50 patients) compared with 12 patients in the control group (40 patients) (Guo 1989; Analysis 5.77) (RR 0.20, 95% CI 0.06 to 0.66);
5 patients in the intervention group (35 patients) compared with 18 patients in the control group (35 patients) (Zhang 1997; Analysis 5.79) (RR 0.28, 95% CI 0.12 to 0.67).
5.77. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 77 nausea/vomiting 7.
5.79. Analysis.
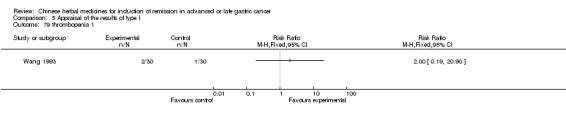
Comparison 5 Appraisal of the results of type I, Outcome 79 thrombopenia 1.
Two trials showed a significant difference in discontinuation during the therapeutic period between the intervention group and the control group, and the comparison result was the following:
3 patients in the intervention group (90 patients) compared with 7 patients in the control group (82 patients) (Xie 2006; Analysis 5.76) (RR 0.39, 95% CI 0.10 to 1.46);
4 patients in the intervention group (40 patients) compared with 8 patients in the control group (30 patients) (Xu 1999; Analysis 5.78) (RR 0.38, 95% CI 0.12 to 1.13).
5.76. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 76 nausea/vomiting 6.
5.78. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 78 nausea/vomiting 8.
Type II (western medicine with TCMHs versus the same TCMHs)
Mortality
The six trials could not be pooled for meta‐analysis.
One trial showed a significant difference in mortality at six months and one year between the intervention group and the control group, and the comparison result was the following, respectively:
7 patients had died in the intervention group (30 patients) compared with 9 patients in the control group (30 patients) (Liu 2002; Analysis 6.5) (RR 0.37, 95% CI 0.18 to 0.74);
17 patients had died in the intervention group (30 patients) compared with 27 patients in the control group (30 patients) (Liu 2002; Analysis 6.6) (RR 0.63, 95% CI 0.45 to 0.88).
6.5. Analysis.
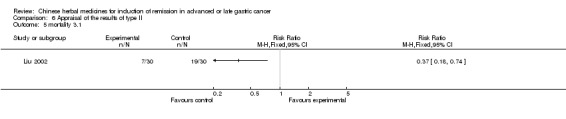
Comparison 6 Appraisal of the results of type II, Outcome 5 mortality 3.1.
6.6. Analysis.
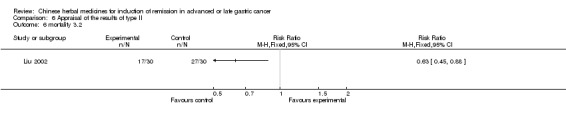
Comparison 6 Appraisal of the results of type II, Outcome 6 mortality 3.2.
One trial showed a significant difference in mortality at one year between the intervention group and the control group, and the comparison result was the following: 22 patients had died in the intervention group (105 patients) compared with 22 patients in the control group (58 patients) (Wang 1998; Analysis 6.1) (RR 0.55, 95% CI 0.34 to 0.91). There was no significant difference at two years and three years between the intervention group and the control group, and the comparison result was the following, respectively: 37 patients had died in the intervention group (105 patients) compared with 26 patients in the control group (58 patients) (Wang 1998; Analysis 6.2) (RR 0.79, 95% CI 0.53 to 1.61); 67 patients had died in the intervention group (105 patients) compared with 42 patients in the control group (58 patients) (Wang 1998; Analysis 6.3) (RR 0.88, 95% CI 0.71 to 1.09).
6.1. Analysis.
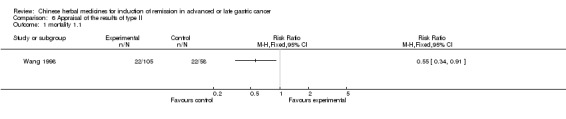
Comparison 6 Appraisal of the results of type II, Outcome 1 mortality 1.1.
6.2. Analysis.
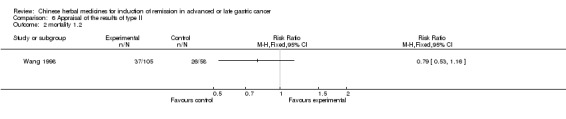
Comparison 6 Appraisal of the results of type II, Outcome 2 mortality 1.2.
6.3. Analysis.
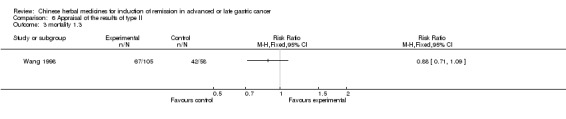
Comparison 6 Appraisal of the results of type II, Outcome 3 mortality 1.3.
One trial showed no significant difference in mortality at six months between the intervention group and the control group, and the comparison result was the following: 7 patients had died in the intervention group (22 patients) compared with 12 patients in the control group (19 patients) (Chen 1997a; Analysis 6.4) (RR 0.41, 95% CI 0.19 to 0.86).
6.4. Analysis.
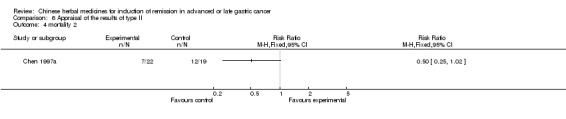
Comparison 6 Appraisal of the results of type II, Outcome 4 mortality 2.
Quality of life (QOL) (< six months)
Of the six individual trials, only one trial with specific data showed a significant difference in Karnofsky score (> 60 points) between the intervention group and the control group after the trial, and the comparison result was the following: 25 patients in the intervention group (33 patients) compared with 10 patients in the control group (26 patients) at six weeks (Zhao 2005; Analysis 6.7) (RR 1.97, 95% CI 1.17 to 3.32).
6.7. Analysis.
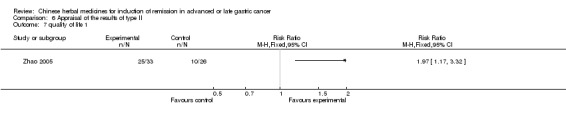
Comparison 6 Appraisal of the results of type II, Outcome 7 quality of life 1.
Rate of short‐term remission
Of the six individual trials, three trials with specific data showed no significant difference in the rate of short‐term remission between the intervention group and the control group after the trial, and the comparison result was the following:
14 patients in the intervention group (22 patients) compared with six patients in the control group (19 patients) at 40 days (Chen 1997a; Analysis 6.8) (RR 2.02, 95% CI 0.97 to 4.20);
14 patients in the intervention group (33 patients) compared with 10 patients in the control group (26 patients) at six weeks (Zhao 2005; Analysis 6.9) (RR 1.10, 95% CI 0.59 to 2.07);
13 patients in the intervention group (30 patients) compared with 11 patients in the control group (30 patients) at 8 to 12 weeks after the therapeutic period (Liu 2002; Analysis 6.10) (RR 1.18, 95% CI 0.63 to 2.20).
6.8. Analysis.
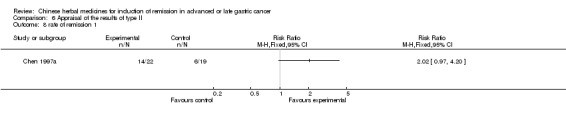
Comparison 6 Appraisal of the results of type II, Outcome 8 rate of remission 1.
6.9. Analysis.
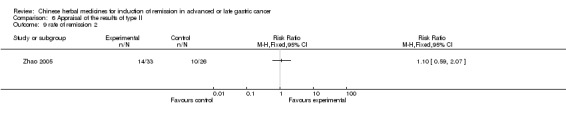
Comparison 6 Appraisal of the results of type II, Outcome 9 rate of remission 2.
6.10. Analysis.
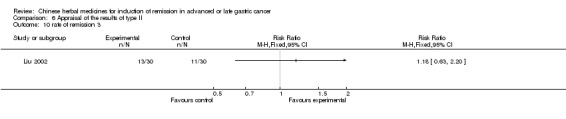
Comparison 6 Appraisal of the results of type II, Outcome 10 rate of remission 3.
Median survival time (MST)
Of the six individual trials, no trial presented specific data on MST so it could not be appraised.
Time to progression (TTP)
Of the six individual trials, no trial presented specific data on TTP so it could not be appraised.
Adverse events
Life threatening
No trial reported adverse events which were life threatening and no patients died of adverse events.
Toxic response
Of the six individual trials, the common severe toxic responses included arrest of bone marrow and gastrointestinal side effects (such as nausea, vomiting, anorexia, etc). Only two trials reported severe adverse events, including arrest of bone marrow in one trial, nausea or vomiting and anorexia in two trials.
Arrest of bone marrow
One trial with specific data showed no significant difference in the arrest of bone marrow between the intervention group and the control group, and the comparison result was the following: 1 patient in the intervention group (33 patients) compared with 4 patients in the control group (26 patients) (Zhao 2005; Analysis 6.12) (RR 0.20, 95% CI 0.02 to 1.66).
6.12. Analysis.
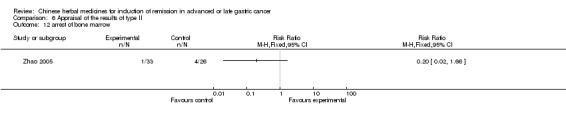
Comparison 6 Appraisal of the results of type II, Outcome 12 arrest of bone marrow.
Nausea or vomiting
One trial with specific data showed no significant difference in nausea and vomiting between the intervention group and the control group, and the comparison result was the following: 0 patients in the intervention group (33 patients) compared with 1 patient in the control group (26 patients) (Zhao 2005; Analysis 6.11) (RR 0.26, 95% CI 0.01 to 6.24).
6.11. Analysis.
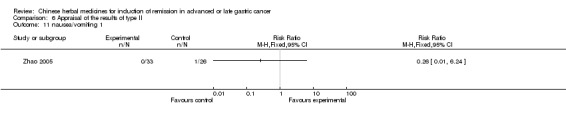
Comparison 6 Appraisal of the results of type II, Outcome 11 nausea/vomiting 1.
Discontinuation of treatment
Of the six individual trials, only one trial with specific data showed a significant difference in discontinuation due to adverse events during the therapeutic period between the intervention group and the control group, and the comparison result was the following: 13 patients in the intervention group (116 patients) compared with 32 patients in the control group (124 patients) (Xu 1989; Analysis 6.13) (RR 0.43, 95% CI 0.24 to 0.79).
6.13. Analysis.
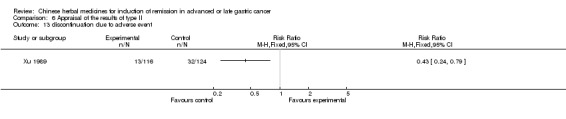
Comparison 6 Appraisal of the results of type II, Outcome 13 discontinuation due to adverse event.
Type III (TCMH versus another TCMH)
Mortality
Two trials could not be pooled for meta‐analysis. One trial with specific data showed a significant difference in mortality at six months between the intervention group and the control group, and the comparison result was the following: 7 patients had died in the intervention group (30 patients) compared with 12 patients in the control group (21 patients) (Shi 2004; Analysis 7.1) (RR 0.41, 95% CI 0.19 to 0.86).
7.1. Analysis.
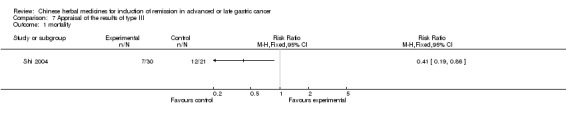
Comparison 7 Appraisal of the results of type III, Outcome 1 mortality.
Quality of life (QOL) (< six months)
Of the two individual trials, only one trial with specific data showed a significant difference in Karnofsky score (> 60 points) between the intervention group and the control group after the trial, and the comparison result was the following: 16 patients in the intervention group (30 patients) compared with 4 patients in the control group (21 patients) at 60 days (Shi 2004; Analysis 7.2) (RR 2.80, 95% CI 1.09 to 7.19).
7.2. Analysis.
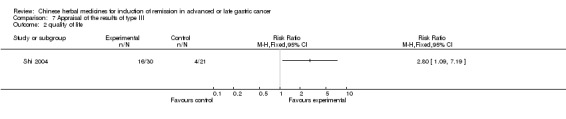
Comparison 7 Appraisal of the results of type III, Outcome 2 quality of life.
Rate of remission (short‐term and long‐term)
Of the two individual trials, no trial presented specific data on rate of remission so it could not be appraised.
Median survival time (MST)
Of the two individual trials, no trial presented specific data on MST so it could not be appraised.
Time to progression (TTP)
Of the two individual trials, no trial presented specific data on TTP so it could not be appraised.
Adverse events
Life threatening
No trial reported life threatening adverse events and no patient died of adverse events.
Toxic response
No trial reported a severe toxic response.
Discontinuation of treatment
No trial reported the discontinuation of treatment.
Type IV (TCMHs versus western medicine)
Mortality
Six trials could not be pooled for meta‐analysis. One trial showed no significant difference in mortality at one year and three years between the intervention group and the control group, and the comparison result was the following, respectively:
13 patients had died in the intervention group (41 patients) compared with 17 patients in the control group (31 patients) (Li 2001; Analysis 8.1) (RR 0.58, 95% CI 0.33 to 1.00);
35 patients had died in the intervention group (41 patients) compared with 29 patients in the control group (31 patients) (Li 2001; Analysis 8.2) (RR 0.91, 95% CI 0.78 to 1.07).
8.1. Analysis.
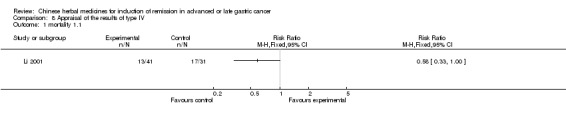
Comparison 8 Appraisal of the results of type IV, Outcome 1 mortality 1.1.
8.2. Analysis.
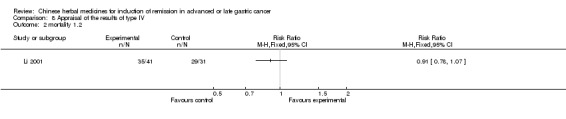
Comparison 8 Appraisal of the results of type IV, Outcome 2 mortality 1.2.
One trial showed no significant difference in mortality at 20 months between the intervention group and the control group, and the comparison result was the following: 15 patients had died in the intervention group (24 patients) compared with 8 patients in the control group (12 patients) (Zhou 2000; Analysis 8.3) (RR 0.94, 95% CI 0.57 to1.56).
8.3. Analysis.
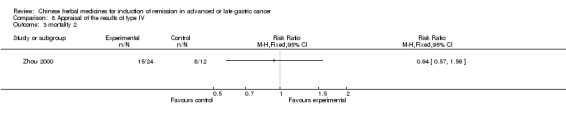
Comparison 8 Appraisal of the results of type IV, Outcome 3 mortality 2.
One trial showed no significant difference in mortality at one and two years between the intervention group and the control group, and the comparison result was the following, respectively: 7 patients had died in the intervention group (62 patients) and 7 patients in the control group (56 patients) (You 2000; Figure 1) (RR 0.90, 95% CI 0.34 to 2.41); 14 patients had died in the intervention group (62 patients) and 18 patients in the control group (56 patients) (You 2000; Figure 2) (RR 0.70, 95% CI 0.39 to 1.28). There was a significant difference in mortality at three, five, and 10 years between the intervention group and the control group, and the comparison result was the following, respectively: 22 patients had died in the intervention group (62 patients) compared with 32 patients in the control group (56 patients) (You 2000) (RR 0.62, 95% CI 0.41 to 0.93); 38 patients had died in the intervention group (62 patients) compared with 56 patients in the control group (56 patients) (You 2000) (RR 0.62, 95% CI 0.51 to 0.75); 57 patients had died in the intervention group (62 patients) compared with 56 patients in the control group (56 patients) (You 2000) (RR 0.92, 95% CI 0.85 to 1.00).
One trial showed no significant difference in mortality at one, two, three, four, or five years between the intervention group and the control group, and the comparison result was the following, respectively:
8 patients died in the intervention group (52 patients) compared with 6 patients in the control group (30 patients) (Jiang 1994) (RR 0.77, 95% CI 0.30 to 2.01);
18 patients died in the intervention group (52 patients) compared with 12 patients in the control group (30 patients) (Jiang 1994) (RR 0.87, 95% CI 0.49 to1.54);
24 patients died in the intervention group (52 patients) compared with 17 patients in the control group (30 patients) (Jiang 1994) (RR 0.81, 95% CI 0.53 to 1.25);
32 patients died in the intervention group (52 patients) compared with 22 patients in the control group (30 patients) (Jiang 1994) (RR 0.84, 95% CI 0.62 to 1.14);
34 patients died in the intervention group (52 patients) compared with 25 patients in the control group (30 patients) (Jiang 1994) (RR 0.78, 95% CI 0.61 to 1.01).
One trial showed no significant difference in mortality at six months and one year between the intervention group and the control group, and the comparison result was the following, respectively: 7 patients had died in the intervention group (39 patients) compared with 8 patients in the control group (39 patients) (Yang 2006) (RR 0.88, 95% CI 0.35 to 2.18); 10 patients had died in the intervention group (39 patients) compared with 17 patients in the control group (39 patients) (Yang 2006) (RR 0.59, 95% CI 0.31 to 1.12). This trial showed a significant difference in mortality at 1.5 years between the intervention group and the control group, and the comparison result was the following: 19 patients had died in the intervention group (39 patients) compared with 28 patients in the control group (39 patients) (Yang 2006) (RR 0.68, 95% CI 0.47 to 0.99).
Quality of life (QOL) (< six months)
Of the six individual trials, only two trials with specific data showed a significant difference in Karnofsky score between the intervention group and the control group after the trial, and the comparison result was the following: Karnofsky score 70.26 ± 6.68 (39 patients) in the intervention group compared with 63.84 ± 6.73 in the control group (39 patients) at two to three months (Yang 2006) (WMD 6.42, 95% CI 3.44 to 9.40); 42 patients in the intervention group (52 patients) compared with 9 patients in the control group (30 patients) at 2 months (Jiang 1994) (RR 2.69, 95% CI 1.53 to 4.72); 20 patients in the intervention group (35 patients) compared with 9 patients in the control group (30 patients) at three weeks (Ye 2009) (RR 3.11, 95% CI 1.11 to 8.70); 20 patients in the intervention group (30 patients) compared with 9 patients in the control group (30 patients) at three weeks (Yang 2010) (RR 4.67, 95% CI 1.57 to 13.87).
Another trial with specific data showed no significant difference in Karnofsky score (> 60 points) between the intervention group and the control group after the trial, and the comparison result was the following: 15 patients in the intervention group (41 patients) compared with 6 patients in the control group (31 patients) at 6 months (Li 2001) (RR 1.89, 95% CI 0.83 to 4.31).
Rate of short‐term remission
Of the seven individual trials, three trials showed a significant difference in the rate of short‐term remission between the intervention group and the control group after the trial, and the comparison result was the following: 3 patients in the intervention group (39 patients) compared with 13 patients in the control group (39 patients) at 8 to 12 weeks (Yang 2006) (RR 0.23, 95% CI 0.09 to 0.75); 21 patients in the intervention group (35 patients) compared with 10 patients in the control group (30 patients) at three weeks (Ye 2009) (RR 3.00, 95% CI 1.09 to 8.29); 21 patients in the intervention group (30 patients) compared with 10 patients in the control group (30 patients) at three weeks (Yang 2010) (RR 4.67, 95% CI 1.57 to 13.87).
Four trials with specific data showed no significant difference in the rate of short‐term remission between the intervention group and the control group after the trial, and the comparison result was the following:
16 patients in the intervention group (41 patients) compared with 5 patients in the control group (31 patients) at six months (Li 2001) (RR 2.42, 95% CI 0.99 to 5.89);
3 patients in the intervention group (24 patients) compared with 2 patients in the control group (12 patients) at two months (Zhou 2000) (RR 0.75, 95% CI 0.14 to 3.90);
5 patients in the intervention group (62 patients) compared with 6 patients in the control group (56 patients) at four months (You 2000) (RR 0.75, 95% CI 0.24 to 2.33);
21 patients in the intervention group (52 patients) compared with 16 patients in the control group (30 patients) at two months (Jiang 1994) (RR 0.76, 95% CI 0.47 to1.21).
Median survival time (MST)
Of the six individual trials, only two trials presented complete data, which showed a significant difference in the median survival time (MST) between the intervention group and the control group, and the comparison results were the following: the MST was 12.68 ± 8.36 months (41 patients) in the intervention group and 7.01 ± 5.32 months (31 patients) in the control group (Li 2001) (MD 5.67, 95% CI 2.50 to 8.84); the MST was 10.51 ± 2.06 months (39 patients) in the intervention group and 7.38 ± 3.24 months (39 patients) in the control group (Yang 2006) (MD 3.13, 95% CI 1.93 to 4.33).
Time to progression (TTP)
Of the six individual trials, no trial presented specific data on TTP so it could not be appraised.
Adverse events
Life threatening
No trial reported life threatening adverse events and no patient died of adverse events.
Toxic response
Of the seven individual trials, only one trial mentioned severe toxic response in the patients of the control group (You 2000) but it could not be appraised due to lack of specific data.
Leukopenia
One trial showed no significant difference in the leukocyte score: 6.1 ± 2.4 (30 patients) in the intervention group and 4.5 ± 2.4 months (30 patients) in the control group at three weeks (Yang 2010) (MD 1.60, 95% CI 0.39 to 2.81).
Thrombopenia
One trial showed no significant difference in the platelet score: 163.9 ± 51.2 (30 patients) in the intervention group and 143.4 ± 49.5 (30 patients) in the control group (Yang 2010) (MD 20.50, 95% CI ‐4.98 to 45.98).
Decrease of haemoglobin
One trial showed a significant difference in the haemoglobin score: 111.2 ± 17.1 (30 patients) in the intervention group and 101.2 ± 16.8 (30 patients) in the control group (Yang 2010) (MD 10.00, 95% CI 1.42 to 18.58).
Discontinuation of treatment
No trial reported discontinuation of treatment.
The outcomes with a statistically significant difference, from the 51 trials, are listed in the 'Additional tables' (Table 13).
9. The outcomes with statistically significant differences from the 53 trials.
| Type I (42 trials) | Type II (6 trials) | Type III (2 trials) | Type IV (7 trials) | Total number of trials | |
| Mortality | 6 | 2 | 1 | 9 | |
| Quality of life | 11 | 1 | 4 | 16 | |
| Rate of remission | 8 | 3 | 11 | ||
| Median survival time | 1 | 2 | 3 | ||
| Time to progression | |||||
| Result in the discontinuation of treatment | 2 | 1 | 3 | ||
| Adverse events (leukopenia) | 5 | 5 | |||
| Adverse events (Thrombopenia) | 4 | 4 | |||
| Adverse events (Decrease of haemoglobin) | 1 | 1 | |||
| Adverse events (nausea/vomiting) | 1 | 1 |
In the 57 trials, follow‐up (from 0.5 to 5 years) was carried out in 16 trials (38.1%, 16/42) in the type I group, three trials (50.0%, 3/6) in the type II group, two trials (100.0%, 2/2) in the type III group, and four trials (57.1%, 4/7) in the type IV group, respectively, but only 13 trials (52%, 13/25) specified the length of follow‐up. All the follow‐ups supported the conclusion that the TCMHs (with western medicine or not) had better long‐term effectiveness (TTP, MST) than the commonly used western medicines for chemotherapy. There were no reports of any toxic effects or side effects of TCMHs and, in fact, one of the curative effects of the TCMHs used in these trials was aimed at the toxic and side effects caused by the chemotherapy itself.
Discussion
Summary of main results
Although the recipes for TCM (including TCMHs) have screened some herbs, such as Radix Astragali seu Hedysari, Rhizoma Atractylodis Macrocephalae, Radix Codonopsis Pilosulae, Rhizoma Zedoariae, etc, the effectiveness of TCM for gastric cancer in an advanced or late stage still needs further confirmation by more trials.
Eighty trials with 6857 advanced or late‐stage gastric cancer patients were identified. Most trials were of low quality and used TCMHs plus chemotherapy compared with the same chemotherapy alone (65 trials). Others included TCMHs plus western therapeutic methods compared with the same TCMHs (type II, seven trials), TCMHs compared with another TCMH (type III, two trials), and TCMHs compared with western therapeutic methods (type IV, six trials). Except for four trials of Huachansu, six trials of Aidi, seven trials of Fufangkushen, three trials of Shenqifuzheng, we could not pool the other 57 results because no more than two used the same intervention or outcomes.
TCMHs with or without chemotherapy in the 57 trials showed a statistically significant difference for the improvement of mortality in nine trials, quality of life in 16 trials, rate of remission in 11 trials, discontinuation from treatment in three trials, leukopenia in five trials, and vomiting and nausea in one trial. The pooled results from the four injections of TCMHs, Huachansu, Aidi, Fufangkushen, and Shenqifuzheng, showed statistically significant differences for improvement of leukopenia; and Huachansu, Aidi, and Fufangkushen for adverse events in the digestive system; but no significant difference in the rate of short‐term remission.
A standard, generally accepted formula of TCM should be used for gastric cancer in an advanced or late stage in large‐scale, randomised, double blind and multi‐centre trials in China.
Overall completeness and applicability of evidence
Although the results, including those of the meta‐analysis and description, implied that TCMHs may have some curative effects for advanced or late‐stage gastric cancer, obtaining more TTP or MST and fewer toxic and side effects than the commonly used western medicine for chemotherapy mentioned above, it is still too early to draw the conclusion that the TCMHs have definitive curative effects for advanced or late‐stage gastric cancer because of the methodological limitations of the data from the trials. Information on allocation concealment, side effects, adverse events, and follow‐up have not been provided adequately for research. All of the primary data should be offered for further analyses, regardless of the positive or negative conclusions of the clinical trials.
Quality of the evidence
The RCTs should be designed strictly, for example to use the same medicine, same dosage, same course in the controlled group; and when including patients with different levels of illness (stage III or IV) the trial should use stratified randomisation. At the same time, the method of allocation concealment for clinical trials should be clarified by the authors. In the 80 included studies, only seven trials (Chen 2009; Jiang 1994; Liu 2009; Wang 1998; Wang 2004; Zhang 2008; Zhang 2010) mentioned that they used a random number table (or drawing straws, envelope concealment), without any special explanation. An urgent mission in the TCM field for gastric cancer is to change the current reporting practices of the study authors. Even if the method of allocation concealment is clear to some, it should be elucidated completely in the article for the purpose of further analysis.
Potential biases in the review process
The journal editors responsible for publication should ask the authors to include information about the methods of the trials and authors should not be permitted to omit these contents. Details of follow‐up and adverse events should also be provided, regardless of whether they occurred or not.
It should be noted that although meta‐analysis could not be carried out for most of the trials on Huachansu, because most of the trials were not strictly RCTs, the TCMH Huachansu has been used widely for many years in China. The main components of Huachansu include extracts from the skin of the Chinese toad.
Agreements and disagreements with other studies or reviews
Because the classical therapeutic regimen for advanced or late‐stage gastric cancer is chemotherapy, and almost all of the researchers in TCM circles are aware of this, choosing it as the active comparator in the control group makes the efficacy of TCHMs more convincing than if they had been compared with placebo. The trials were all designed as equivalence trials and no placebo controlled trials were carried out. Some researchers believe their self‐made formulas of TCMHs for gastric cancer in an advanced or late stage to be more effective than some patented TCMHs, so in the type II group some commonly used patented medicines of TCMHs were chosen as the active comparators in the control group. Other relevant reviews about TCMHs for breast cancer (Zhang 2007), TCMHs for colorectal cancer (Wu 2008), and TCMHs for lung cancer (Rui 2008) all showed that TCMHs may alleviate the chemotherapy‐related side effects or adverse events, but the evidence is too limited to make any confident conclusions. More high quality studies are needed.
Authors' conclusions
Implications for practice.
This review did not provide assured evidence concerning the effectiveness of TCMHs in improving the quality of life or rate of remission, alleviating the toxic and side effects caused by the chemotherapy, delaying the time to progression, prolonging the median survival times, or reducing short‐term mortality. Limited and weak evidence showed that Huachansu, Aidi, Fufangkushen, and Shenqifuzheng, when used together with chemotherapy, improved leukopenia; and Huachansu, Aidi, and Fufangkushen also improved the adverse events in the digestive system caused by chemotherapy but did not improve the rate of short‐term remission (complete remission or partial remission). Limitations were due to most of the included studies being of low quality and the scarcity of valid samples. Large, well designed clinical trials are required urgently before any confident conclusions can be drawn about the value of TCMHs for advanced or late‐stage gastric cancer.
At present, the general evidence is insufficient to suggest that clinical practice should be changed on the basis of these results.
Implications for research.
A standardised formula of TCMHs should be developed and used for all trials of advanced or late‐stage gastric cancer in China. Data from large, randomised, double blind, multi‐centre trials are required to confirm the benefit and safety of TCMHs for treatment of advanced or late‐stage gastric cancer. These trials should use standardised outcome measures for efficacy and should include an assessment of adverse events. All the trials should provide adequate follow‐up periods so that long‐term efficacy and safety can be assessed.
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2011 | New search has been performed | Twenty‐three new trials were identified and incorporated into the meta‐analyses. |
| 8 October 2011 | New citation required but conclusions have not changed | TCMHs combined with or without chemotherapy in the 57 trials showed statistically significant difference for the improvement of mortality in 9 trials, quality of life in 16 trials, rate of remission in 11 trials, leukopenia in 5 trials. The pooled results from the four injections of TCMHs, Huachansu, Aidi, Fufangkushen, Shenqifuzheng showed statistically significant difference for the improvement of leukopenia, and Huachansu, Aidi, Fufangkushen for adverse events in the digestive system, but no significant difference of the rate of short‐term remission. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 4 January 2011 | Amended | Review withdrawn |
| 21 September 2010 | Amended | Contact details updated. |
| 30 September 2008 | Amended | Converted to new review format. |
Notes
The review was withdrawn in January 2011 due to an outdated literature search.
Acknowledgements
We thank Janet Lilleyman, Cathy Bennett and Karin Dearness, Coordinators of the Cochrane UGPD Group, for advice on writing and revising this protocol, and thank the Danish Cancer Society for funding this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Phytotherapy explode all trees in MeSH #2 MeSH descriptor Drugs, Chinese Herbal explode all trees in MeSH #3 MeSH descriptor Medicine, Herbal explode all trees in MeSH #4 MeSH descriptor Plants, Medicinal explode all trees in MeSH #5 MeSH descriptor Medicine, Traditional explode all trees #6 ((traditional or chinese or oriental or alternative or complementary) and medicine*) or herb* or plant* ? #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 MeSH descriptor Gastric cancer explode all trees #9 Gastric cancer #10 (#8 OR #9) #11 (#7 AND #10)
Appendix 2. MEDLINE search strategy
1. exp Medicine, Traditional/ 2. exp Drugs, Chinese Herbal/ 3. exp Medicine, Herbal/ 4. exp Phytotherapy/ 5. exp Plants, Medicinal/ 6. (((traditional or chinese or oriental or alternative or complementary) and medicine*) or herb* or plant*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Gastric cancer/ 9. gastric cancer.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 10. 8 or 9 11. 7 and 10 12. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 13. 11 and 12
Appendix 3. EMBASE search strategy
1. exp Traditional Medicine/ 2. exp Medicinal Plant/ 3. Plant Medicinal Product/ 4. exp Phytotherapy/ 5. (((traditional or chinese or oriental or alternative or complementary) and medicine*) or herb* or plant*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. 1 or 2 or 3 or 4 or 5 7. exp Gastric cancer/ 8. Gastric cancer.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 9. 8 or 7 10. 6 and 9 11. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 12. 11 and 10
Appendix 4. Chinese Bio‐Medicine Database
(((traditional OR chinese OR oriental OR alternative OR complementary) AND medicine*) OR herb* OR plant*) AND (Gastric cancer OR Gastric carcinoma OR Gastric tumour)
Data and analyses
Comparison 1. Appraisal of the results of Huachansu in the short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 the rate of complete remission and partly remission | 7 | 448 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.17] |
| 2 the toxic and side effects in digestive system after chemotherapy (no special data in trial of Zhang 2006) | 6 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 3 the toxic and side effects of leukopenia after chemotherapy(no special data in trial of Zhang 2006) | 6 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.21, 0.50] |
1.1. Analysis.
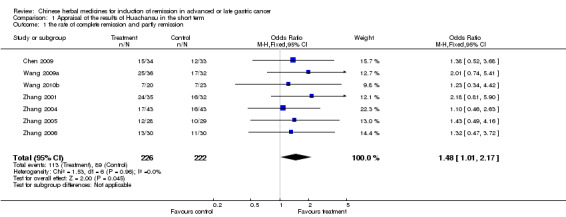
Comparison 1 Appraisal of the results of Huachansu in the short term, Outcome 1 the rate of complete remission and partly remission.
1.2. Analysis.
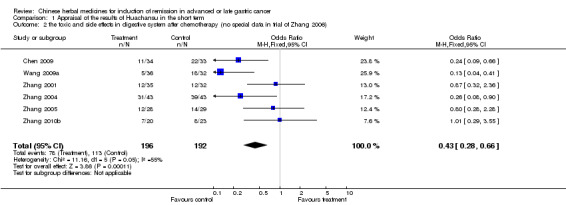
Comparison 1 Appraisal of the results of Huachansu in the short term, Outcome 2 the toxic and side effects in digestive system after chemotherapy (no special data in trial of Zhang 2006).
1.3. Analysis.
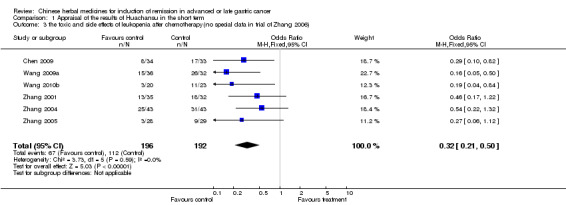
Comparison 1 Appraisal of the results of Huachansu in the short term, Outcome 3 the toxic and side effects of leukopenia after chemotherapy(no special data in trial of Zhang 2006).
Comparison 2. Appraisal of the results of Aidi in the short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 the rate of complete remission and partly remission(no special data in trial of Zhang 2009) | 5 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.94, 2.41] |
| 2 the toxic and side effects in digestive system after chemotherapy | 6 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.20, 0.54] |
| 3 the toxic and side effects of leukopenia after chemotherapy(no special data in trial of Jia 2003, Zhang 2009) | 4 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.23, 0.80] |
2.1. Analysis.
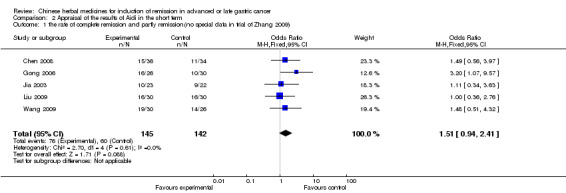
Comparison 2 Appraisal of the results of Aidi in the short term, Outcome 1 the rate of complete remission and partly remission(no special data in trial of Zhang 2009).
2.2. Analysis.
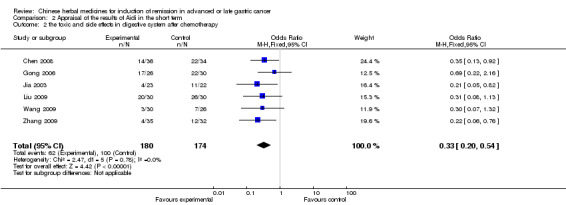
Comparison 2 Appraisal of the results of Aidi in the short term, Outcome 2 the toxic and side effects in digestive system after chemotherapy.
2.3. Analysis.
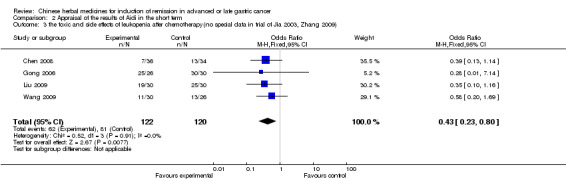
Comparison 2 Appraisal of the results of Aidi in the short term, Outcome 3 the toxic and side effects of leukopenia after chemotherapy(no special data in trial of Jia 2003, Zhang 2009).
Comparison 3. Appraisal of the results of Fufangkushen in the short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 the rate of complete remission and partly remission(no special data in trial of Fu 2011) | 6 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.83, 1.79] |
| 2 the toxic and side effects in digestive system after chemotherapy(no special data in trial of Fu 2011, Liu 2009a, Zhang 2010) | 4 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.26, 0.69] |
| 3 the toxic and side effects of leukopenia after chemotherapy | 7 | 503 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.25, 0.56] |
3.1. Analysis.
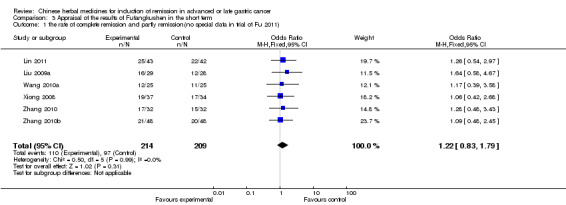
Comparison 3 Appraisal of the results of Fufangkushen in the short term, Outcome 1 the rate of complete remission and partly remission(no special data in trial of Fu 2011).
3.2. Analysis.
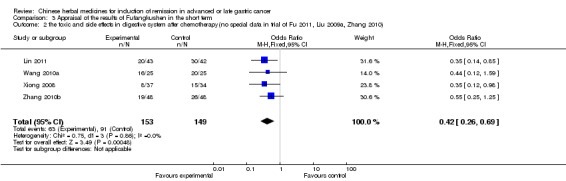
Comparison 3 Appraisal of the results of Fufangkushen in the short term, Outcome 2 the toxic and side effects in digestive system after chemotherapy(no special data in trial of Fu 2011, Liu 2009a, Zhang 2010).
3.3. Analysis.
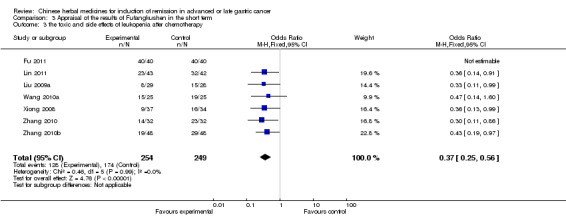
Comparison 3 Appraisal of the results of Fufangkushen in the short term, Outcome 3 the toxic and side effects of leukopenia after chemotherapy.
Comparison 4. Appraisal of the results of Shenqifuzheng in the short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 the rate of complete remission and partly remission | 3 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.78, 2.81] |
| 2 the toxic and side effects in digestive system after chemotherapy | 3 | 153 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.18, 7.24] |
| 3 the toxic and side effects of leukopenia after chemotherapy | 3 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.74] |
4.1. Analysis.
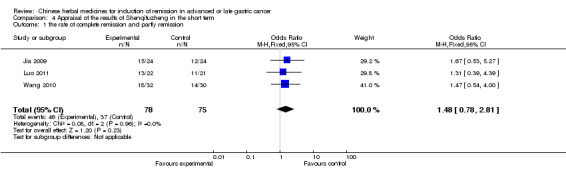
Comparison 4 Appraisal of the results of Shenqifuzheng in the short term, Outcome 1 the rate of complete remission and partly remission.
4.2. Analysis.
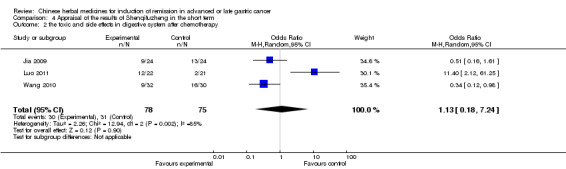
Comparison 4 Appraisal of the results of Shenqifuzheng in the short term, Outcome 2 the toxic and side effects in digestive system after chemotherapy.
4.3. Analysis.
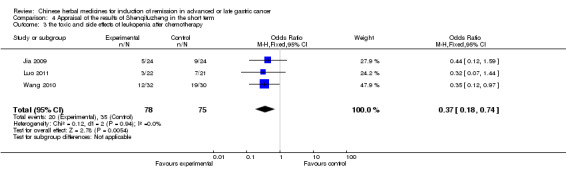
Comparison 4 Appraisal of the results of Shenqifuzheng in the short term, Outcome 3 the toxic and side effects of leukopenia after chemotherapy.
Comparison 5. Appraisal of the results of type I.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 mortality 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 mortality 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 mortality 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 mortality 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 mortality 6‐2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 mortality 6‐1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 mortality 6‐3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 mortality 7‐1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 mortality 7‐2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 mortality 7‐3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 mortaliyt 8‐1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 mortality 8‐2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 mortality 8‐3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 quality of life 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 qualitiy of life 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 quality of life 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17 quality of life 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18 quality of life 6 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19 quality of life 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20 quality of life 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21 quality of life 9 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22 quality of life 10 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23 quality of life 11 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24 quality of life 12 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25 quality of life 13 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26 quality of life 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 27 quality of life 15 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 28 quality of life 16 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 29 quality of life 17 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 30 quality of life 18 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 31 quality of life 19 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 32 quality of life 20 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 5.5 [0.43, 10.57] |
| 33 quality of life 21 | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.48, 4.89] |
| 34 quality of life 22 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 5.39 [2.27, 8.51] |
| 35 quality of life 23 | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 14.07 [12.09, 16.05] |
| 36 rate of remission 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 37 rate of remission 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 38 rate of remission 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 39 rate of remission 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 40 rate of remission 6 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 41 rete of remission 8 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 42 rate of remission 9 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 43 rate of remission 10 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 44 rate of remission 11 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 45 rate of remission 12 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 46 rate of remission 13 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 47 rate of remission 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 48 rate of remission 15 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 49 rate of remission 16 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 50 rate of remission 17 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 51 rate of remission 18 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 52 rate of remission 19 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 53 rate of remission 20 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 54 rate of remission 21 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 55 rate of remission 22 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 56 rate of remission 23 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 57 rate of remission 24 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 58 rate of remission 25 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 59 rate of remission 26 | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.37, 3.95] |
| 60 rate of remission 27 | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.41, 4.06] |
| 61 rate of remission 28 | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.47, 3.61] |
| 62 rate of remission 29 | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.47, 5.19] |
| 63 rate of remission 30 | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.50, 3.02] |
| 64 median survival times 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 65 leukopenia 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 66 leukopenia 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 67 leukopenia 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 68 leukopenia 5 | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.18, 0.78] |
| 69 leukopenia 6 | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.94] |
| 70 leukopenia 7 | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.59] |
| 71 leukopenia 8 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [1.53, 2.47] |
| 72 nausea/vomiting 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 73 nausea/vomiting 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 74 nausea/vomiting 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 75 nausea/vomiting 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 76 nausea/vomiting 6 | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.57] |
| 77 nausea/vomiting 7 | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.00] |
| 78 nausea/vomiting 8 | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 0.99] |
| 79 thrombopenia 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 80 thrombopenia 2 | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.91] |
| 81 thrombopenia 3 | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.14, 0.44] |
| 82 thrombopenia 4 | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.06, 0.94] |
| 83 thrombopenia 5 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 13.27 [1.53, 25.01] |
| 84 diarrhea 1 | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.11, 1.19] |
| 85 decrease of hemoglobin 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 86 decrease of hemoglobin 2 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 15.87 [3.40, 28.34] |
| 87 decrease of hemoglobin 3 | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [0.23, 0.77] |
| 88 damage of liver and/or kidney function 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 89 damage of liver and/or kidney function 2 | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.05, 1.55] |
| 90 damage of liver and/or kidney function 3 | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.03, 1.01] |
| 91 damage of liver and/or kidney function 4 | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.13, 1.00] |
| 92 discontinuation due to adverse event 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 93 discontinuation due to adverse event 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 94 discontinuation due to adverse event 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 95 discontinuation due to adverse event 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.2. Analysis.
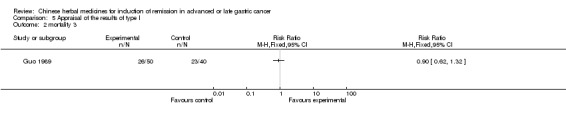
Comparison 5 Appraisal of the results of type I, Outcome 2 mortality 3.
5.17. Analysis.
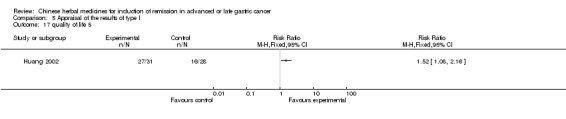
Comparison 5 Appraisal of the results of type I, Outcome 17 quality of life 5.
5.36. Analysis.
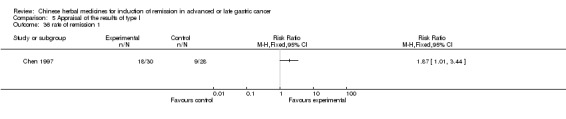
Comparison 5 Appraisal of the results of type I, Outcome 36 rate of remission 1.
5.40. Analysis.
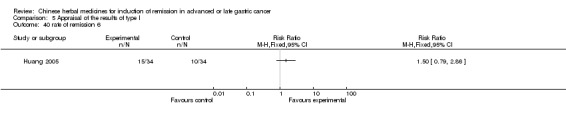
Comparison 5 Appraisal of the results of type I, Outcome 40 rate of remission 6.
5.54. Analysis.
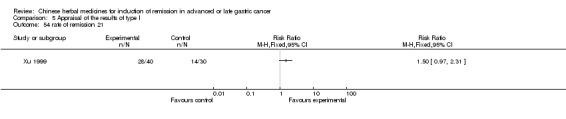
Comparison 5 Appraisal of the results of type I, Outcome 54 rate of remission 21.
5.60. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 60 rate of remission 27.
5.64. Analysis.
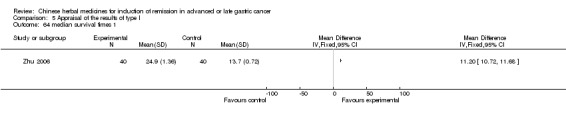
Comparison 5 Appraisal of the results of type I, Outcome 64 median survival times 1.
5.71. Analysis.
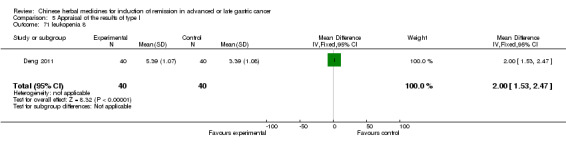
Comparison 5 Appraisal of the results of type I, Outcome 71 leukopenia 8.
5.72. Analysis.
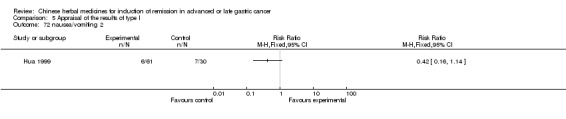
Comparison 5 Appraisal of the results of type I, Outcome 72 nausea/vomiting 2.
5.73. Analysis.
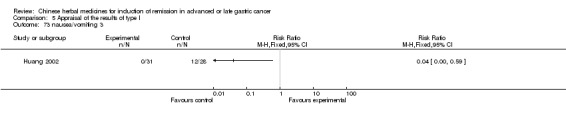
Comparison 5 Appraisal of the results of type I, Outcome 73 nausea/vomiting 3.
5.80. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 80 thrombopenia 2.
5.81. Analysis.
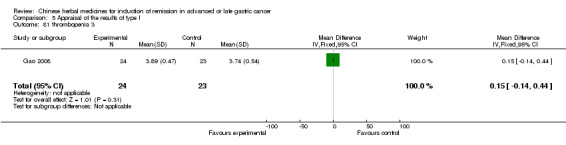
Comparison 5 Appraisal of the results of type I, Outcome 81 thrombopenia 3.
5.82. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 82 thrombopenia 4.
5.83. Analysis.
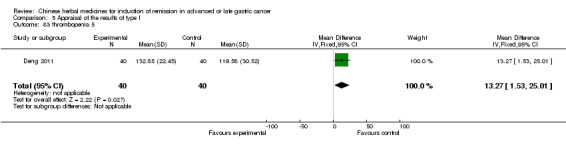
Comparison 5 Appraisal of the results of type I, Outcome 83 thrombopenia 5.
5.84. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 84 diarrhea 1.
5.85. Analysis.
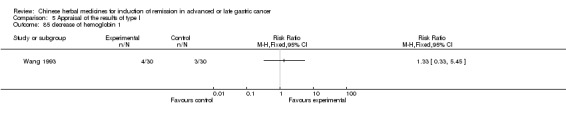
Comparison 5 Appraisal of the results of type I, Outcome 85 decrease of hemoglobin 1.
5.86. Analysis.
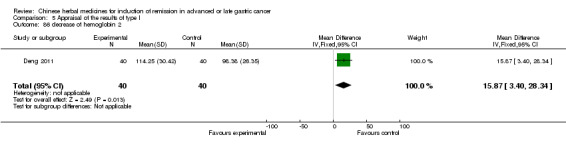
Comparison 5 Appraisal of the results of type I, Outcome 86 decrease of hemoglobin 2.
5.87. Analysis.
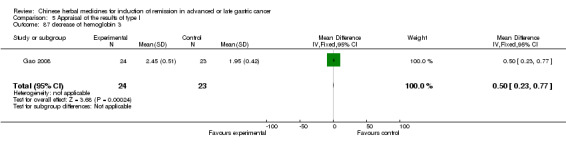
Comparison 5 Appraisal of the results of type I, Outcome 87 decrease of hemoglobin 3.
5.88. Analysis.
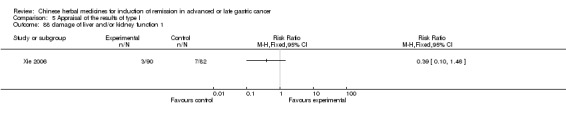
Comparison 5 Appraisal of the results of type I, Outcome 88 damage of liver and/or kidney function 1.
5.89. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 89 damage of liver and/or kidney function 2.
5.90. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 90 damage of liver and/or kidney function 3.
5.91. Analysis.

Comparison 5 Appraisal of the results of type I, Outcome 91 damage of liver and/or kidney function 4.
5.92. Analysis.
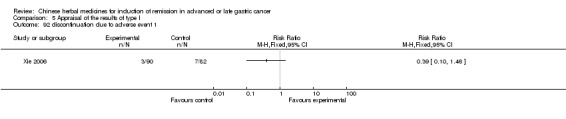
Comparison 5 Appraisal of the results of type I, Outcome 92 discontinuation due to adverse event 1.
5.93. Analysis.
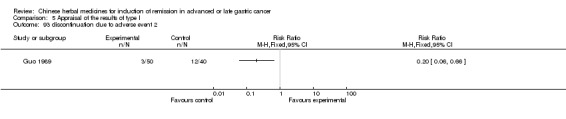
Comparison 5 Appraisal of the results of type I, Outcome 93 discontinuation due to adverse event 2.
5.94. Analysis.
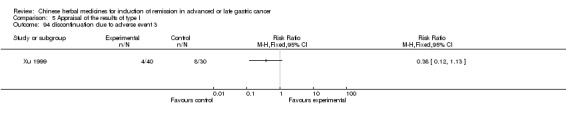
Comparison 5 Appraisal of the results of type I, Outcome 94 discontinuation due to adverse event 3.
5.95. Analysis.
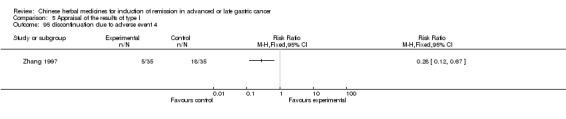
Comparison 5 Appraisal of the results of type I, Outcome 95 discontinuation due to adverse event 4.
Comparison 6. Appraisal of the results of type II.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 mortality 1.1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 mortality 1.2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 mortality 1.3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 mortality 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 mortality 3.1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 mortality 3.2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 quality of life 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 rate of remission 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 rate of remission 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 rate of remission 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 nausea/vomiting 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 arrest of bone marrow | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 discontinuation due to adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Appraisal of the results of type III.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 quality of life | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Appraisal of the results of type IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 mortality 1.1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 mortality 1.2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 mortality 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 mortality 3.1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 mortality 3.2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 mortality 3.3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 mortality 3.4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 mortality 3.5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 mortality 4.1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 mortality 4.2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 mortality 4.3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 mortality 5.1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 mortality 5.2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 mortality 5.3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 mortality 5.4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 mortality 5.5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17 quality of life 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18 quality of life 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19 quality of life 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20 quality of life 4 | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.11 [1.11, 8.70] |
| 21 quality of life 5 | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.67 [1.57, 13.87] |
| 22 rate of remission 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23 rate of remission 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24 rate of remission 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25 rate of remission 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26 rate of remission 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 27 rate of remission 6 | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.09, 8.29] |
| 28 rate of remission 7 | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.67 [1.57, 13.87] |
| 29 Leukopenia 1 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [0.39, 2.81] |
| 30 Thrombopenia 1 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 20.5 [‐4.98, 45.98] |
| 31 Decrease of haemoglobin 1 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [1.42, 18.58] |
| 32 median survival time 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 33 median survival time 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
8.4. Analysis.
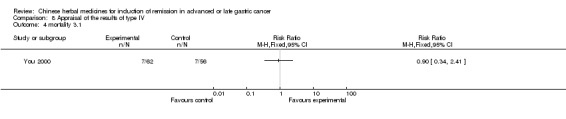
Comparison 8 Appraisal of the results of type IV, Outcome 4 mortality 3.1.
8.5. Analysis.
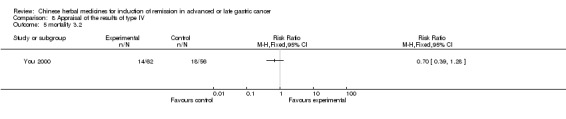
Comparison 8 Appraisal of the results of type IV, Outcome 5 mortality 3.2.
8.6. Analysis.
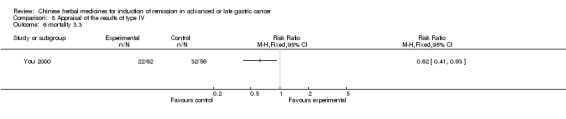
Comparison 8 Appraisal of the results of type IV, Outcome 6 mortality 3.3.
8.7. Analysis.
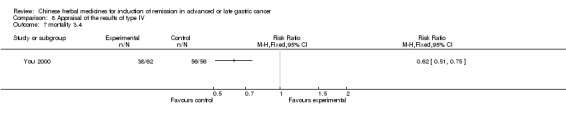
Comparison 8 Appraisal of the results of type IV, Outcome 7 mortality 3.4.
8.8. Analysis.
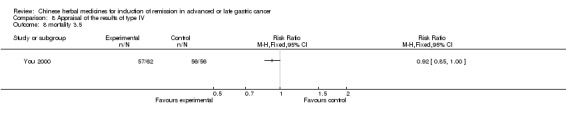
Comparison 8 Appraisal of the results of type IV, Outcome 8 mortality 3.5.
8.9. Analysis.
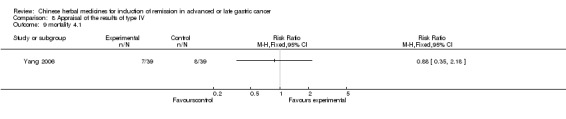
Comparison 8 Appraisal of the results of type IV, Outcome 9 mortality 4.1.
8.10. Analysis.
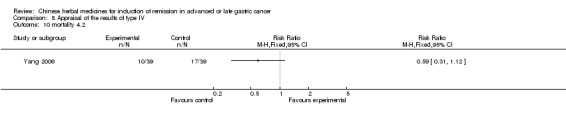
Comparison 8 Appraisal of the results of type IV, Outcome 10 mortality 4.2.
8.11. Analysis.
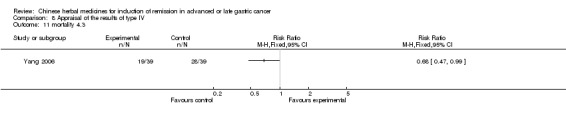
Comparison 8 Appraisal of the results of type IV, Outcome 11 mortality 4.3.
8.12. Analysis.
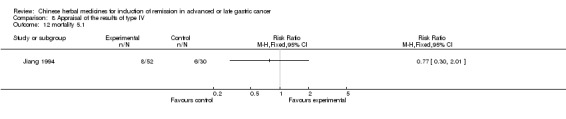
Comparison 8 Appraisal of the results of type IV, Outcome 12 mortality 5.1.
8.13. Analysis.
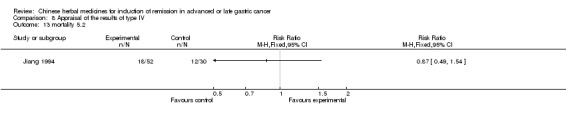
Comparison 8 Appraisal of the results of type IV, Outcome 13 mortality 5.2.
8.14. Analysis.
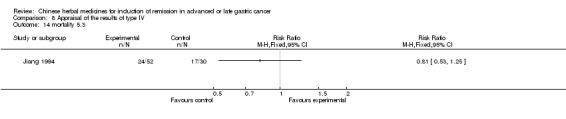
Comparison 8 Appraisal of the results of type IV, Outcome 14 mortality 5.3.
8.15. Analysis.
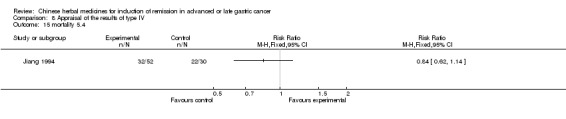
Comparison 8 Appraisal of the results of type IV, Outcome 15 mortality 5.4.
8.16. Analysis.
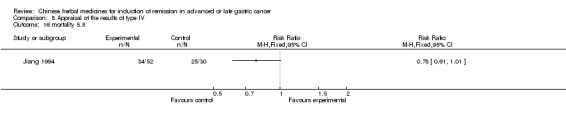
Comparison 8 Appraisal of the results of type IV, Outcome 16 mortality 5.5.
8.17. Analysis.
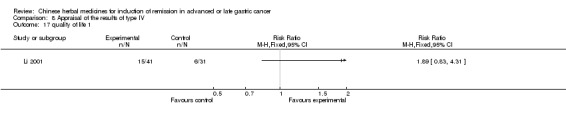
Comparison 8 Appraisal of the results of type IV, Outcome 17 quality of life 1.
8.18. Analysis.
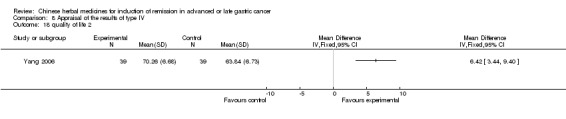
Comparison 8 Appraisal of the results of type IV, Outcome 18 quality of life 2.
8.19. Analysis.
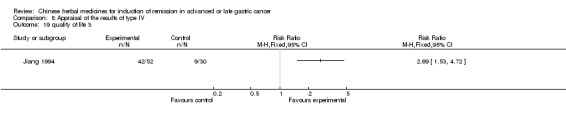
Comparison 8 Appraisal of the results of type IV, Outcome 19 quality of life 3.
8.20. Analysis.

Comparison 8 Appraisal of the results of type IV, Outcome 20 quality of life 4.
8.21. Analysis.

Comparison 8 Appraisal of the results of type IV, Outcome 21 quality of life 5.
8.22. Analysis.
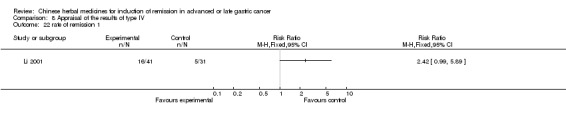
Comparison 8 Appraisal of the results of type IV, Outcome 22 rate of remission 1.
8.23. Analysis.
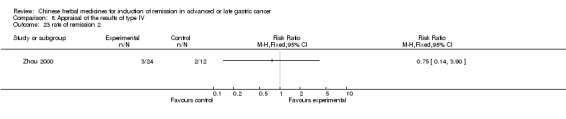
Comparison 8 Appraisal of the results of type IV, Outcome 23 rate of remission 2.
8.24. Analysis.
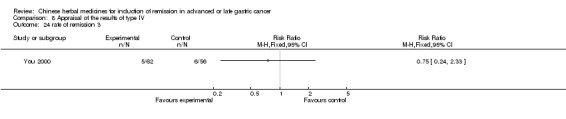
Comparison 8 Appraisal of the results of type IV, Outcome 24 rate of remission 3.
8.25. Analysis.
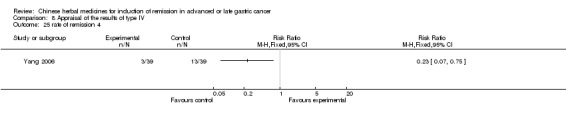
Comparison 8 Appraisal of the results of type IV, Outcome 25 rate of remission 4.
8.26. Analysis.
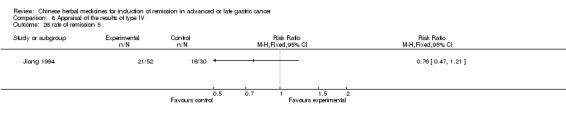
Comparison 8 Appraisal of the results of type IV, Outcome 26 rate of remission 5.
8.27. Analysis.

Comparison 8 Appraisal of the results of type IV, Outcome 27 rate of remission 6.
8.28. Analysis.

Comparison 8 Appraisal of the results of type IV, Outcome 28 rate of remission 7.
8.29. Analysis.
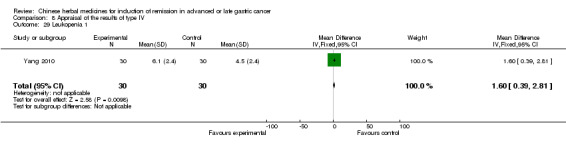
Comparison 8 Appraisal of the results of type IV, Outcome 29 Leukopenia 1.
8.30. Analysis.
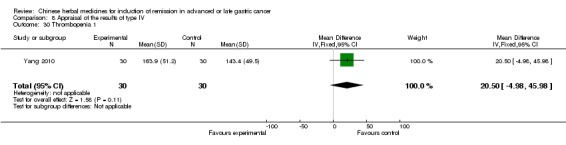
Comparison 8 Appraisal of the results of type IV, Outcome 30 Thrombopenia 1.
8.31. Analysis.
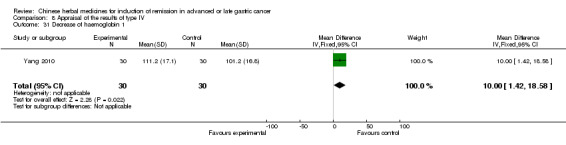
Comparison 8 Appraisal of the results of type IV, Outcome 31 Decrease of haemoglobin 1.
8.32. Analysis.
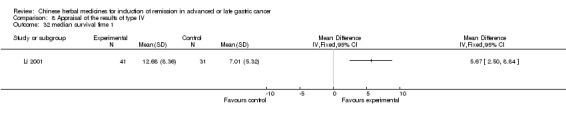
Comparison 8 Appraisal of the results of type IV, Outcome 32 median survival time 1.
8.33. Analysis.
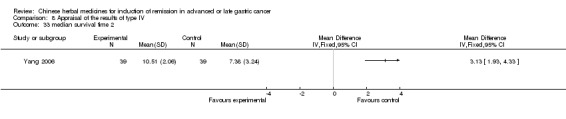
Comparison 8 Appraisal of the results of type IV, Outcome 33 median survival time 2.
Comparison 9. Sensitivity analyses for Huachansu.
9.1. Analysis.
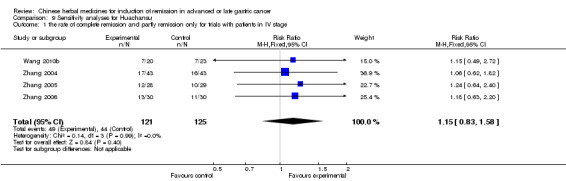
Comparison 9 Sensitivity analyses for Huachansu, Outcome 1 the rate of complete remission and partly remission only for trials with patients in IV stage.
9.2. Analysis.
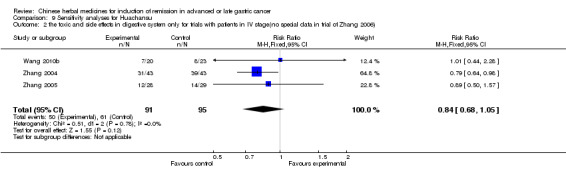
Comparison 9 Sensitivity analyses for Huachansu, Outcome 2 the toxic and side effects in digestive system only for trials with patients in IV stage(no special data in trial of Zhang 2006).
9.3. Analysis.
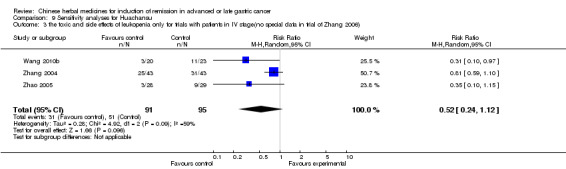
Comparison 9 Sensitivity analyses for Huachansu, Outcome 3 the toxic and side effects of leukopenia only for trials with patients in IV stage(no special data in trial of Zhang 2006).
9.4. Analysis.
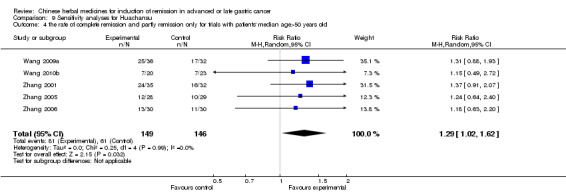
Comparison 9 Sensitivity analyses for Huachansu, Outcome 4 the rate of complete remission and partly remission only for trials with patients' median age>50 years old.
9.5. Analysis.
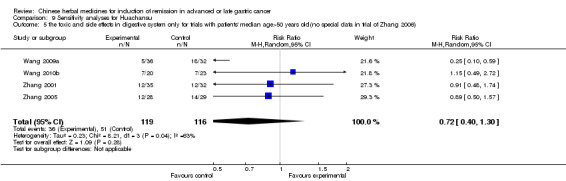
Comparison 9 Sensitivity analyses for Huachansu, Outcome 5 the toxic and side effects in digestive system only for trials with patients' median age>50 years old(no special data in trial of Zhang 2006).
9.6. Analysis.
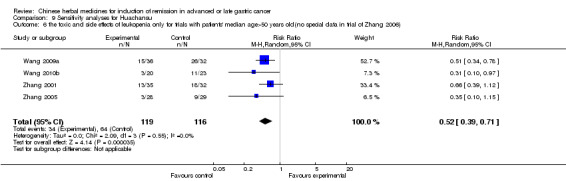
Comparison 9 Sensitivity analyses for Huachansu, Outcome 6 the toxic and side effects of leukopenia only for trials with patients' median age>50 years old(no special data in trial of Zhang 2006).
9.7. Analysis.
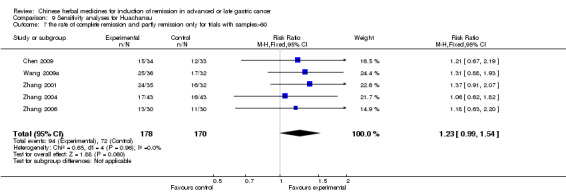
Comparison 9 Sensitivity analyses for Huachansu, Outcome 7 the rate of complete remission and partly remission only for trials with samples>60.
9.8. Analysis.
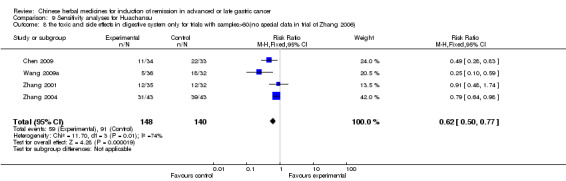
Comparison 9 Sensitivity analyses for Huachansu, Outcome 8 the toxic and side effects in digestive system only for trials with samples>60(no special data in trial of Zhang 2006).
9.9. Analysis.
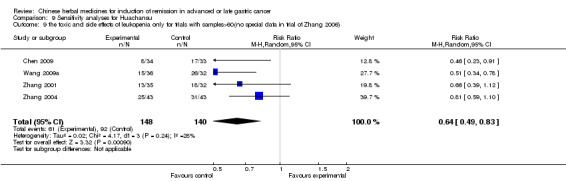
Comparison 9 Sensitivity analyses for Huachansu, Outcome 9 the toxic and side effects of leukopenia only for trials with samples>60(no special data in trial of Zhang 2006).
9.10. Analysis.
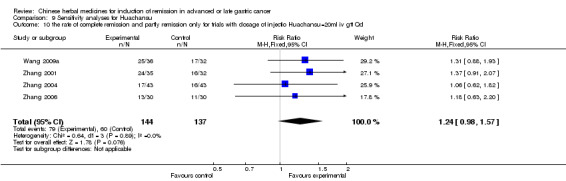
Comparison 9 Sensitivity analyses for Huachansu, Outcome 10 the rate of complete remission and partly remission only for trials with dosage of injectio Huachansu=20ml iv gtt Qd.
9.11. Analysis.
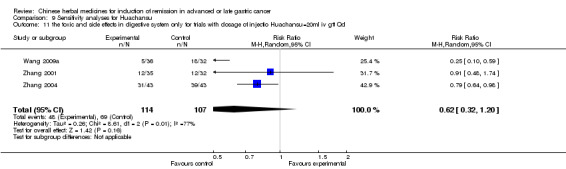
Comparison 9 Sensitivity analyses for Huachansu, Outcome 11 the toxic and side effects in digestive system only for trials with dosage of injectio Huachansu=20ml iv gtt Qd.
9.12. Analysis.
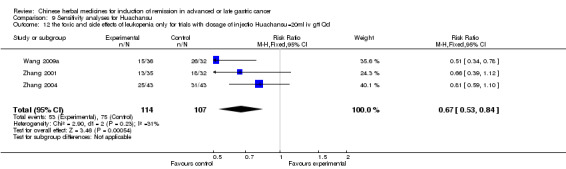
Comparison 9 Sensitivity analyses for Huachansu, Outcome 12 the toxic and side effects of leukopenia only for trials with dosage of injectio Huachansu=20ml iv gtt Qd.
Comparison 10. Sensitivity analyses for Aidi.
10.1. Analysis.
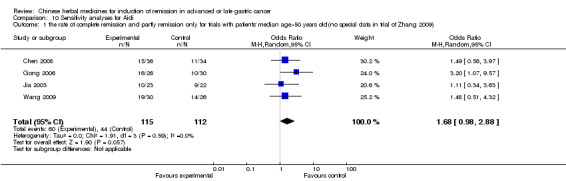
Comparison 10 Sensitivity analyses for Aidi, Outcome 1 the rate of complete remission and partly remission only for trials with patients' median age>50 years old(no special data in trial of Zhang 2009).
10.2. Analysis.
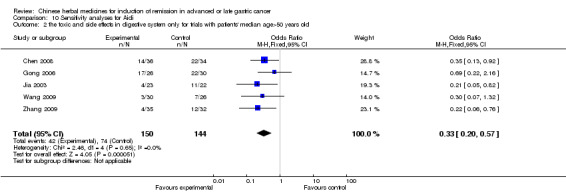
Comparison 10 Sensitivity analyses for Aidi, Outcome 2 the toxic and side effects in digestive system only for trials with patients' median age>50 years old.
10.3. Analysis.
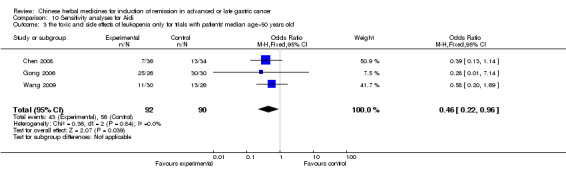
Comparison 10 Sensitivity analyses for Aidi, Outcome 3 the toxic and side effects of leukopenia only for trials with patients' median age>50 years old.
10.4. Analysis.
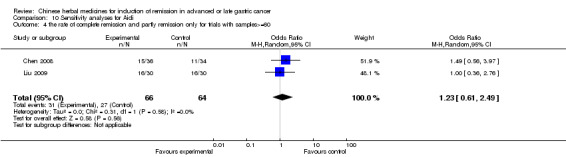
Comparison 10 Sensitivity analyses for Aidi, Outcome 4 the rate of complete remission and partly remission only for trials with samples>=60.
10.5. Analysis.
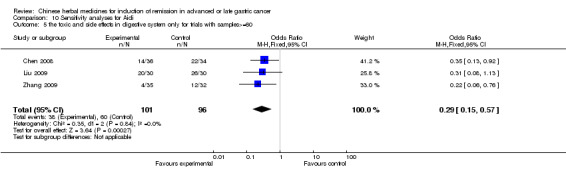
Comparison 10 Sensitivity analyses for Aidi, Outcome 5 the toxic and side effects in digestive system only for trials with samples>=60.
10.6. Analysis.
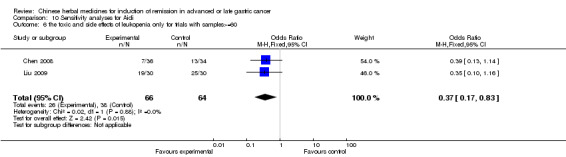
Comparison 10 Sensitivity analyses for Aidi, Outcome 6 the toxic and side effects of leukopenia only for trials with samples>=60.
10.7. Analysis.
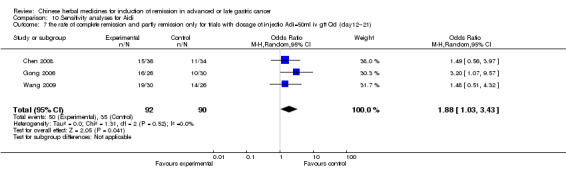
Comparison 10 Sensitivity analyses for Aidi, Outcome 7 the rate of complete remission and partly remission only for trials with dosage of injectio Adi=50ml iv gtt Qd (day12˜21).
10.8. Analysis.
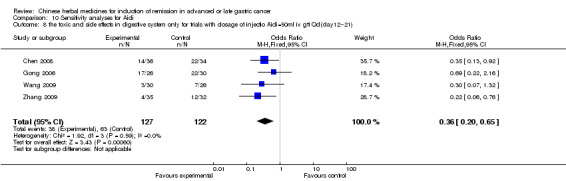
Comparison 10 Sensitivity analyses for Aidi, Outcome 8 the toxic and side effects in digestive system only for trials with dosage of injectio Aidi=50ml iv gtt Qd(day12˜21).
10.9. Analysis.
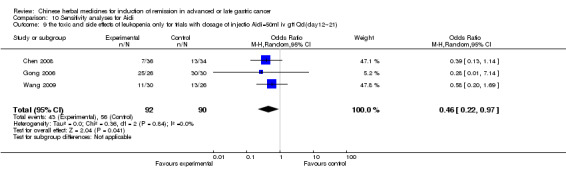
Comparison 10 Sensitivity analyses for Aidi, Outcome 9 the toxic and side effects of leukopenia only for trials with dosage of injectio Aidi=50ml iv gtt Qd(day12˜21).
Comparison 11. Sensitivity analyses for Fufangkushen.
11.1. Analysis.

Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 1 the rate of complete remission and partly remission only for trials with patients in IV stage.
11.2. Analysis.

Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 2 the toxic and side effects in digestive system only for trials with patients in IV stage.
11.3. Analysis.

Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 3 the toxic and side effects of leukopenia only for trials with patients in IV stage.
11.4. Analysis.
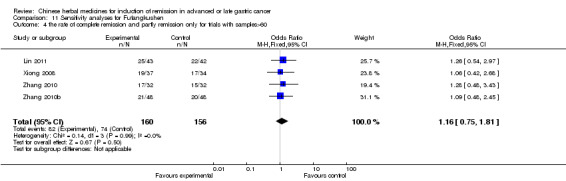
Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 4 the rate of complete remission and partly remission only for trials with samples>60.
11.5. Analysis.
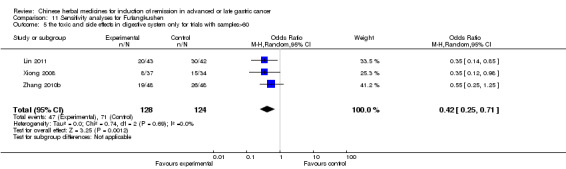
Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 5 the toxic and side effects in digestive system only for trials with samples>60.
11.6. Analysis.
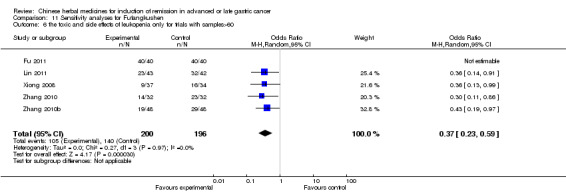
Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 6 the toxic and side effects of leukopenia only for trials with samples>60.
11.7. Analysis.
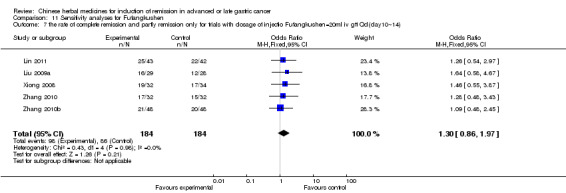
Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 7 the rate of complete remission and partly remission only for trials with dosage of injectio Fufangkushen=20ml iv gtt Qd(day10˜14).
11.8. Analysis.
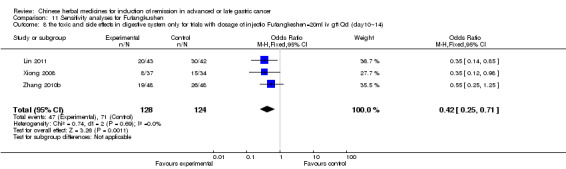
Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 8 the toxic and side effects in digestive system only for trials with dosage of injectio Fufangkeshen=20ml iv gtt Qd (day10˜14).
11.9. Analysis.
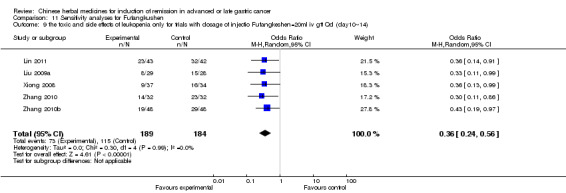
Comparison 11 Sensitivity analyses for Fufangkushen, Outcome 9 the toxic and side effects of leukopenia only for trials with dosage of injectio Fufangkeshen=20ml iv gtt Qd (day10˜14).
Comparison 12. Sensitivity analyses for Shenqifuzheng.
12.1. Analysis.

Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 1 the rate of complete remission and partly remission only for trials with patients in IV stage.
12.2. Analysis.

Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 2 the toxic and side effects in digestive system only for trials with patients in IV stage.
12.3. Analysis.

Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 3 the toxic and side effects of leukopenia only for trials with patients in IV stage.
12.4. Analysis.
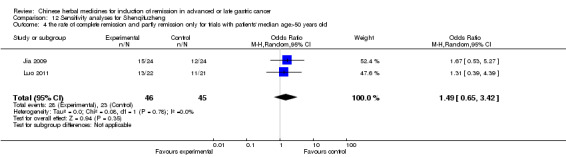
Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 4 the rate of complete remission and partly remission only for trials with patients' median age>50 years old.
12.5. Analysis.
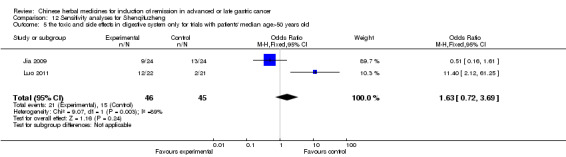
Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 5 the toxic and side effects in digestive system only for trials with patients' median age>50 years old.
12.6. Analysis.
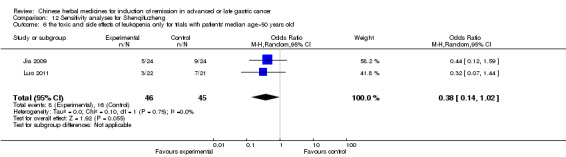
Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 6 the toxic and side effects of leukopenia only for trials with patients' median age>50 years old.
12.7. Analysis.

Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 7 the rate of complete remission and partly remission only for trials with samples>60.
12.8. Analysis.

Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 8 the toxic and side effects in digestive system only for trials with samples>60.
12.9. Analysis.

Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 9 the toxic and side effects of leukopenia only for trials with samples>60.
12.10. Analysis.
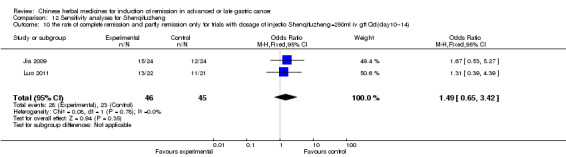
Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 10 the rate of complete remission and partly remission only for trials with dosage of injectio Shenqifuzheng=250ml iv gtt Qd(day10˜14).
12.11. Analysis.
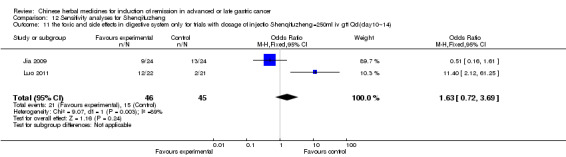
Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 11 the toxic and side effects in digestive system only for trials with dosage of injectio Shenqifuzheng=250ml iv gtt Qd(day10˜14).
12.12. Analysis.
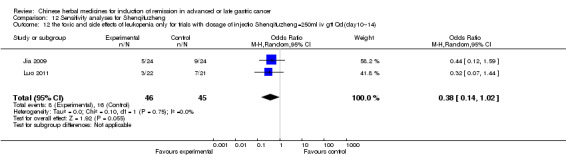
Comparison 12 Sensitivity analyses for Shenqifuzheng, Outcome 12 the toxic and side effects of leukopenia only for trials with dosage of injectio Shenqifuzheng=250ml iv gtt Qd(day10˜14).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cao 1992.
| Methods | RCT, method unspecified | |
| Participants | IP: 20 cases; M: unclear, F: unclear; CP1: 20 cases; M: unclear, F: unclear; CP2: 20 cases; M: unclear, F: unclear. | |
| Interventions | IP: 5‐FU 500mg iv gtt + MMC 20mg iv gtt + VCR 1mg iv gtt Biw X 5 weeks + Shenqi injecta 20ml iv gtt qd X 5 weeks; CP1: Shenqi injecta 20ml iv gtt qd X 5 weeks; CP2: 5‐FU 500mg iv gtt qd X 4 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; immune function change of body weight | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | High risk | Four rating scales for cognition listed in Methods, but only two reported. |
Cao 1997.
| Methods | RCT, method unspecified | |
| Participants | IP: 12 cases; M: 11 cases, F: 1 cases; CP I: 12 cases; M: 10 cases, F: 2 cases; CP2: 12 cases; M: 9 cases, F: 3 cases. | |
| Interventions | IP: Lanxiangxiru 300mg/m² iv qd day 1˜5; 5‐FU 0.5/m² iv qd day6˜10; acupuncture 30min, Tiw X 6˜8 weeks; CP1: Lanxiangxiru 300mg/m² iv qd day1˜5;5‐FU 0.5/m² iv qd day 6˜10 X 6˜8 weeks; CP2: 5‐FU 0.5/m² iv qd day 1˜5; X 6˜8 weeks; 100ml (enema) qd X 1˜2month Xiaokuitang II 4 pills Tid X 1˜2 month. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy. | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Chen 1997.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 22 cases, F: 8 cases; CP: 28 cases; M: 21 cases, F: 7 cases. | |
| Interventions | IP: 5‐FU 750mg iv gtt day 1˜5 + CDDP 30mg iv gtt day 1˜3 +ADM40mg iv gtt day1, 8X6 weeks + Pingxiao Capsule 6# tid X60 days; CP: 5‐FU 750mg iv gtt day1˜5 + CDDP 30mg iv gtt day 1˜3 +ADM40mg iv gtt day1, 8X6 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; MST; rate of remission | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Chen 1997a.
| Methods | RCT, method unspecified | |
| Participants | IP: 22 cases; M: 15 cases, F: 7 cases; CP: 19 cases; M: 14 cases, F: 5 cases. | |
| Interventions | IP: CPT8mg iv qd 10 days X 2 + TCMHs po qd 10 days X 2; CP: TCMHs po qd 10 days X 2. | |
| Outcomes | toxic and side effects after chemotherapy; MST; rate of remission | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Chen 2005.
| Methods | RCT, method unspecified | |
| Participants | IP: 64 cases; M: 42 cases, F: 22 cases; CP: 64 cases; M: 48 cases, F: 16 cases. | |
| Interventions | IP: OXA iv gtt day 1,8 + CF 50mg day1˜5 + 5‐FU 500˜750mg iv gtt day1˜5 + Cidan Capsule 5# tid X 4 weeks; CP: OXA iv gtt day 1,8 + CF 50mg day1˜5 + 5‐FU 500˜750mg iv gtt day1˜5 X 4 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; MST; rate of remission | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: Random sampling |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/128 missing from the study |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Chen 2008.
| Methods | RCT, method unspecified | |
| Participants | IP: 36 cases; M: unclear, F: unclear;
CP: 34 cases; M: unclear, F: unclear; M: 38 cases, F 32 cases. |
|
| Interventions | IP: L‐OHP (85mg/m²) iv gtt day1 + CF (200mg/m² ) iv gtt day1˜2 + 5‐FU (400mg/m²) iv gtt day1 (5‐FU (600mg/m²) iv gtt day2) + Aidi injecta 1.5g iv gtt day1˜12 X 6 weeks; CP: L‐OHP (85mg/m²) iv gtt day1+ CF (200mg/m² ) iv gtt day1˜2 + 5‐FU (400mg/m²) iv gtt day1 (5‐FU (600mg/m²) iv gtt day2) X 6 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; MST; rate of remission | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Chen 2009.
| Methods | RCT, draw by lot | |
| Participants | IP: 34 cases; M: 20 cases, F: 14 cases; CP: 33 cases; M: 20 cases, F: 13 cases. | |
| Interventions | IP: Huachansu 30ml iv gtt qd X 21days + Taxol (175mg/m²) iv gtt day1+ CDDP (200mg/m² ) iv gtt day1˜5 + 5‐FU (600mg/m²) iv gtt day1˜5 X 6 weeks; CP: Taxol (175mg/m² ) iv gtt day1 + CDDP (200mg/m²) iv gtt day1˜5 + 5‐FU (600mg/m²) iv gtt day1˜5 X 6 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; MST; rate of remission | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: draw by lot |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Deng 2001.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: unclear, F: unclear; CP: 26 cases; M: unclear, F: unclear. | |
| Interventions | IP: 5‐FU 500 mg/m² iv gtt qdXd1‐3; MMC 8 mg/m², iv gtt d1; Lanxiangxiru 400ml , iv gtt d1‐10 X 3 weeks; CP: 5‐FU 500 mg/m² iv gtt qdXd1‐3; MMC 8 mg/m², iv gtt d1 X 3 weeks. | |
| Outcomes | Blood count; immune function | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Deng 2011.
| Methods | RCT, method unspecified | |
| Participants | IP: 40 cases; M: unclear, F: unclear;
CP: 40 cases; M: unclear, F: unclear; M: 54 cases, F: 26 cases. |
|
| Interventions | IP: Shenfu injecta 50ml iv gtt day1˜7 + Taxol (135mg/m² ) iv gtt day1+ CF (400mg/m² ) iv gtt day1 + 5‐FU (2400mg/m² ) iv gtt day1˜2 + CDDP 40mg iv gtt day2˜3 X 4 weeks; CP: Taxol (135mg/m² ) iv gtt day1+ CF (400mg/m² ) iv gtt day1 + 5‐FU (2400mg/m² ) iv gtt day1˜2 + CDDP 40mg iv gtt day2˜3 X 4 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; rate of remission; blood count | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Du 2010.
| Methods | RCT, method unspecified | |
| Participants | IP: 40 cases; M: 26 cases, F:14 cases;
CP1: 40 cases; M: 24 case, F:16 cases; CP2: 40 cases; M: 25 case, F:14 cases. |
|
| Interventions | IP: Hyperthermia + TCMHs 200ml b.i.d day1˜14 + L‐OHP (100mg/m² ) iv gtt day1+ CF (200mg/m² ) iv gtt day1˜5 + 5‐FU (500mg/m² ) iv gtt day1˜5 X 6 weeks;
CP1: Hyperthermia + L‐OHP (100mg/m² ) iv gtt day1+ CF (200mg/m² ) iv gtt day1˜5 + 5‐FU (500mg/m² ) iv gtt day1˜5 X 6 weeks; CP2: L‐OHP (100mg/m² ) iv gtt day1+ CF (200mg/m² ) iv gtt day1˜5 + 5‐FU (500mg/m² ) iv gtt day1˜5 X 6 weeks. |
|
| Outcomes | MST; rate of remission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Fu 2011.
| Methods | RCT, method unspecified | |
| Participants | IP: 40 cases; M: 20cases, F: 20 cases; CP: 40 cases; M: 21 cases, F: 19 cases. | |
| Interventions | IP: L‐OHP (85mg/m² ) iv gtt day1+ CF (200mg/m² ) iv gtt day1 + 5‐FU (2600mg/m² ) iv gtt day1 X 4weeks + Fufangkushen injecta 20ml iv gtt day1˜28; CP: L‐OHP (85mg/m² ) iv gtt day1+ CF (200mg/m² ) iv gtt day1 + 5‐FU (2600mg/m² ) iv gtt day1 X 4 weeks. |
|
| Outcomes | pain, toxic and side effects after chemotherapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Gao 2008.
| Methods | RCT, method unspecified | |
| Participants | IP: 24 cases; M: 13 cases, F: 11 cases; CP: 23 cases; M: 12 cases, F: 11 cases. | |
| Interventions | IP: L‐OXA (70mg/m²) iv gtt day1+ CF (400mg/m²) iv gtt day1 + 5‐FU (500mg/m²) iv gtt day1˜5 X 3˜4 weeks + Mojisankeli 2 bags b.i.d X 3 months; CP: L‐OXA (70mg/m²) iv gtt day1+ CF (400mg/m²) iv gtt day1 + 5‐FU (500mg/m²) iv gtt day1˜5 X 3˜4 weeks. |
|
| Outcomes | toxic and side effects after chemotherapy; rate of remission; blood count; MST | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Gong 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 26 cases; M: 15 cases, F:11 cases; CP: 30 cases; M: 16 cases, F: 14 cases. | |
| Interventions | IP: Taxol 135mg/m², iv, day1; 5‐Fu + 500mg/m², iv (4h) day1˜5 + CF100mg/m², iv day1˜5 + CDDP 30mg/m² iv gtt day1˜3 + Aidi 50ml iv gtt qd x 12 weeks; CP: Taxol 135mg/m², iv, day1; 5‐Fu + 500mg/m², iv (4h) day1˜5 + CF100mg/m², iv day1˜5 + CDDP 30mg/m² Ii gtt day1˜3 X 12 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; TTP; MST; rate of remission | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Guan 2001.
| Methods | RCT, method unspecified | |
| Participants | IP: 58 cases; M: unclear, F: unclear; CP: 40 cases; M: unclear, F: unclear. | |
| Interventions | CP: 5‐FU 500mg/m² iv gtt qd d1˜5 + ADM 30˜50mg/m² iv d1 + MMC 8mg/m² iv d1 + Lanxiangxiru 400ml iv gtt d1˜10X3 weeks: CP: 5‐FU 500mg/m² iv gtt qd d1˜5 + ADM 30˜50mg/m² iv d1 + MMC 8mg/m² iv d1 X 3 weeks. | |
| Outcomes | Blood count; immune function | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Guo 1989.
| Methods | RCT, method unspecified | |
| Participants | IP: 50 cases; M: 40 cases, F:10 cases; CP: 40 cases; M: 28 cases, F: 12 cases. | |
| Interventions | IP: 5‐FU 0.5‐1.0 iv gtt qd d1˜5 + MMC 4mg iv gtt d1 + VCR 2mg iv d1˜2 X4˜6 weeks + Jianpiyishenchongji 1 packet bid X 6˜8 weeks; CP: 5‐FU 0.5‐1.0 iv gtt qd d1˜5 + MMC 4mg iv gtt d1 + VCR 2mg iv d1˜2 X 4˜6 weeks. | |
| Outcomes | blood count; immune function; MST; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/40 missing from control group; 3/50 missing from intervention group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Hu 2011.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 18 cases, F: 12 cases; CP: 30 cases; M: 17 cases, F: 13 cases. | |
| Interventions | IP: L‐OXA (130mg/m²) iv gtt day1 + CF (120mg/m²) iv gtt day1˜5 + 5‐FU (350mg/m²) iv gtt day1˜5 X 6˜9 weeks + Fuzheng Xiao'ai Prescription I one dosage q.d X 3˜6 weeks; CP: L‐OXA (130mg/m² ) iv gtt day1 + CF (120mg/m² ) iv gtt day1˜5 + 5‐FU (350mg/m² ) iv gtt day1˜5 X 6˜9 weeks. |
|
| Outcomes | toxic and side effects after chemotherapy; MST; rate of remission; blood count | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: random by envelope |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Hua 1999.
| Methods | RCT, method unspecified | |
| Participants | IP: 61 cases; M: 42 cases, F: 19 cases; CP: 30 cases; M: 23 cases, F: 7 cases. | |
| Interventions | IP: PDD 120˜150mg iv qw1 + MMC 6mg iv qw w1‐2+5‐FU 500mg iv gtt biw w2‐3 X 9weeks + Fufangxiansu Capsule 1.48˜2.22 tid X 12 weeks; CP: PDD 120˜150mg iv qw w1 + MMC 6mg iv qw w1‐2 + 5‐FU 500mg iv gtt biw w2‐3 X 9 weeks. | |
| Outcomes | blood count; immune function; rate of remission; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Huang 2002.
| Methods | RCT, method unspecified | |
| Participants | IP: 31 cases; M 24 cases, F: 7 cases; CP: 28 cases; M 23 cases, F: 5 cases. | |
| Interventions | IP: MMC 6 mg/ m², iv gtt,qw+5‐Fu 10 mg/ kg, iv gtt,qw; CF 300 mg/ m², iv, biwX4 weeks+Chansu injecta 10ml iv gtt qdX4 weeks; CP: MMC 6 mg/ m², iv gtt, qw+5‐Fu 10 mg/ kg iv gtt,qw; CF 300 mg/ m², iv, biwX4 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL; improvement of clinical symptoms | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Huang 2005.
| Methods | RCT, method unspecified | |
| Participants | the total number of the patients was 68 cases. IP: cases were unclear; CP: cases were unclear. | |
| Interventions | IP:CF 200mg/m² iv gtt d1˜5 + 5‐FU 300mg iv gtt d1˜5 + DDP 30mg iv gtt d3˜5 X 9 weeks + Lanxiangxiru 0.6 iv gtt d1˜5 X 9 weeks; CP: CF 200mg/m² iv gtt d1˜5 + 5‐FU 300mg iv gtt d1˜5 + DDP 30mg iv gtt d3˜5 X 9 weeks. | |
| Outcomes | rate of remission in short; immune function; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Jia 2003.
| Methods | RCT, method unspecified | |
| Participants | IP: 23 cases; M: unclear, F: unclear; CP: 22 cases; M: unclear, F: unclear; CP2: 20 cases; M: unclear, F: unclear. | |
| Interventions | IP: 5‐FU 500mg/m² iv gtt day1˜5 + DDP 50mg iv gtt day1˜3 X 6 weeks + Aidi 50mg iv gtt qd X 42 days; CP: 5‐FU 500mg/m² iv gtt day1˜5 + DDP 50mg iv gtt d1˜3 X 6 weeks. | |
| Outcomes | rate of remission in short; immune function; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Jia 2009.
| Methods | RCT, method unspecified | |
| Participants | IP: 24 cases; M: 14 cases, F: 10 cases; CP: 24 cases; M: 13 cases, F: 11 cases. | |
| Interventions | IP: Shenqifuzheng injecta 250ml iv gtt day1˜10 + L‐OXA (85mg/m²) iv gtt day1+ CF (200mg/m² ) iv gtt day1˜2 + 5‐FU (600mg/m²) (5‐FU 400mg/m² iv day1˜2 ) iv gtt day1˜2 X 8 weeks; CP: L‐OXA (85mg/m²) iv gtt day1 + CF (200mg/m²) iv gtt day1˜2 + 5‐FU (600mg/m²) (5‐FU 400mg/m² iv day1˜2) iv gtt day1˜2 X 8 weeks. |
|
| Outcomes | toxic and side effects after chemotherapy; MST; rate of remission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Jiang 1994.
| Methods | RCT, the special method was drawing straws | |
| Participants | IP: 52 cases; M: unclear, F: unclear; CP: 30 cases; M: unclear, F: unclear; CP2: 20 cases; M:unclear, F unclear. | |
| Interventions | IP: Jianpixiao'aisan one dosage bid X 8 weeks; CP:5‐FU 300mg/m² iv gtt biw + ADM 30mg/m² iv w1,w4 + MMC 3mg/m² iv qw X 6 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL; improvement of clinical symptoms in short term | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: the method of sequence generation was drawing straws. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: 4/82 missing from the study. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Li 2001.
| Methods | RCT, method unspecified | |
| Participants | IP: 32 cases; M: unclear, F: unclear; CP1: 31 cases; M: unclear, F: unclear; CP2: 41 cases; M: unclear, F: unclear; CP2: 20 cases; M: unclear, F: unclear. | |
| Interventions | IP: VP‐16 120mg/m² iv gtt d1˜3 + CF 300mg/m² iv gtt d1˜3 + 5‐FU 500mg/m² iv gtt d1˜3 + Jinlongshekoufuye 30ml tid X 6 months; CP1: VP‐16 120mg/m² iv gtt d1˜3 + CF 300mg/m² iv gtt d1˜3 + 5‐FU 500mg/m² iv gtt d1˜3X6 months; CP2: Jinlongshekoufuye 30ml tid X 6 months. | |
| Outcomes | rate of remission in short term; MST; life span; tumour markers | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: random by envelope. |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Li 2002.
| Methods | RCT, method unspecified | |
| Participants | IP: 35 cases; M: 24 cases, F: 11 cases; CP: 33 cases; M: 18 cases, F: 15 cases. | |
| Interventions | IP: ADM 20˜40mg iv d1,d7 + CDDP 40˜60mg iv gtt d2,d8 + VP‐16 100mg iv gtt d4£6 + TCMHs po X 3 weeks; CP: ADM 20˜40mg iv d1,d7 + CDDP 40˜60mg iv gtt d2,d8 + VP‐16 100mg iv gtt d4£6 X 3 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Lin 2011.
| Methods | RCT, method unspecified | |
| Participants | IP: 43 cases; M: unclear, F: unclear; CP: 42 cases; M: unclear, F: unclear; M: unclear, F: unclear. | |
| Interventions | IP: Fufangkushen injecta 20ml iv gtt day1˜10 + L‐OXA (130mg/m²) iv gtt day1+ Docetaxe (75mg/m²) iv gtt day1 + 5‐FU (1500mg/m²) iv gtt day1, 8 X 9 weeks; CP: L‐OXA (130mg/m²) iv gtt day1 + Docetaxe (75mg/m²) iv gtt day1 + 5‐FU (1500mg/m²) iv gtt day1, 8 X 9 weeks. |
|
| Outcomes | toxic and side effects after chemotherapy; QOL; MST | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Liu 2002.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 26 cases, F: 4 cases; CP: 30 cases; M: 25 cases, F: 5 cases. | |
| Interventions | IP: MMC 10mg/m² iv d1 + ADM 30mg/m² iv d1,d8 + 5‐FU 500mg iv gtt d2˜6 X 8˜12 weeks+TCMHs one dosage qd X 2 weeks; CP: MMC 10mg/m² iv d1 + ADM 30mg/m² iv d1,d8 + 5‐FU 500mg iv gtt d2˜6 X 8˜12 weeks. | |
| Outcomes | QOL; rate of remission in short term; MST in half a year | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Liu 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 38 cases; M: 29 cases, F: 9 cases; CP: 36 cases; M: 28 cases, F: 8 cases. | |
| Interventions | IP: DDP 80mg/m² iv gtt d1 + CF200mg/m² iv gtt d1˜5 + 5‐FU 300mg/m² iv gtt d1˜5 + Jiaweibazhentang one dosage 100ml bid X 9 weeks; CP: DDP 80mg/m² iv gtt d1 + CF200mg/m² iv gtt d1˜5 + 5‐FU 300mg/m² iv gtt d1˜5 X 9 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; QOL; rate of remission in short term | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Liu 2006a.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 18 cases, F: 12 cases; CP: 30 cases; M: 17 cases, F: 13 cases. | |
| Interventions | IP: MMC 4mg/m² iv gtt d1,d8 + 5‐FU 500mg iv gtt d1˜5 + DDP 50mg/m² iv gtt d1˜5 + Tianlongheji 50ml tidX6 weeks; CP: MMC 4mg/m² iv gtt d1,d8 + 5‐FU 500mg iv gtt d1˜5 + DDP 50mg/m² iv gtt d1˜5 X 6 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; QOL; rate of remission in short term; change of body weight | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Liu 2009.
| Methods | RCT, draw by lots | |
| Participants | IP: 30 cases; M: 16 cases, F:14 cases; CP: 30 cases; M: 18 cases, F: 12 cases. | |
| Interventions | IP: Aidi injecta 50ml iv gtt day1˜10 + Taxol (175mg/m²) iv gtt day1+ CDDP (20mg/m²) iv gtt day1˜5 + 5‐FU (600mg/m²) iv gtt day1˜5 X 8 weeks; CP: Taxol (175mg/m²) iv gtt day1+ CDDP (20mg/m²) iv gtt day1˜5 + 5‐FU (600mg/m²) iv gtt day1˜5 X 8 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; rate of remission in short term | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: draw by lots. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Liu 2009a.
| Methods | RCT, method unspecified | |
| Participants | IP: 29 cases; M: unclear, F: unclear; CP: 28 cases; M: unclear, F: unclear; M: 38 cases, F: 19 cases. | |
| Interventions | IP: Fufangkushen injecta 20ml iv gtt day1˜14 + L‐OXA (130mg/m²) iv gtt day1+ CF (100mg/m²) iv gtt day1˜5 + Tegafur (1000mg/m²) iv gtt day1˜5 X 6 weeks; CP: L‐OXA (130mg/m²) iv gtt day1+ CF (100mg/m²) iv gtt day1˜5 + Tegafur (1000mg/m²) iv gtt day1˜5 X 6 weeks. |
|
| Outcomes | toxic and side effects after chemotherapy; rate of remission in short term | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no patients missing from each group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Luo 2011.
| Methods | RCT, method unspecified | |
| Participants | IP: 22 cases; M: 15 cases, F: 7 cases; CP: 21 cases; M: 13 cases, F: 8 cases. | |
| Interventions | IP: Shenqifuzheng injecta 250ml iv gtt day1˜14 + L‐OHP (130mg/m²) iv gtt day1+ CF (100mg/m²) iv gtt day1˜5 + 5‐FU (200mg/m²) iv gtt day1˜5 X 6 weeks; CP: L‐OHP (130mg/m²) iv gtt day1 + CF (100mg/m²) iv gtt day1˜5 + 5‐FU (200mg/m²) iv gtt day1˜5 X 6 weeks. |
|
| Outcomes | toxic and side effects after chemotherapy; rate of remission in short term; QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Lv 1999.
| Methods | RCT, method unspecified | |
| Participants | IP: 48 cases; M: 28 cases, F: 20 cases; CP: 34 cases; M: 19 cases, F: 15 cases. | |
| Interventions | IP: MMC6˜8mg iv qw + 5‐FU 300mg/m² iv gtt biw (or ADM 20˜30mg/m² iv w1,w4+MMC6˜8mg iv qw + 5‐FU 300mg/m² iv gtt biw) + TCMHs (no special therapeutic period); CP: MMC6˜8mg iv qw + 5‐FU 300mg/m² iv gtt biw (or ADM 20˜30mg/m² iv w1,w4 + MMC6˜8mg iv qw + 5‐FU 300mg/m² iv gtt biw) (no special therapeutic period). | |
| Outcomes | QOL; rate of remission in short term | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/48 missing from intervention group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Niu 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 60 cases; M 36 cases, F: 24 cases; CP: 60 cases; M unclear, F: unclear. | |
| Interventions | IP: U FTM¡¢ FAM¡¢EA P¡¢EL F¡¢FM (no special dosage and therapeutic period)+TCMHs one dosage qdX40 days; CP: U FTM¡¢ FAM¡¢EA P¡¢EL F¡¢FM (no special dosage and therapeutic period). | |
| Outcomes | rate of remission in short term | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Peng 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 45 cases; M: 28 cases, F: 17 cases; CP: 43 cases; M: 25 cases, F: 18 cases. | |
| Interventions | IP:CF 200mg iv gtt d1˜5 + 5‐FU 500mg iv gtt d1˜5 X 3 weeks + Qingyufuzhengtang one dosage 150ml bid X 3 months. | |
| Outcomes | immune function | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Rao 1994.
| Methods | RCT, method unspecified | |
| Participants | IP: 64 cases; M: 40 cases, F: 24 cases; CP: 17 cases; M: 10 cases, F: 7 cases. | |
| Interventions | IP: MMC4˜6mg iv gtt qw + 5‐FU 500˜750mg iv gtt biw + VCR 1mg (or Ara‐C 50mg) iv gtt qw + Shengxuetang one dosage bid X 12˜24 weeks; CP: MMC4˜6mg iv gtt qw + 5‐FU 500˜750mg iv gtt biw + VCR 1mg(or Ara‐C 50mg) iv gtt qw X 12˜24 weeks. | |
| Outcomes | MST | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: draw by lots. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/81 missing from the study. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Shao 1998.
| Methods | RCT, method unspecified | |
| Participants | IP: 94 cases; M: unclear, F: unclear; CP: 49 cases; M: unclear, F: unclear. | |
| Interventions | IP: FUfangzhenjianye 40ml tid X 3 months; CP: Pingxiao Capsule 1.2 tid X f3 months. | |
| Outcomes | rate of remission in short term; MST in five years; immune function; improvement of clinical symptoms; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Shi 2004.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 18 cases, F: 12 cases; CP: 30 cases; M: 15 cases, F: 15 cases. | |
| Interventions | IP: Huachansu 20ml iv gtt qd + TCMHs one dosage X 2 months; CP: TCMHs one dosage X 2 months. | |
| Outcomes | MST in half a year; QOL; immune function | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Si 2004.
| Methods | RCT, method unspecified | |
| Participants | IP: 31 cases; M: 21 cases, F: 10 cases; CP: 31 cases; M: 18 cases, F: 13 cases. | |
| Interventions | IP: 5‐FU 500mg iv gtt d1˜5 + DDP 20mg iv gtt d1˜5 + CF100mg iv gtt d1˜5+TCMHs X 12 weeks; CP: 5‐FU 500mg iv gtt d1˜5 + DDP 20mg iv gtt d1˜5 + CF100mg iv gtt d1˜5 X 12 weeks. | |
| Outcomes | QOL; rate of remission in short term; improvement of clinical symptoms | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: be in hospital order |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Sun 1999.
| Methods | RCT, method unspecified | |
| Participants | IP: 77 cases; M: 59 cases, F: 18 cases; CP: 46 cases; M: 35 cases, F: 11 cases. | |
| Interventions | IP: PDD + MMC + 5‐FU + Yangweikangliuchongji (no special dosage and therapeutic period); CP: PDD + MMC + 5‐FU (no special dosage and therapeutic period). | |
| Outcomes | QOL; rate of remission in short term; immune function; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Tian 1999.
| Methods | RCT, method unspecified | |
| Participants | IP: 14 cases; M: unclear, F: unclear; CP: 17 cases; M: unclear, F: unclear; CP1: 11 cases; M: unclear, F: unclear. | |
| Interventions | IP: VP16 0. 1 iv d1˜5,CF 30mg. iv d1˜5, 5‐FU 0.5 iv d1˜5X4 weeks + Lanxiangxiru 80ml ivgtt X 15 days; CP: VP16 0. 1 iv d1˜5,CF 30mg. iv d1˜5, 5‐FU 0.5 iv d1˜5 X 4 weeks; CP1: Lanxiangxiru 80ml iv gtt qd X 15 days. | |
| Outcomes | rate of remission in short term; multi‐drug resistance | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 1993.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 23 cases, F: 7 cases; CP: 30 cases; M: 28 cases, F: 2 cases. | |
| Interventions | IP: UFT 4# tid + VP‐16 100mg iv gtt qw + CDDP 40mg iv qw X 8 weeks + Jiandujianpitang one dosage bid X 8 weeks; CP: 5‐FU500mg iv gtt biw + MMC 8mg iv w1,w2,w5,w6 X 45 days. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; improvement of clinical symptoms | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | simple randomisation. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 1998.
| Methods | RCT, the special method is random number table | |
| Participants | IP: 105 cases; M: 63 cases, F: 42 cases; CP: 86 cases; M: 56 cases, F: 30 cases; CP1: 58 cases; M: 37 cases, F: 21 cases. | |
| Interventions | IP: UFT 3 #,tid X 8 weeks, MMC 120˜160 mg/ kg iv qw X 6˜8 weeks + Fuzhengkang'aichongji one dosage bid X 3 months; CP: UFT 3 #,tid X 8 weeks, MMC 120˜160 mg/ kg iv qw X 6˜8 weeks; CP1: Fuzhengkang'aichongji one dosage bid X 3 months. | |
| Outcomes | rate of remission in short term; QOL; MST in three years; toxic and side effects after chemotherapy; immune function | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | the method of sequence generation was offered by random number table. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2002.
| Methods | RCT, method unspecified | |
| Participants | IP: 90 cases; M: 69 cases, F: 21 cases; CP: 40 cases; M: 32 cases, F: 8 cases. | |
| Interventions | IP: Jianpixiao'aitang one dosage bid X 3 months + VP‐16 0.1ivgtt d1˜3 + CF 0.1 iv gtt d1˜5 + 5‐FU750mg iv gtt d1˜5 X 9˜12 weeks; CP: VP‐16 0.1ivgtt d1˜3 + CF 0.1 iv gtt d1˜5 + 5‐FU750mg iv gtt d1˜5 X 9˜12 weeks. | |
| Outcomes | rate of remission in short term; MST; QOL; blood count; improvement of clinical symptoms | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2004.
| Methods | RCT, the special method is envelope concealment | |
| Participants | IP: 38 cases; M: 23 cases, F: 15 cases; CP: 30 cases; M: 19 cases, F: 11 cases. | |
| Interventions | IP: Yadanziyouru injecta 30ml iv gtt qdX30˜90days + HCPT10˜12mg/m² + CF100mg/m² iv gtt d1˜5 + 5‐FU500mg/m² iv gtt d1˜5 X 9˜12 weeks; CP: HCPT10˜12mg/m² + CF100mg/m² iv gtt d1˜5 + 5‐FU500mg/m² iv gtt d1˜5 X 9˜12 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | the method of sequence generation was envelope concealment. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2004a.
| Methods | RCT, method unspecified | |
| Participants | IP: 24 cases; M: 15 cases, F: 9 cases; CP: 22 cases; M: 14 cases, F: 8 cases. | |
| Interventions | IP: Jianpihuayuheji 200ml bid X 2˜3 months + L‐OHP 130mg/m² iv gtt d1 + CF 100mg iv gtt d1˜5 + 5‐FU 0.5 iv gtt d1˜5 X 8˜12 weeks; CP: L‐OHP 130mg/m² iv gtt d1 + CF 100mg iv gtt d1˜5 + 5‐FU 0.5 iv gtt d1˜5 X 8˜12 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; MST; improvement of clinical symptoms | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2009.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 19 cases, F: 11 cases; CP: 26 cases; M: 17 cases, F: 9 cases. | |
| Interventions | IP: Aidi injecta 50ml iv gtt day1˜15 + 5‐FU 0.5 iv day1 + ADM 20mg iv day1 + MMC 20mg iv day1 X 12 weeks; CP: 5‐FU 0.5 iv day1 + ADM 20mg iv day1+ MMC 20mg iv day1 X 12 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2009a.
| Methods | RCT, method unspecified | |
| Participants | IP: 36 cases; M: unclear, F: unclear; CP: 32 cases; M: unclear, F: unclear; M: 48 cases, F: 20 cases. | |
| Interventions | IP: Huachansu 20ml iv gtt qd X 28 weeks + L‐OHP (85mg/m² ) iv gtt day1+ CF (100mg/m² ) iv gtt day1˜2 + 5‐FU (400mg/m² ) iv day1˜2 + 5‐FU (600mg/m² ) iv gtt day1˜2 X 8 weeks; CP: L‐OHP (85mg/m² ) iv gtt day1+ CF (100mg/m² ) iv gtt day1˜2 + 5‐FU (400mg/m² ) iv day1˜2 + 5‐FU (600mg/m² ) iv gtt day1˜2 X 8 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; tumour markers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2010.
| Methods | RCT, method unspecified | |
| Participants | IP: 32 cases; M: unclear, F: unclear; CP: 30 cases; M: unclear, F: unclear; M: 29 cases, F: 33 cases. | |
| Interventions | IP: Shenqifuzheng injecta 250ml iv gtt qd X 31days + Radiotherapy (No specific dosage) X 31days + LF regimen (day1˜4, day28˜31, No specific dosage); CP: Radiotherapy (No specific dosage) X 31days + LF regimen (day1˜4, day28˜31, No specific dosage). |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; body weight loss | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2010a.
| Methods | RCT, toss of a coin | |
| Participants | IP: 25 cases; M: 18 cases, F: 7 cases; CP: 25 cases; M: 16 cases, F: 9 cases. | |
| Interventions | IP: Fufangkushen injecta 20ml iv gtt day1˜21 + Docetaxel ( 30mg/m²) iv gtt day1,8 + CDDP (20mg/m²) iv gtt day1˜5 + 5‐FU (750mg/m²) iv gtt day1˜5 X 6 weeks; CP: Docetaxel ( 30mg/m²) iv gtt day1,8 + CDDP (20mg/m²) iv gtt day1˜5 + 5‐FU (750mg/m²) iv gtt day1˜5 X 6 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | the method of sequence generation was tossed of a coin. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wang 2010b.
| Methods | RCT, method unspecified | |
| Participants | IP: 20 cases; M: unclear, F: unclear; CP: 23 cases; M: unclear, F: unclear; M: 27 cases, F: 16 cases. | |
| Interventions | IP: Huachansu 10˜20ml iv gtt day1˜10 + L‐OHP (85˜100mg/m²) iv gtt day1+ CF (200mg/m²) iv gtt day1˜2 + 5‐FU (400mg/m²) iv day1˜2 + 5‐FU (600mg/m²) iv gtt day1˜2 X 16 weeks; CP: L‐OHP (85˜100mg/m²) iv gtt day1+ CF (200mg/m²) iv gtt day1˜2 + 5‐FU (400mg/m²) iv day1˜2 + 5‐FU (600mg/m²) iv gtt day1˜2 X 1weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wu 1999.
| Methods | RCT, method unspecified | |
| Participants | IP: 57 cases; M: 36 cases, F: 21 cases; CP: 46 cases; M: 34 cases, F: 12 cases. | |
| Interventions | IP: MF¡¢FAP¡¢ELF regimen + TCMHs (no special dosage and therapeutic period); CP: MF¡¢FAP¡¢ELF regimen (no special dosage and therapeutic period). | |
| Outcomes | MST in five years | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wu 2000.
| Methods | RCT, method unspecified | |
| Participants | IP: 32 cases; M: 24 cases, F: 8 cases; CP: 30 cases; M: 23 cases, F: 7 cases. | |
| Interventions | IP: FAM¡¢EAP¡¢ HELF X 6˜8 weeks (no special dosage) + Fuzhenggongjian Capsule 11.6 tid X 6˜8 weeks; CP: FAM¡¢EAP¡¢ HELF X 6˜8 weeks (no special dosage). | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL; MST | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Wu 2000a.
| Methods | RCT, method unspecified | |
| Participants | IP: 32 cases; M: 27 cases, F: 5 cases; CP: 36 cases; M: 29 cases, F: 7 cases. | |
| Interventions | IP: Lanxiangxiru 400mg iv gtt qd d1˜5 + 5‐FU 0.5 iv gtt qd d1˜5 + ADM 40mg/m² iv d1 + MMC8˜10mg iv d1 X 6˜8 weeks; CP: 5‐FU 0.5 iv gtt qd d1˜5 + ADM 40mg/m² iv d1 + MMC8˜10mg iv d1 X 6˜8 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Xie 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 90 cases; M: 52 cases, F: 38 cases; CP: 82 cases; M: 48 cases, F: 34 cases. | |
| Interventions | IP:OXA 100mg iv gtt d1,d8 + 5‐FU0.75 iv gtt + CF 100mg iv gtt d2˜6 X 3 weeks + Lailikangkeli 24g bid X 28 days; CP:OXA 100mg iv gtt d1,d8 + 5‐FU0.75 iv gtt + CF 100mg iv gtt d2˜6 X 3 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Xiong 2008.
| Methods | RCT, method unspecified | |
| Participants | IP: 37 cases; M: unclear, F: unclear; CP: 34 cases; M: unclear, F: unclear; M: 48 cases, F: 23 cases. | |
| Interventions | IP: Aishu (Fufangkeshen injecta) 20ml iv gtt day1˜10 + Taxol (130mg/m²) iv gtt day1+ L‐OHP (135mg/m²) iv gtt day2 X 9 weeks; CP: Taxol (130mg/m²) iv gtt day1+ L‐OHP (135mg/m²) iv gtt day2 X 9 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | High risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Xu 1989.
| Methods | RCT, method unspecified | |
| Participants | IP: 116 cases; M: 89 cases, F: 27 cases; CP: 124 cases; M: 93 cases, F: 31 cases. | |
| Interventions | IP: MMC 4mg iv d1 + 5‐FU 0.5 iv gtt qd d1˜5 + VCR 1mg iv d1 + Yangxuetang 100ml bid X 4˜6 weeks; CP: MMC 4mg iv d1 + 5‐FU 0.5 iv gtt qd d1˜5 + VCR 1mg iv d1 X 4˜6 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; immune function; blood count; MST | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/116 missing from intervention group; 32/124 missing from control group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Xu 1993.
| Methods | RCT, method unspecified | |
| Participants | IP: 51 cases; M: 45 cases, F: 6 cases; CP: 45 cases; M: 40 cases, F: 5 cases. | |
| Interventions | IP: MMC6˜8mg iv qw˜biw + VCR 1mg iv qw˜biw + 5‐FU 0.5˜0.7 iv gtt qw˜biw X 6˜8 weeks + Aifukang 8g bid X 60 days; CP: MMC6˜8mg iv qw˜biw + VCR 1mg iv qw˜biw + 5‐FU 0.5˜0.7 iv gtt qw˜biw X 6˜8 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; MST in 5 years | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Xu 1999.
| Methods | RCT, method unspecified | |
| Participants | IP: 40 cases; M: 26 cases, F: 14 cases; CP: 30 cases; M: 22 cases, F: 8 cases. | |
| Interventions | IP: VP‐16 100mg iv gtt qd d1˜5 + 5‐FU 1.0 iv gtt qd d1˜5 + CF30mg iv gtt qd d1˜5 (or ADM 30mg iv qd d1,d7 + PDD 40mg iv qd d2,d8 + VP‐16 100mg iv gtt qd d4˜6) X 6 weeks + Fuzhenghuoxuejiedufang one dosage bid X 10 weeks; CP: VP‐16 100mg iv gtt qd d1˜5 + 5‐FU 1.0 iv gtt qd d1˜5 + CF30mg iv gtt qd d1˜5 (or ADM 30mg iv qd d1,d7 + PDD 40mg iv qd d2,d8 + VP‐16 100mg iv gtt qd d4˜6) X 6 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; rate of remission in short term; complete rate of chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/40 missing from intervention group; 8/30 missing from control group. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Yang 2005.
| Methods | RCT, method unspecified | |
| Participants | IP: 40 cases; M: 28 cases, F: 12 cases; CP: 40 cases; M: 30 cases, F: 10cases. | |
| Interventions | IP: VP‐16 100mg iv gtt qd d1˜5 + CF 30mg iv gtt qd d1˜5 + 5‐FU 0.75‐1.0 iv gtt qd d1˜5 + PDD 60mg celiac injection qw˜biw X 8 weeks + Shenfu injecta 60ml iv gtt qd X 10 days; CP: VP‐16 100mg iv gtt qd d1˜5 + CF 30mg iv gtt qd d1˜5 + 5‐FU 0.75‐1.0 iv gtt qd d1˜5 + PDD 60mg celiac injection qw˜biw X 8 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; rate of remission in short term | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Yang 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 39 cases; M: 23 cases, F: 16 cases; CP: 39 cases; M: 22 cases, F: 17 cases. | |
| Interventions | IP: Weitiao III 200ml bid X 2˜3 months; CP: CF 100mg iv gtt d1 + 5‐FU 0.5 iv gtt qd d1˜5 + L‐OHP 150˜200mg iv gtt d1 X 8˜12 weeks. | |
| Outcomes | QOL; MST in 1.5 years; rate of remission in short term; improvement of clinical symptoms | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Yang 2010.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 18 cases, F: 12 cases; CP: 30 cases; M: 18 cases, F: 12 cases. | |
| Interventions | IP: Shenqifuzheng injecta 250ml iv gtt qd X 3 weeks; CP: Nengliangheji (placebo) 250ml iv gtt qd X 3 weeks | |
| Outcomes | QOL; improvement of clinical symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Ye 2009.
| Methods | RCT, method unspecified | |
| Participants | IP: 35 cases; M: 22 cases, F: 13 cases; CP: 30 cases; M: 18 cases, F: 12 cases. | |
| Interventions | IP: Shenqifuzheng injecta 250ml iv gtt qd X 3 weeks; CP: Nengliangheji (placebo) 250ml iv gtt qd X 3 weeks. | |
| Outcomes | QOL; improvement of clinical symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
You 2000.
| Methods | RCT, method unspecified | |
| Participants | IP: 62 cases; M: 40 cases, F: 22 cases; CP: 58 cases; M: 33 cases, F: 25 cases; CP1: 56 cases; M: 34 cases, F22 cases. | |
| Interventions | IP: Weitiaopinghengfang one dosage bid X 4 weeks; CP: Xiaozheng Capsule 1g qd˜bid X 4 weeks; CP2: FAM regimen(no special dosage) X 4 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; immune function; MST in 10 years | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
You 2005.
| Methods | RCT, method unspecified | |
| Participants | IP: 62 cases; M: 50 cases, F: 12 cases; CP: 152 cases; M: 102 cases, F: 50 cases; CP1: 32 cases; M: 28 cases, F4 cases. | |
| Interventions | IP: 5‐FU 0.5 iv gtt qd d1˜5 + ADM 50˜60mg iv d1 + MMC 10mg iv d1(or OXA 150mg iv gtt d1 + CF 100mg iv gtt qd d1˜5 + 5‐FU 0.5 iv gtt qd d1˜5) X 56 days + Fuzhengheweiheji 30ml tid X 56 days; CP: Fuzhengheweiheji 30ml tid X 56 days; CP1: TCMHs one dosage bid X 56 days. | |
| Outcomes | rate of remission in short term; QOL; table of life span | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 1997.
| Methods | RCT, method unspecified | |
| Participants | IP: 35 cases; M: unclear, F: unclear; CP: 35 cases; M: unclear, F: unclear. | |
| Interventions | IP: MMC8mg, 5‐Fu750mg,V CR1mg (no special therapeutic period) + Shengyutang (no special therapeutic period); CP: MMC8mg, 5‐Fu750mg,V CR1mg (no special therapeutic period). | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; immune function; blood count | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2001.
| Methods | RCT, method unspecified | |
| Participants | IP: 35 cases; M: 28 cases, F: 7 cases; CP: 32 cases; M: 23 cases, F: 9 cases. | |
| Interventions | IP: VP‐16 100mg iv gtt qd d1˜5 + CF 100mg iv gtt qd d1˜5 + 5‐FU 0.5 iv gtt qd d1˜5 X 4 weeks + Huachansu 20ml iv gtt qd X 6 weeks; CP: VP‐16 100mg iv gtt qd d1˜5 + CF 100mg iv gtt qd d1˜5 + 5‐FU 0.5 iv gtt qd d1˜5 X 4 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2004.
| Methods | RCT, method unspecified | |
| Participants | IP: 43 cases; M: 26 cases, F: 43 cases; CP: 43 cases; M: 27 cases, F: 16 cases. | |
| Interventions | IP: Huachansu 20ml iv gtt qd X 15days + HCPT 7mg/m² iv gtt qd d1˜5 + CF 200mg iv gtt qd d1˜5 + 5‐FU 0.5 iv gtt qd d1˜5 X 3 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: be in hospital order. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2005.
| Methods | RCT, method unspecified | |
| Participants | IP: 28 cases; M: unclear, F: unclear; CP: 29 cases; M: unclear, F: unclear. | |
| Interventions | IP: Huachansu 50ml iv gtt qdX15 days + L‐OHP 130mg/m² iv gtt d1 + CF 200mg iv gtt qd d1˜3 + 5‐FU 0.5 iv gtt qd d1˜3 X 3 weeks; CP: L‐OHP 130mg/m² iv gtt d1 + CF 200mg iv gtt qd d1˜3 + 5‐FU 0.5 iv gtt qd d1˜3 X 3 weeks. | |
| Outcomes | toxic and side effects after chemotherapy; MST; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: be in hospital order. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2005a.
| Methods | RCT, method unspecified | |
| Participants | IP: 27 cases; M: unclear, F: unclear; CP: 29 cases; M: unclear, F: unclear. | |
| Interventions | IP: HCPT 10˜15mg iv gtt qd d1˜4 + 5‐FU 0.3 iv gtt qd d1˜3 + CF 100mg iv gtt qd d1˜3 X 6 weeks + Jinlong Capsule 4# tid X 6 weeks; CP: HCPT 10˜15mg iv gtt qd d1˜4 + 5‐FU 0.3 iv gtt qd d1˜3 + CF 100mg iv gtt qd d1˜3 X 6 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; improvement of clinical symptoms | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: unclear, F: unclear; CP: 30 cases; M: unclear, F: unclear. | |
| Interventions | IP: CHPT 5mg iv gtt qd d1˜5 X 3 weeks + Huachansu 20ml iv gtt qd X 3 weeks; CP: CHPT 5mg iv gtt qd d1˜5 X 3 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: be in hospital order. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2008.
| Methods | RCT, random number table | |
| Participants | IP: 36 cases; M: 24 cases, F: 12 cases; CP: 36 cases; M: 23 cases, F: 13 cases. | |
| Interventions | IP: Shenlingbaizhusanjiawei 100ml b.i.d X 15days + CF (200mg/m² ) iv gtt day1˜5 + CDDP (100mg/m² ) iv gtt day2 + 5‐FU (400mg/m² ) iv day1˜5 X 2 weeks; CP: CF (200mg/m² ) iv gtt day1˜5 + CDDP (100mg/m² ) iv gtt day2 + 5‐FU (400mg/m² ) iv day1˜5 X 2 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | the method of sequence generation was random number table. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2009.
| Methods | RCT, method unspecified | |
| Participants | IP: 35 cases; M: 20 cases, F: 15 cases; CP: 32 cases; M: 19 cases, F: 13 cases | |
| Interventions | IP: Aidi injecta 50ml iv gtt qd + L‐OXA (100mg/m²) iv gtt day1+ LV (200mg/m²) iv gtt day1˜2 + 5‐FU (400mg/m²) iv day1˜2 + 5‐FU (600mg/m²) iv day1˜2 X 9 weeks; CP: L‐OXA (100mg/m²) iv gtt day1+ LV (200mg/m²) iv gtt day1˜2 + 5‐FU (400mg/m²) iv day1˜2 + 5‐FU (600mg/m²) iv day1˜2 X 9 weeks. |
|
| Outcomes | toxic and side effects after chemotherapy; QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2010.
| Methods | RCT, random number table | |
| Participants | IP: 32 cases; M: 20 cases, F: 12 cases; CP: 32 cases; M: 19 cases, F: 13 cases. | |
| Interventions | IP: Fufangkushen injecta 20ml iv gtt day1˜10 + EPI (50mg/m²) iv gtt day1 + CDDP (60mg/m²) iv gtt day1 + 5‐FU (600mg/m²) iv day1˜5 X 3 weeks; CP: EPI (50mg/m² ) iv gtt day1 + CDDP (60mg/m²) iv gtt day1 + 5‐FU (600mg/m²) iv day1˜5 X 3 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL; pain; liver function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number table. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2010a.
| Methods | RCT, method unspecified | |
| Participants | IP: 22 cases; M: 14 cases, F: 8 cases; CP: 23 cases; M: 13 cases, F: 9 cases. | |
| Interventions | IP: Kanglaite 250ml iv gtt day1˜15 + Taxol (135mg/m²) iv gtt day1+ CDDP (200mg/m²) iv gtt day1˜5 + 5‐FU (500˜700mg/m²) iv gtt day1˜5 X 8 weeks; CP: Taxol (135mg/m²) iv gtt day1+ CDDP (200mg/m²) iv gtt day1˜5 + 5‐FU (500˜700mg/m²) iv gtt day1˜5 X 8 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; immune function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhang 2010b.
| Methods | RCT, method unspecified | |
| Participants | IP: 22 cases; M: 14 cases, F: 8 cases; CP: 23 cases; M: 13 cases, F: 9 cases. | |
| Interventions | IP: Aishu (Fufangkushen injecta) 20ml iv gtt day1˜14 + CDDP (20mg/m² ) iv gtt day1˜5 + CF(200mg/m²) iv gtt day1˜5 + 5‐FU (500mg/m²) iv gtt day1˜5 X 6˜ 8 weeks; CP: CDDP (20mg/m²) iv gtt day1˜5 + CF(200mg/m²) iv gtt day1˜5 + 5‐FU (500mg/m²) iv gtt day1˜5 X 6˜ 8 weeks. |
|
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | not offered by the authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhao 2005.
| Methods | RCT, method unspecified | |
| Participants | IP: 33 cases; M: unclear, F: unclear; CP: 26 cases; M: unclear, F: unclear. | |
| Interventions | IP: VP ‐ 16 80¡«100mg/m²,iv gtt qd d1˜5 + CF200mg/m²,iv gtt qd d1˜5 + 5‐FU500mg/m² iv gtt qd d1 ‐ 5 X 6 weeks + Shengmai injecta 50ml iv gtt qdX14 days; CP: VP ‐ 16 80¡«100mg/m²,iv gtt qd d1˜5 + CF200mg/m²,iv gtt qd d1˜5 + 5‐FU500mg/m² iv gtt qd d1 ‐ 5 X 6 weeks. | |
| Outcomes | rate of remission in short term; toxic and side effects after chemotherapy; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zheng 1999.
| Methods | RCT, method unspecified | |
| Participants | IP: 28 cases; M: 19 cases, F: 9 cases; CP: 22 cases; M: 13 cases, F: 9 cases. | |
| Interventions | IP: 5‐FU 0.6 iv gtt qd d1˜5 + MMC8˜10mg iv d1 + ADM 30˜40mg iv d1 X 12˜16 weeks + TCMHs 0. 5 dosage qd X 6˜8 weeks; CP: 5‐FU 0.6 iv gtt qd d1˜5 + MMC8˜10mg iv d1 + ADM 30˜40mg iv d1 X 12˜16 weeks. | |
| Outcomes | rate of remission in short term | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhou 2000.
| Methods | RCT, method unspecified | |
| Participants | IP: 24 cases; M: unclear, F: unclear; CP: 12 cases; M: unclear, F: unclear. | |
| Interventions | IP: Kangyan I 50ml iv gtt X 60 days; CP: MMC 6˜8mg iv d1 + 5‐FU 0.5 iv gtt d1˜5 X 2 months. | |
| Outcomes | QOL; immune function; toxic and side effects after chemotherapy; life span | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhu 2005.
| Methods | RCT, method unspecified | |
| Participants | IP: 30 cases; M: 24 cases, F: 6 cases; CP: 30 cases; M: 20 cases, F: 10 cases. | |
| Interventions | IP: Sanwubaisanjiaweifang 7.04˜10.56 bid + chemotherapy (no special dosage) X 4 weeks; CP: chemotherapy(no special dosage) X 4 weeks. | |
| Outcomes | rate of remission in short term; QOL | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
Zhu 2006.
| Methods | RCT, method unspecified | |
| Participants | IP: 40 cases; M: 22 cases, F: 18 cases; CP: 40 cases; M: 21 cases, F: 19 cases. | |
| Interventions | IP: VP‐16 200 mg/ m² + ADM 60 mg/ m² + carboplatin 200 mg/ m² intra‐aterial chemotherapy Qow+Fuzhengkang'aichongji 60g bid X 2 months; CP: VP‐16 200 mg/ m² + ADM 60 mg/ m² + carboplatin 200 mg/ m² intra‐aterial chemotherapy Qow X 2 months. | |
| Outcomes | rate of remission in short term; immune function; QOL; MST | |
| Notes | IP: interventional group CP: control group M: male F: female | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | the method of sequence generation was not offered by the authors. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not offered by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none of them lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the rating scales for cognition listed in Methods reported. |
IP: interventional group CP: control group M: male F: female
QOL: quality of life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ben 2010 | this article was not a RCT, and the selected patients in the clinical trial had no TNM stage |
| Bu 2001 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Cao 2010 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Chen 1997b | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Chen 2004 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Chen 2011 | this article was not a clinical RCT |
| Cui 2009 | this article was not a clinical RCT |
| Da 2003 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Deng 2003 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Gao 2003 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Gao 2006 | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Gao 2011 | the selected patients in the clinical trial had no description of TNM stage |
| Ge 2010 | the selected patients in the clinical trial had no stage of TNM, and the diagnostic standard did not match the new TNM descriptive stage for late gastric cancer, so the trial should be excluded |
| Gu 2006 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Guo 2003 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Han 2005 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| He 2010 | the selected patients in the clinical trial had no description of TNM stage |
| Hu 2001 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Hu 2009 | the selected patients in the clinical trial had no TNM stage, and the illness of some selected patients in the clinical trial were not in late or advanced stage |
| Hu 2010 | the illness of some selected patients in the clinical trial were not in late or advanced stage |
| Huang 2002a | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Huang 2008 | this article was not a RCT |
| Huang 2009 | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Huo 2009 | the selected patients in the clinical trial had no description of TNM stage |
| Jiang 2011 | the selected patients in the clinical trial had no description of TNM stage |
| Jiao 2001 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Ke 2010 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Lai 2010 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Li 2006 | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Li 2006a | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Li 2008 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Li 2011 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Liu 1999 | it was not a clinical trial of TCM for gastric cancer |
| Liu 2002a | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Liu 2003 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Liu 2009b | the selected patients in the clinical trial had no TNM stage |
| Liu 2011 | the selected patients in the clinical trial had I, II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Lu 1998 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Lu 2010 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Luo 2009 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Mo 2010 | it was not a clinical RCT trial |
| Ni 2005 | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Ni 2010 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Pan 2009 | the selected patients in the clinical trial had no description of TNM stage |
| Qin 2010 | the selected patients in the clinical trial had II stage of TNM |
| Qiu 1992 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Qu 1997 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Qu 2010 | the selected patients in the clinical trial had no description of TNM stage |
| Ren 2008 | this article was not a clinical RCT |
| Shi 2010 | this article was not a clinical RCT |
| Shu 2010 | the selected patients in the clinical trial had no description of TNM stage |
| Shu 2010a | the selected patients in the clinical trial had no description of TNM stage |
| Su 1993 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Sun 2010 | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Tian 2006 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Tian 2011 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Wang 1996 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Wang 2003 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Wang 2004b | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Wang 2006 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Wang 2008 | the selected patients in the clinical trial had no TNM stage, and this article was not a RCT |
| Wang 2008a | the selected patients in the clinical trial had no TNM stage |
| Wang 2009b | the selected patients in the clinical trial had no TNM stage |
| Wang 2010c | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Wang 2011 | this article was not a clinical RCT |
| Wen 1997 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Wu 1992 | it was not a clinical trial of TCM for gastric cancer |
| Wu 2004 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Wu 2009 | this article was not a clinical RCT |
| Wu 2010 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Xie 2004 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Xiong 2006 | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Xu 1999a | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage. |
| Xu 2005 | the selected patients in the clinical trial had I, II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Yan 2010 | the selected patients in the clinical trial had I, II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Yang 1998 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Yang 2006a | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Ye 2008 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Yin 1996 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Yin 1999 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| You 2009 | this article was not a clinical RCT |
| Yu 2006 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhang 1987 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhang 2000 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhang 2009a | the selected patients in the clinical trial had no TNM stage |
| Zhang 2010c | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Zhao 1991 | the selected patients in the clinical trial were not in late or advanced stage, so the trial should be excluded |
| Zhao 2009 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhao 2011 | the selected patients in the clinical trial had II stage of TNM, besides III and IV stage of TNM, so the trial should be excluded |
| Zheng 1996 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zheng 1996a | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhou 2000a | the same Traditional Chinese Medicine (TCM) was given in intervention group and control group in the clinical, and another physical therapeutic method was used in the intervention group with the TCM, so this clinical trial should be excluded because of not belonging to the field of TCM for gastric cancer |
| Zhou 2000b | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhu 1996 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhu 1999 | the selected patients in the clinical trial had no TNM stage, and the illness of some patients were not in late or advanced stage |
| Zhu 2004 | it was not a clinical trial of TCM for gastric cancer, but for ileus caused by gastric cancer |
| Zhu 2008 | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
| Zhu 2009 | the selected patients in the clinical trial had no TNM stage |
| Zhu 2009a | it was not a clinical RCT trial with same interventional formula of TCM for gastric cancer |
Contributions of authors
Linlin Zhu and Jinlin Yang revised the review.
Tao Gan designed and revised previous versions of the review, and controlled the overall quality of the review.
Tao Gan and Zongying Wu wrote the first draft of the review.
Ling Tian and Zongying Wu performed handsearches, retrieved papers, and extracted data.
Yiping Wang conceived the idea for the review and gave some suggestions for the review.
Sources of support
Internal sources
-
Chinese Cochrane Center, Huaxi Hospital of Sichuan University, China.
Provided the data, information and cost‐free search for this review.
External sources
-
Danish Cancer Society, Denmark.
Provided the funding for this review.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cao 1992 {published data only}
- Cao Y, Li N, Zhang L, Hua L, Li Z. Clinical observation about association with Western and Traditional Chinese Medicines treating 70 patients with advanced gastric cancer. Clinical Journal of Traditional Chinese Medicine 1992;2(3):8‐9. [Google Scholar]
Cao 1997 {published data only}
- Cao J, Xiao X, Tang X. Observation of effect about Elemene plus fluorouracil and acupuncturing Shenque treat advanced gastric carcinoma. Chinese Clinic of Oncology 1997;24(7):549‐50. [Google Scholar]
Chen 1997 {published data only}
- Chen N, Jin Y, Lai Y. Observation of effect Pingxiao capsule combined chemotherapy treats advanced gastric cancer. Straits Pharmacy 1997;9(1):66‐7. [Google Scholar]
Chen 1997a {published data only}
- Chen N, Jin Y, Liu Y, Chen Y. Observation of effect about hydroxycamptothecin combined with Chinese medicine treating advanced gastric carcinoma of 41 cases. Fujian Journal of Medicine 1997;19(2):82. [Google Scholar]
Chen 2005 {published data only}
- Chen P, Gao H, Song J, Zheng W. ELF connected with Cidan capsule treat gastric carcinoma of 64 cases. Chinese Clinic of Oncology 2005;32(8):466‐7. [Google Scholar]
Chen 2008 {published data only}
- Chen NJ, Wu DH, Lai YQ, Chen YY. Combining AIDI and FOLFOX4 treated advanced gastric cancer. Guangming Journal of Chinese Medicine 2008;23(11):1768‐9. [Google Scholar]
Chen 2009 {published data only}
- Chen HM. The effect of Huachansu with TPF regimen for late gastric cancer. Journal of Emergency in Traditional Chinese Medicine 2009;18(1):35‐6. [Google Scholar]
Deng 2001 {published data only}
- Deng W, Guan C. Elemene plus chemotherapy influence on immunity of older progressive gastric carcinoma. Journal of Guangdong Medical College 2001;19(5):350. [1005‐4057(2001)‐05‐0350‐01] [Google Scholar]
Deng 2011 {published data only}
- Deng ZJ, Cao HN, Gao TN. Shenfu Injection with PCF chemotherapy for late or advanced gastric cancer in 40 cases. Contemporary Medicine 2011;17(12):116‐8. [Google Scholar]
Du 2010 {published data only}
- Du CJ, Wang TL, Guo YS. Clinical observation of quality of life in patients with gastric carcinoma treated by combination of traditional Chinese medicine, chemotherapy and hyperthermia treatment. Hebei Journal of Traditional Chinese Medicine 2010;32(9):1299‐301. [Google Scholar]
Fu 2011 {published data only}
- Fu JW, Deng Y, Huang W. Compound matrine injection with chemotherapy in the treatment of pain from advanced or late gastric cancer in 40 patients. Journal of Emergency in Traditional Chinese Medicine 2011;20(1):152‐3. [Google Scholar]
Gao 2008 {published data only}
- Gao P, Fang XH, Zhang Q, Yang F, Yang ZB, Zhang XC, et al. Clinical Random Control Study on Advanced Gastric Cancer with Mijisan Granule Infusion Combined with FLO Plan. Journal of Zhejiang Chinese Medicine College 2008;32(6):756‐8. [Google Scholar]
Gong 2006 {published data only}
- Gong L, Jiang C, Zhao Y. Aidi combining with chemotherapy treat progressive gastric carcinoma. Journal of Oncology 2006;12(5):424‐5. [Google Scholar]
Guan 2001 {published data only}
- Guan C, He G, Yin Z. Effect of elemene emulsion combined chemotherapy on immunity of gastric cancer patients in progressive stage. Chinese Clinic of Oncology 2001;28(2):123‐4. [1000‐8179(2001)‐02‐0123‐02] [Google Scholar]
Guo 1989 {published data only}
- Guo L, Chen G. Observation of efficacy about association with Western and Traditional Chinese Medicines treats 90 patients with advanced gastric carcinoma. Fujian Medicine Journal 1999;11(1):19‐20. [Google Scholar]
Hu 2011 {published data only}
- Hu Y, Hou AJ, Zhang HW, Huang YL, Gu WF. Clinical observation of Fuzheng Xiao'ai prescription I combined with OFL regimen for treatment advanced gastric cancer. Journal of New Chinese Medicine 2011;43(2):104‐6. [Google Scholar]
Hua 1999 {published data only}
- Hua B, Wang A, Hou W. Clinical study on treatment of mid‐late stage gastric carcinoma by composite Xiansu capsule combined with chemotherapy. Journal of Combination of Traditional Chinese Medicine with Western Medicine 1999;19(8):470‐3. [PubMed] [Google Scholar]
Huang 2002 {published data only}
- Huang Z, Si Z, Luo Y, Huang N. Clinical efficacy of Toad Venom injection combined with chemotherapy in treating 31 patients with medium and advanced gastric cancer. Hebei Journal of Traditional Chinese Medicine 2002;24(3):163‐5. [Google Scholar]
Huang 2005 {published data only}
- Huang X, Song A. Clinical research on elemene combined chemotherapy treating gastric cancer. Chinese Traditonal Medicine 2005;5(3):40‐1. [Google Scholar]
Jia 2003 {published data only}
- Jia L, Zhu S, Li P, Zhang K, Wan D, Cai G, et al. Therapeutic effect observation about Aidi, Cisplatin and Fluorouracil treating gastric carcinoma. Journal of China Oncology 2003;25(5):517. [Google Scholar]
Jia 2009 {published data only}
- Jia JW, Liu YQ. The effect of Shenqi Fuzheng injection combined with FOLFOX4 regimen in the treatment of advanced gastric cancer. The Practical Journal of Cancer 2009;24(3):273‐5. [Google Scholar]
Jiang 1994 {published data only}
- Jiang Y. Clinical observation about invigorating the spleen and removing cancer ingredient treating advanced gastric carcinoma 52 cases. Human Journal of Traditional Chinese Medicine 1994;10(4):3‐5. [Google Scholar]
Li 2001 {published data only}
- Li X, Wei P. Effect observation about Jinlongshe for oral use treating advanced gastric carcinoma. Hubei Journal of Traditional Chinese Medicine 2001;23(11):3‐5. [Google Scholar]
Li 2002 {published data only}
- Li S, He L, Song Y. A clinical report of Yiqihuoxue Recipe combinating with FAP regimen for improving the living qualities in patients with gastric cancer in advanced or late stage. Journal of Sichuan of Traditional Chinese Medicine 2002;20(12):33‐4. [Google Scholar]
Lin 2011 {published data only}
- Lin CL, Ge JH. Clinical research of gastric cancer at the late stage treated with Compound Spophora Flavescens injection combined with chemotherapy. World Journal of Integrated Traditional and Western Medicine 2011;6(4):316‐8. [Google Scholar]
Liu 2002 {published data only}
- Liu Y, Zhou J. Association with Western and Traditional Chinese Medicines treats 30 patients with advanced gastric cancer. Journal of Shandong Traditional Chinese Medicine 2002;21(3):164‐5. [0257‐358X(2002)03‐0164‐02] [Google Scholar]
Liu 2006 {published data only}
- Liu H, Zeng B, Xu L. Observation of decoction of eight ingredients combined with chemotherapy treating advanced gastric carcinoma. Modern Journal of Integrated Traditional Chinese and Western Medicine 2006;15(24):3366. [Google Scholar]
Liu 2006a {published data only}
- Liu J, Hu D, Fan H, Niu L. Clinical observation of Tianlong treating advanced gastric cancer of 30 cases. Hebei Traditional Chinese Medicine 2006;28(10):739‐40. [1002‐2619(2006)10‐739‐02] [Google Scholar]
Liu 2009 {published data only}
- Liu LH, Zhang CX. Clinical efficacy of Aidi injection combined with TPF in the treatment of advanced gastric carcinoma. China Modern Doctor 2009;47(29):16‐7. [Google Scholar]
Liu 2009a {published data only}
- Liu SL, Ding C, Gu XX. Compound matrine injection with chemotherapy in the treatment of 29 patients with advanced gastric cancer. Chinese Journal of Coal Industry Medicine 2009;12(10):1566‐7. [Google Scholar]
Luo 2011 {published data only}
- Luo PF. The effect of Shenqi Fuzheng injection combined with chemotherapy for advanced gastric cancer. Modern Medicine & Health 2011;27(8):1170‐1. [Google Scholar]
Lv 1999 {published data only}
- Lv B, Yang J. Analysis of efficacy about association Western and Traditional Chinese medicines treating 48 patients with progressive and advanced gastric cancer. Chinese Traditional Treatment 1999;2(2):24‐5. [Google Scholar]
Niu 2006 {published data only}
- Niu H, Mu Y. Complex prescription of the TCM and chemotherapy treat 120 patients with advanced gastric carcinoma. Shanxi Traditional Chinese Medicine 2006;26(9):1034‐5. [Google Scholar]
Peng 2006 {published data only (unpublished sought but not used)}
- Peng Z, Zhang Y, Chen M. Strengthing‐body‐resistance decoction effect on immunity of gastric cancer patients. Liaoning Journal of Traditional Chinese Medicine 2006;33(11):1459. [1000‐1719(2006)11‐1459‐01] [Google Scholar]
Rao 1994 {published data only}
- Rao F, Yu R, Hu Y, Wang Y, Jin J, Tian Z. Long‐term effect observation Shengxuetang combined with chemotherapy treats advanced gastric cancer. Chinese Journal of Traditional Chinese Medicine 1994;14(6):366. [Google Scholar]
Shao 1998 {published data only}
- Shao J. clinical study of compound Zhenjian liquid in treating advanced gastric cancer. Journal of Traditional Chinese Medicine 1998;39(8):479‐80. [Google Scholar]
Shi 2004 {published data only}
- Shi L, Ye Y, Luo S, Chen J, Yan X. Effect on life quality and immune about Huachansu combined with Chinese medical differentiation of symptoms and signs in treating gastric carcinoma. Journal of Zhejiang Chinese Medicine College 2004;28(6):20‐1. [Google Scholar]
Si 2004 {published data only}
- Si H, Shang G, Lu W. Observation of efficacy about TCM and chemo treating advanced gastric cancer. Jiangsu Journal of Traditional Chinese Medicine 2004;35(258):40‐1. [Google Scholar]
Sun 1999 {published data only}
- Sun G, Li J. Clinical and experimental research about Qiao‐Wei‐Kang‐Liu dissolved medicines combined with chemotherapy treating advanced gastric cancer. Medical Research Communication 1999;28(6):6‐7. [Google Scholar]
Tian 1999 {published data only}
- Tian R, Yang J, Zhang B, Li G. Clinical observations of gastric cancer patients treated with chemotherapy and Lanxiangxiru as a MDR mediator treated with chemotherapy and Elemene as a MDR mediator. Cancer Research on Prevention and Treatment 1999;26(3):215‐6. [Google Scholar]
Wang 1993 {published data only}
- Wang R, Wu D, Jin Y, Chen J, Liu Y, Huang P, et al. The short‐term effects of Jianpijiandu Decoction plus chemotherapy for advanced gastric cancer. Fujian Journal of Traditional Chinese Medicine 1993;24(5):23‐6. [Google Scholar]
Wang 1998 {published data only}
- Wang G, Zhu J, Xu W, Wang Y, Zhou A. Clinical and experimental studies on Fuzheng granula combined with chemotherapy in advanced gastric cancer. World Chinese Journal of Digestology 1998;6(3):214‐8. [ISSN1007‐9319 CN 14‐1218/R] [Google Scholar]
Wang 2002 {published data only}
- Wang X, Wang R, Dai H. Invigorating the spleen and removing cancer ingredient combined with chemotherapy treat older advanced gastric carcinoma of 90 cases. Shandong Journal of Chinese Medicine 2002;21(9):527‐8. [0257‐358X(2002)09‐0527‐02] [Google Scholar]
Wang 2004 {published data only}
- Wang P. Observation effect in chemotherapy, radiotherapy and Yadanzi oil treating advanced gastric carcinoma. Journal of Practical Oncology 2004;19(1):78‐9. [1001‐1692(2004)01‐0078‐02] [Google Scholar]
Wang 2004a {published data only}
- Wang R, Pan Y, Ye Z. Clinical observation about Invigorating the spleen and dissolving petechia mixtura combined with chemotherapy treating advanced gastric carcinoma of 24 cases. Jiangsu Traditional Chinese Medicine 2004;25(11):22‐3. [1672‐397X(2004)11‐0022‐02] [Google Scholar]
Wang 2009 {published data only}
- Wang ZL, Shen HL, Zhou G, Li ZY. The effects of Aidi injection to assist selective infusion chemotherapy in treatment of advanced gastric cancer. Journal of Zhejiang Chinese Medical University 2009;33(2):219‐20. [Google Scholar]
Wang 2009a {published data only}
- Wang YH. The effect of Huachansu with chemotherapy for advanced or late gastric cancer. Jaingxi Journal of Traditional Chinese Medicine 2009;40(4):31‐2. [Google Scholar]
Wang 2010 {published data only}
- Wang JF. The effect of Shenqi Fuzheng injection combined with chemotherapy and radiotherapy for local advanced gastric cancer. Chinese Journal of Traditional Medical Science and Technology 2010;17(6):537‐8. [Google Scholar]
Wang 2010a {published data only}
- Wang J, Wang KM, Wang ZX, Lu BB, Li J. Compound matrine injection combined with DCF chemotherapy in the treatment of 25 patients with advanced gastric carcinoma. Chinese Journal of New Drugs 2010;19(17):1585‐8. [Google Scholar]
Wang 2010b {published data only}
- Wang WM, Li CF, Yao RJ. The clinical effect of Huachansu with chemotherapy for late gastric cancer. Clinical Journal of Traditional Chinese Medicine 2010;22(4):314‐5. [Google Scholar]
Wu 1999 {published data only}
- Wu Y, Cai M, Zhu X. Following up of 5 years about Western and Chinese Traditional Medicines treating advanced gastric cancer. Journal of Nanjing University of Traditional Chinese Medicine 1999;15(2):124. [Google Scholar]
Wu 2000 {published data only}
- Wu Y. Analysis of the therapeutic effect of Fuzhenggongjian capsule on the late gastric carcinoma under chemotherapy. Journal of Zhengjiang Medical College 2000;10(4):656‐7. [Google Scholar]
Wu 2000a {published data only}
- Wu C, Li Q, Zhao Y, Li Q. Controlled observation on Elemene plus chemotherapy treating advanced gastric cancer. Journal of North Sichuan Medical College 2000;15(3):12‐3. [1005‐3697(2000)03‐0012‐02] [Google Scholar]
Xie 2006 {published data only}
- Xie C. Observation of effect Lailikang granula combined with chemotherapy treat advanced gastric carcinoma. Modern Medicine 2006;22(6):882‐3. [1009‐5519(2006)‐06‐0882‐02] [Google Scholar]
Xiong 2008 {published data only}
- Xiong LG. Clinical observation of Yanshu injection combined with paclitaxel and oxaliplatin in the treatment of advanced gastric cancer. The Practical Journal of Cancer 2008;23(3):276‐7. [Google Scholar]
Xu 1989 {published data only}
- Xu Y. The effects of Yangxue decoction plus chemotherapy for late gastric cancer after operation. Journal of Practical Oncology 1989;3(1):54‐6. [Google Scholar]
Xu 1993 {published data only}
- Xu D, Zhang X. Clinical observation about Ai‐Fu‐Kang plus chemotherapy treating patients with progressive gastric cancer after operation. Shanxi Traditional Chinese Medicine 1993;9(4):14. [Google Scholar]
Xu 1999 {published data only}
- Xu Y. Clinical efficacy study Fuzhenghuoxue combined with chemotherapy in treating advanced gastric cancer. Hebei Journal of Traditional Chinese Medicine 1999;21(6):329‐31. [Google Scholar]
Yang 2005 {published data only}
- Yang Z, Yuan X, Li G. Shenfu combining with ELFP treat advanced gastric carcinoma of 40 cases. Chinese Medical Science Study 2005;18(7):26‐7. [Google Scholar]
Yang 2006 {published data only}
- Yang Z, You J. No 3 readjust of Chinese medicine treat advanced gastric carcinoma by differentiation of symptoms and signs. Liaoning Journal of Traditional Chinese Medicine 2006;33(11):1434‐5. [1000‐1719(2006)11‐1434‐02] [Google Scholar]
Yang 2010 {published data only}
- Yang SP, Du B. The effect of Shenqi Fuzheng injection for advanced or late gastric cancer in old patients. Chinese Community Doctors 2010;12:87. [Google Scholar]
Ye 2009 {published data only}
- Ye SF, Ye B, Wu M. The effect of Shenqi Fuzheng injection for advanced or late gastric cancer in old patients. Journal of Wenzhou Medical College 2009;39(2):172‐3. [Google Scholar]
You 2000 {published data only}
- You J, Zhao J. Readjusting and balancing methods of doctor Zhao treat advanced gastric cancer of 176 cases. Jilin Traditional Chinese Medicine 2000;20(5):10‐11. [Google Scholar]
You 2005 {published data only}
- You J, Zhao J, Zhou L. Clinical study about Fuzhenghewei in treating fourth stage gastric cancer. Jiangsu Chinese Medicine 2005;26(11):11‐3. [Google Scholar]
Zhang 1997 {published data only}
- Zhang F. Clinic research that Shengyutang treats adverse reaction after advanced gastric cancer patients receive chemotherapy. Journal of Changchun College of Traditional Chinese Medicine 1997;13(61):18. [Google Scholar]
Zhang 2001 {published data only}
- Zhang C, Wang Q. Huachansu combined with chemotherapy treat advanced 35 cases of gastric carcinoma. Journal of Anhui Traditional Chinese Medicine College 2001;20(4):18‐9. [1000‐2219(2001)‐04‐0018‐01] [Google Scholar]
Zhang 2004 {published data only}
- Zhang R, Chen C, Shen B, Zhou D, Zhang G. Clinical observation of Huachansu combined with chemotherapy treating advanced gastric carcinoma. Chinese Clinical Oncology 2004;9(3):269‐70. [1009‐0460(2004)‐03‐0269‐02] [Google Scholar]
Zhang 2005 {published data only}
- Zhang Y, Zhu M, Cao T, Zhang P, Yao L, Huang H. Observation of curative effect of Huachansu combined with chemotherapy treating progressive and advanced gastric carcinoma. Henan Journal of Oncology 2005;18(5):359‐60. [1003‐1464(2005)05‐0359‐02] [Google Scholar]
Zhang 2005a {published data only}
- Zhang J, Wang H, Zhang Y. Jinlong capsule combined with HFL treat advanced gastric carcinoma. Capital Medicine 2005;9(23):33. [Google Scholar]
Zhang 2006 {published data only}
- Zhang Z, Wang Y, Rong D. Late effect of Huachansu combined with hydroxy. Practical Journal of Medicine and Pharmaceuticals 2006;23(7):794‐5. [Google Scholar]
Zhang 2008 {published data only}
- Zhang H, Su ZX. Curative effect of Shenling Baizhu powder on chemotherapy‐induced toxicity in advanced gastric cancer. Journal of TCM University of Hunan 2008;28(2):51‐3, 56. [Google Scholar]
Zhang 2009 {published data only}
- Zhang AX. Aidi injection with chemotherapy for advanced or late gastric cancer. Journal of Medical Theory & Practice 2009;22(10):1214. [Google Scholar]
Zhang 2010 {published data only}
- Zhang JJ, Wei JH, Wei S, Wei AF, Lan C, Zhang F'E. ECF with compound injection of radix sophorae flavescentis for late gastric cancer in 32 patients. Guangji Medical Journal 2010;32(5):533‐5. [Google Scholar]
Zhang 2010a {published data only}
- Zhang L, Wang YF. The effect and immune function of Kanglaite with chemotherapy for advanced or late gastric cancer. Acta Universitatis Medicinalis Nanjin (Natural Science) 2010;30(11):1657‐9. [Google Scholar]
Zhang 2010b {published data only}
- Zhang MJ, Zuo CF. Observation of clinical efficacy of Yanshu injection combined with NP chemotherapy in treatment of advanced gastric cancer. Chinese Journal of Clinical Oncology and Rehabilitation 2010;17(6):531‐3. [Google Scholar]
Zhao 2005 {published data only}
- Zhao Z, Liao Z, Zhao X. The clinic research of the effect of Sheng Mai injection combined with the chemotherapy in the elderly patients with advanced gastric cancer. Modern Oncology 2005;13(3):387‐8. [1672‐4992(2005)03‐387‐02] [Google Scholar]
Zheng 1999 {published data only}
- Zheng L, Sun K. Research of efficacy about Western and Traditional Chinese Medicines treating progressing and advanced gastric cancer. Heilongjiang Medicine and Pharmacy 1999;22(1):86‐7. [Google Scholar]
Zhou 2000 {published data only}
- Zhou R, Wu L, Ni A, Xu Z. Clinical observation of intravenous drip of "Anti‐inflammation1" in treating 24 patients with mid and late gastric carcinoma. Shanghai Journal of Traditional Chinese Medicine 2000;34(8):15‐6. [1007‐1334(2000)08‐0015‐02] [Google Scholar]
Zhu 2005 {published data only}
- Zhu H, Zhu Z, Xu L. White Powder of Three Ingredients Medicine treats progressive gastric cancer of 30 cases. Journal of Nanjing Traditional Chinese Medicine University 2005;21(5):284‐5. [1000‐5005(2005)05‐0284‐02] [Google Scholar]
Zhu 2006 {published data only}
- Zhu J, Song M, Wang L, Sun Q, Zhu L, Fang C. Immunoregulation and short‐term therapeutic effect of super‐selective intra‐arteral chemotherapy combined with traditional Chinese drugs on gastric cancer patients. Journal of Chinese Integrative Medicine 2006;4(5):478‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ben 2010 {published data only}
- Ben BJ. The effect of Kang'ai injection with chemotherapy for late gastric cancer in 38 old patients. Shanxi Journal of Traditional Chinese Medicine 2010;31(5):546‐7. [Google Scholar]
Bu 2001 {published data only}
- Bu X. The effects of Xindekang on apoptosis of advanced stage stomach cancers. Herald of Medicine 2001;20(10):616‐7. [1004‐0781(2001)10‐0616‐02] [Google Scholar]
Cao 2010 {published data only}
- Cao J. The analysis of curative effect of traditional Chinese medicine combined with FOLFOX‐4 chemotherapy on treatment for the patients with advanced gastric cancer. Liaoning Journal of Traditional Chinese Medicine 2010;37(3):493‐5. [Google Scholar]
Chen 1997b {published data only}
- Chen X. Long‐term observation of effect about Yi‐Ai‐San treating advanced gastric cancer. Journal of New Chinese Medicine 1997;29(1):34. [Google Scholar]
Chen 2004 {published data only}
- Chen J, Chen Q, Huang Z, Luo X, Zhang Y, Huang X. Therapeutic effect observation about Aidi treating the senior advanced gastric carcinoma of 32 cases. Journal of West China Pharmacy 2004;19(2):157‐8. [Google Scholar]
Chen 2011 {published data only}
- Chen QS. The effect of Yiqijianpi decoction combined with FOLFOX regimen for advanced or late gastric cancer in 30 patients. Yunnan Journal of Traditional Chinese Medicine and Materia Medica 2011;32(4):45‐7. [Google Scholar]
Cui 2009 {published data only}
- Cui P. The effect of Huachansu for late gastric cancer. Journal of Liaoning Medical University 2009;30(4):333‐4. [Google Scholar]
Da 2003 {published data only}
- Da N, Ren D, Shuai S, Guo C, Fu Y, Long D. Efficacy of Yishou Xiaozhen Powder in patients with advanced gastric cancer. Medical Journal of West China 2003;1(1):67‐9. [Google Scholar]
Deng 2003 {published data only}
- Deng F, Chen Y. Wei‐kang combined with CF and 5‐Fu treats advanced gastric cancer of 30 cases. Straits Pharmacy 2003;15(1):54. [1006‐3765(2003)‐01‐0054‐01] [Google Scholar]
Gao 2003 {published data only}
- Gao P. Clinical observation of conclusion Huweixiaoji prescription combined with FF207 treating older patient with advanced gastric carcinoma. Journal of Henan University of Chinese Medicine 2003;2(18):35‐6. [1006‐3234(2003)03‐0035‐02] [Google Scholar]
Gao 2006 {published data only}
- Gao S, Wang X, Pan Q. Eight dainties drops combining with enterocoelia chemotherapy treat progressive postoperative gastric carcinoma. Journal of Northern China Coal Medical College 2006;8(2):199‐200. [Google Scholar]
Gao 2011 {published data only}
- Gao J. Avanica oil joint MF/CF clinical observation of advanced gastric cancer chemotherapy. Medical Journal of Chinese Peoples' Health 2011;23(9):1108‐9. [Google Scholar]
Ge 2010 {published data only}
- Ge YL. Clinical efficacy of Aidi injection combined with MF/CF in the treatment of advanced gastric carcinoma. Chinese Journal of Ethnomedicine and Ethnopharmacy 2010;19(10):31‐2. [Google Scholar]
Gu 2006 {published data only}
- Gu W, Jiao J. Clinical observation that Pulse‐activating Injection plus chemotherapy treats advanced gastric cancer of 36 cases. New Journal of Traditional Chinese Medicine 2006;38(10):59‐60. [0256‐7415(2006)10‐0059‐02] [Google Scholar]
Guo 2003 {published data only}
- Guo Y, Li Y, Huang L, Jiao Z. Clinical study of compound Xuanju capsules combined with chemotherapy in treating moderate and advanced gastric cancer. Chinese Journal of Integrated Traditional and Western Medicine 2003;23(12):941‐2. [Google Scholar]
Han 2005 {published data only}
- Han Y, Si P. Clinical observation in chemotherapy combined with herbs treating 30 cases of progressive gastric cancer after operation. Shanxi Journal of Traditional Chinese Medicine 2005;21(2):13. [1000‐7156(2005)02‐0013‐01] [Google Scholar]
He 2010 {published data only}
- He GJ. The effect of Yiqijianpiyangyin Decoction combined with chemotherapy for advanced or late gastric cancer. Liaoning Journal of Traditional Chinese Medicine 2010;37(12):2400‐1. [Google Scholar]
Hu 2001 {published data only}
- Hu P. Fuzhenghewei combined with HFC treat gastric cancer of 90 cases. Journal of Nanjing Chinese Medicine University 2001;17(5):274. [Google Scholar]
Hu 2009 {published data only}
- Hu PP. The effect of Fuzhenghewei Decoction with OLF therapeutic regimen for late gastric caner in 30 patients. Shanxi Journal of Traditional Chinese Medicine 2009;30(1):6‐7. [Google Scholar]
Hu 2010 {published data only}
- Hu PP, Wang WS, Xue Q. The effect of Jianpisanjie Decoction with FOLFOX regimen for late gastric cancer in 28 patients. Journal of New Chinese Medicine 2010;42(6):76‐7. [Google Scholar]
Huang 2002a {published data only}
- Huang Z, Li H, Zhang Z, Tan Z, Lu Y, Chen C, et al. Jianpixiaojitang combined with chemotherapy treat advanced gastric carcinoma of 30 cases. Shanxi Journal of Traditional Chinese Medicine 2002;18(4):35‐6. [1000‐7156(2002)04‐0035‐02] [Google Scholar]
Huang 2008 {published data only}
- Huang XN, You JL. The effect of Weitiao III with paclitaxel for advanced or late gastric cancer. Journal of Liaoning University of Traditional Chinese Medicine 2008;10(11):113‐4. [Google Scholar]
Huang 2009 {published data only}
- Huang ZF, Liu JB, Li HZ, Huang CJ. Effect of combination of Fufang Ku Shen Zhusheye and chemotherapy for treatment of 30 advanced gastric cancer patients. West China Medical Journal 2009;24(11):2883‐5. [Google Scholar]
Huo 2009 {published data only}
- Huo Y, Cheng G. The effect of Xiao'aiping combined with chemotherapy for advanced or late gastric cancer. Chinese Community Doctors 2009;11(18):138. [Google Scholar]
Jiang 2011 {published data only}
- Jiang H. The effect of traditional Chinese medicine with western medicine for advanced or late gastric cancer. Heilongjiang Journal of Traditional Chinese Medicine 2011;53(1):8. [Google Scholar]
Jiao 2001 {published data only}
- Jiao G, Zhou C, Xu G, Chen B, Sun C. The clinical research on Curcum in and chlorophyll on auxiliary therapy to gastric cancer. Acta Nutrinenta Sinica 2001;23(3):237‐8. [Google Scholar]
Ke 2010 {published data only}
- Ke YF, Yi J. The effect of Aidi injectio with chemotherapy for late gastric cancer. Public Medical Forum Magazine 2010;14:1107‐8. [Google Scholar]
Lai 2010 {published data only}
- Lai YQ, Chen NJ, Wu DH, Yu JP. The clinical effect of self‐made Jianpiyishen Decoction with chemotherapy for late gastric cancer in 25 patients. Fujian Journal of Traditional Chinese Medicine 2010;41(5):18‐9. [Google Scholar]
Li 2006 {published data only}
- Li R. Clinical observation about Yan‐Shu injection combined with chemotherapy treating mild and advanced gastric cancer. Chinese Community Doctor 2006;8(16):60. [Google Scholar]
Li 2006a {published data only}
- Li H, Pan L. Clinical research about TCM and neoadjuvant chemotherapy treating progressive gastric cancer. Jiangsu Traditional Chinese Medicine 2006;27(3):25‐7. [1672‐397x(2006)03‐0025‐03] [Google Scholar]
Li 2008 {published data only}
- Li XY. 58 Cases of late gastric cancer treated with decoction for stomach cancer. Journal of Henan University of Chinese Medicine 2008;23(2):50, 52. [Google Scholar]
Li 2011 {published data only}
- Li ZQ. The effect of Shenlingbaizhusan with chemotherapy for late gastric cancer in 27 patients. Shanxi Journal of Traditional Chinese Medicine 2011;32(1):4‐6. [Google Scholar]
Liu 1999 {published data only}
- Liu X, Zhou M, Xu F, Lu Y. Jinkehuaier granula combined with FAM treat advanced gastric carcinoma of 38 cases. Zhejiang Oncology 1999;5(3):189. [Google Scholar]
Liu 2002a {published data only}
- Liu Y, He J. Clinical observation about No3 Kangwei combined with chemotherapy treating advanced gastric carcinoma. Journal of Shandong University of Traditional Chinese Medicine 2002;26(6):444. [1007‐659x(2002)06‐0444‐01] [Google Scholar]
Liu 2003 {published data only}
- Liu B, Wang L, Zhou H, Qian H. Clinical observation of Huishengtai capsule treating advanced gastric carcinoma. Journal of Chengdu University of Traditional Chinese Medicine 2003;26(2):9‐11. [Google Scholar]
Liu 2009b {published data only}
- Liu TC, Zheng RS, Yu XQ. Clinical study on patients with advanced gastric cancer treated with KangAiPingWan and FED combination chemotherapy. Chinese Journal of General Practice 2009;7(9):921‐3. [Google Scholar]
Liu 2011 {published data only}
- Liu YH, Huang J, Wang Y, Zheng ZS. The effect of Astragalus polysaccharides injectio with chemotherapy for advanced or late gastric cancer. Journal of Practical Medicine 2011;27(3):516‐8. [Google Scholar]
Lu 1998 {published data only}
- Lu H, Lin X. Huachansu combined with chemotherapy treating progressing and advanced gastric carcinoma of 70 cases. Practical Journal of Chinese Medicine 1998;11(13):1201. [Google Scholar]
Lu 2010 {published data only}
- Lu ZF. Clinical observation of treating gastric cancer with CTM‐WM therapy. Chinese Journal of Clinical Rational Drug Use 2010;3(11):20‐1. [Google Scholar]
Luo 2009 {published data only}
- Luo ZY. The effect of traditional Chinese Medicine with chemotherapy for advanced or late gastric cancer. China Practical Medicine 2009;4(23):163‐4. [Google Scholar]
Mo 2010 {published data only}
- Mo YY. A clinical analysis of Kang'ai injection with oxaliplatin, calcium folinate, fluorouracil for advanced or late gastric cancer. Journal of Chinese Practical Diagnosis and Therapy 2010;24(10):1008‐9. [Google Scholar]
Ni 2005 {published data only}
- Ni M, Chen Z, Peng Y. Clinical study of intraperitoneal chemotherapy plus ShiQuanDaBu Drink on advanced gastric cancer. Chinese Clinical Oncology 2005;10(3):288‐90. [1009‐0460(2005)03‐0288‐03] [Google Scholar]
Ni 2010 {published data only}
- Ni YQ. Effect of combination of traditional Chinese medicine and lentinan on cellular immunity in patients with medium and advanced gastric cancer. Hebei Journal of Traditional Chinese Medicine 2010;32(8):1136‐8. [Google Scholar]
Pan 2009 {published data only}
- Pan SJ, Yin CC, Feng YL. Clinical observation on Wuzhuyu soup for the treatment of vomit of later gastric cancer of 32 cases. Liaoning Journal of Traditional Chinese Medicine 2009;36(9):1519‐20. [Google Scholar]
Qin 2010 {published data only}
- Qin ZQ, Lu LQ, Yuan GR, Wu GQ, Xue Q, Zhao TW, et al. Elemene combined OLF program of treatment of advanced gastric cancer. Chinese Archives of Traditional Chinese Medicine 2010;28(2):435‐7. [Google Scholar]
Qiu 1992 {published data only}
- Qiu J, Jia J, Yang J, Zheng J, Zheng J, Tang L, et al. Investigation of invigorating the spleen in treating advanced gastric carcinoma. Journal of Chinese Medicine 1992;33(8):23‐5. [Google Scholar]
Qu 1997 {published data only}
- Qu T, Si H. TCM and chemotherapy treat 86 patients with advanced gastric cancer. Fujian Journal of Traditional Chinese Medicine 1997;28(2):1‐2. [Google Scholar]
Qu 2010 {published data only}
- Qu XY, Yang CZ. The effect of Fupihualiu Decoction for advanced or late gastric cancer in 32 patients. Shanxi Journal of Traditional Chinese Medicine 2010;31(1):9‐10. [Google Scholar]
Ren 2008 {published data only}
- Ren LX, Wang YH, Ha MH. The clinical effect of Huchansu for late gastric cancer. China Journal of Chinese Materai Medica 2008;33(12):1474‐5. [Google Scholar]
Shi 2010 {published data only}
- Shi XL, Sun Y, Wang Y, Zhang XX, Han J, Zhou Y. Clinical study on "Changweiqing Liquid" plus chemotherapy in treating advanced gastric cancer of spleen deficiency and phlegm damp stagnation. Shanghai Journal of Traditional Chinese Medicine 2010;44(11):43‐5. [Google Scholar]
Shu 2010 {published data only}
- Shu P, Wang Z. The inhibition effect of Shenqijianwei Decoction for VEGF‐A in late or advanced gastric cancer. Liaoning Journal of Traditional Chinese Medicine 2010;37(10):1969‐70. [Google Scholar]
Shu 2010a {published data only}
- Shu P. The inhibition effect of Shenqijianwei Decoction for VEGF‐C in late or advanced gastric cancer. Shanxi Journal of Traditional Chinese Medicine 2010;31(9):1110‐1. [Google Scholar]
Su 1993 {published data only}
- Su J, Su D, Li M, Zhang L, Zhou P, Mi K, et al. Study on attenuation effect of A‐L tonic drink against UFTM‐associated systemic toxicity in patients with advanced gastric cancer. Chinese Oncology Clinic 1993;20(12):929‐31. [Google Scholar]
Sun 2010 {published data only}
- Sun ZX, Li DG, Lv SJ, Liu JX. The effect of Huazhuojiedu Decoction with chemotherapy for advanced or late gastric cancer in 32 patients. Hebei Journal of Traditional Chinese Medicine 2010;32(9):1305‐6. [Google Scholar]
Tian 2006 {published data only}
- Tian J. Study of efficacy of Fuzhengkangai combined with chemotherapy in treating advanced gastric cancer. Chinese Civilian Treatment 2006;14(11):16‐7. [Google Scholar]
Tian 2011 {published data only}
- Tian CT, Han LY. The effect of self‐made Jianpiyishen Decoction with chemotherapy for late gastric cancer in 42 patients. Shanxi Journal of Traditional Chinese Medicine 2011;32(5):518‐20. [Google Scholar]
Wang 1996 {published data only}
- Wang R, Wang J. Clinical observation of Qilong stomach‐calming decoction combined with chemotherapy treating advanced gastric cancer. Shandong Journal of Traditional Chinese Medicine 1996;15(6):248‐9. [Google Scholar]
Wang 2003 {published data only}
- Wang Z, Qing H, Dong G, Wang W. KangLaiTe effects on apoptosis and hyperplasia of gastric cancer cells. Journal of the Fourth Military Medical University 2003;24(4):1. [1000‐2790(2003)04‐·â3‐01] [Google Scholar]
Wang 2004b {published data only}
- Wang M. Poeder for regulating the function of stomach relieves toxic reaction of EAP treating progressive gastric cancer. Fujian Medical Journal 2004;26(3):128‐9. [1002‐2600(2004)03‐0128‐02] [Google Scholar]
Wang 2006 {published data only}
- Wang D, Zhang L, Li S, Zhang Y. Clinical observation about Traditional Chinese Medicine and chemotherapy treating gastric cancer. Journal of Liaoning University of Traditional Chinese Medicine 2006;8(5):100‐1. [Google Scholar]
Wang 2008 {published data only}
- Wang LJ, Li XD, Zhang HZ. The effect of Kang'ai Injectio with chemotherapy for late gastric cancer. Chinese Medicine Modern Distance Education of China 2008;6(4):358‐9. [Google Scholar]
Wang 2008a {published data only}
- Wang X'E, Jiang H, Yang H, Xie GM. The effect of self‐made Jianpisanjie Decoction with chemotherapy for late gastric cancer. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine 2008;18(8):500‐1. [Google Scholar]
Wang 2009b {published data only}
- Wang YQ, Shi YL, Shi YY. The effect of Shaolifoshoukunbu capsule with chemotherapy for advanced or late gastric cancer. Henan Journal of Traditional Chinese Medicine 2009;29(2):172. [Google Scholar]
Wang 2010c {published data only}
- Wang PP, Lv ZH, Zhang YJ, Sun CX, Xing XR. The effect of Huazhuoheweisanjie Decoction with FOLFOX regimen for advanced or late gastric cancer in 36 patients. Chinese Journal of Integrated Traditional and Western Medicine on Digestion 2010;18(6):403‐4. [Google Scholar]
Wang 2011 {published data only}
- Wang G, Guan YP. The effect of traditional Chinese medicine with western medicine for late gastric cancer. Contemporary Medicine 2011;17(4):231. [Google Scholar]
Wen 1997 {published data only}
- Wen E, Xiong Y. Association with Western and Chinese Traditional Medicines treats 24 patients with advanced gastric cancer. Journal of Jianxi College of Traditional Chinese Medicine 1997;9(3):12‐3. [Google Scholar]
Wu 1992 {published data only}
- Wu Q. Efficancy of Snake venom preparation in patients of gastric cancer after operation. Chinese Journal of Clinical Oncology 1992;19(1):63. [Google Scholar]
Wu 2004 {published data only}
- Wu Y, Guo M. Observation of efficacy about association with Western and Traditional Chinese Medicines treating 92 patients with advanced gastric cancer. Journal of Practical Traditional Chinese Internal Medicine 2004;18(3):207. [1671‐7813(2004)03‐0207‐01] [Google Scholar]
Wu 2009 {published data only}
- Wu L, Yang Y. A clinical study of treating advanced gastric cancer with the combination of Kangai injection and chemotherapy. Proceeding of Clinical Medicine 2009;18(7):493‐6. [Google Scholar]
Wu 2010 {published data only}
- Wu JP, Wang LJ, Zhang ZH, Jia HR, Zhao YH. The clinical effect of Shenshexiaoliu with chemotherapy decoction for late gastric cancer in 44 cases. Hebei Journal of Traditional Chinese Medicine 2010;32(11):1674‐5. [Google Scholar]
Xie 2004 {published data only}
- Xie X, Zheng Z, Li J, Liu D, Liu Y. Effects of xeloda combined with carmofur in the improvement of quality of life in patients with advanced carcinoma of stomach: A randomized controlled observation. Chinese Journal of Clinical Rahabilitation 2004;8(14):2608‐9. [1671‐5926(2004)14‐2608‐02] [Google Scholar]
Xiong 2006 {published data only}
- Xiong M, Yu Q, Tang X. Effect of the traditional Chinese medicine "San Juan Cu Diao Fa" for advanced gastric cancer. Chinese Oncology 2006;15(12):883‐4. [1004‐0242(2006)12‐0883‐02] [Google Scholar]
Xu 1999a {published data only}
- Xu A, Liu J. Huangshen instant herbal medicines combined with chemotherapy treat advanced gastric carcinoma. Guangdong Medical Journal 1999;20(2):150‐1. [Google Scholar]
Xu 2005 {published data only}
- Xu Z, Liu J, Zhang L. Chinese herbal medicine combined with intraperitoneal chemotherapy for treating intermediate and later gastric carcinoma: a report of 69 cases. Chinese Medical Guide: Medical Journal 2005;3(1):94‐6. [1671‐8194(2005)01‐0094‐03] [Google Scholar]
Yan 2010 {published data only}
- Yan R, Zhang MY, She DJ, Shen GM, Ding Y, Su ZT. The clinical effect of Xiaxing Decoction for advanced or late gastric cancer in 20 patients with Tanyufujie type. Hebei Journal of Traditional Chinese Medicine 2010;32(4):512‐3. [Google Scholar]
Yang 1998 {published data only}
- Yang Z, Zhu F, Yang S. Apply of Shuganjianwei tablet in treating advanced gastric cancer. Journal of Practical Traditional Chinese Medicine 1998;14(2):13‐4. [Google Scholar]
Yang 2006a {published data only}
- Yang J. Association with Western and Chinese traditional medicines treats 24 patients with advanced gastric cancer. Anhui Medicine 2006;27(4):335‐6. [Google Scholar]
Ye 2008 {published data only}
- Ye GC, Yuan WB, Liu LW. Clinical research on treating gastric cancer with Chinese Traditional Medicine. Journal of Zhejiang College of Traditional Chinese Medicine 2008;32(1):73‐4. [Google Scholar]
Yin 1996 {published data only}
- Yin Z, Zhang Y, Xie Z, Guan C. Elemene plus fluorouracil in the treatment of advanced gastric cancer. Chinese Clinic of Oncology 1996;23(11):810‐2. [Google Scholar]
Yin 1999 {published data only}
- Yin X, Yu X, Gao Z, Peng C, Tian J, Liu Y, et al. study on therapeutic effect of treating moderate and advanced state gastric cancer by the compound Jiaogulan capsules. Journal of Heze Medical College 1999;11(4):1‐3. [R243 R511 .605] [Google Scholar]
You 2009 {published data only}
- You JL, Huang XN. The effect of Fuzhenghewei Decoction with chemotherapy for advanced or late gastric cancer. Shanxi Journal of Traditional Chinese Medicine 2009;30(9):1112‐4. [Google Scholar]
Yu 2006 {published data only}
- Yu W, Xu Y, Li T, Chen H, Xu X. Clinical observation that Pulse‐activating injection plus chemotherapy treat advanced gastric cancer. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine 2006;16(3):159‐60. [Google Scholar]
Zhang 1987 {published data only}
- Zhang X, Zhang M, Xia D. Clinical observation about Aidean treating advanced stage gastric carcinoma. Journal of Jilin University Medicine Edition 1987;29(5):395. [Google Scholar]
Zhang 2000 {published data only}
- Zhang Y. Association with Western and Traditional Chinese Medicines treats 24 patients with metastatic hepatic carcinoma after operation of gastric carcinoma. Liver Ailment of Journal of Traditional Chinese Medicine 2000;10(1):56‐7. [Google Scholar]
Zhang 2009a {published data only}
- Zhang L, Xiong JP. Kangai injection combined with chemotherapy in advanced gastric cancer. Cancer Research on Prevention and Treatment 2009;36(4):328‐30. [Google Scholar]
Zhang 2010c {published data only}
- Zhang H'O, Fang WY, Huang MH, Hu GH, Wang WR, Huang HQ, Ye CR. The improvement of living quality from Traditional Chinese Medicine combined with westerm medicine for advanced or late gastric cancer. Fujian Journal of Traditional Chinese Medicine 2010;41(4):8‐9. [Google Scholar]
Zhao 1991 {published data only}
- Zhao G, Wu Z, Fu H, Ren Z, Li Y. The effects and side‐effects of Shenqifuzheng Decoction for late gastric cancer after operation in 83 patients. Liberation Army Medical Journal 1991;16(5):379‐81. [Google Scholar]
Zhao 2009 {published data only}
- Zhao JG, Xiong JP, Zhang L. Aidi injection combined with chemotherapy in advanced gastric cancer. Journal of Jiangxi University of TCM 2009;21(1):17‐9. [Google Scholar]
Zhao 2011 {published data only}
- Zhao SP, Li XH. Clinical experience of first‐line chemotherapy combined Tianzhicao capsule with the treatment of advanced gastric cancer. Chinese Medicine Modern Distance Education of China 2011;9(5):148‐9. [Google Scholar]
Zheng 1996 {published data only}
- Zheng W, Zheng J. Chinese herbs and chemotherapy by celiac arterial cannula treat advanced gastric cancer. Intermediate Medical Journal 1996;31(6):54‐5. [Google Scholar]
Zheng 1996a {published data only}
- Zheng W, Zheng J. TCM and interventional chemotherapy treat 40 patients with advanced cancer. New Digestive Disease Journal 1996;4(12):717. [Google Scholar]
Zhou 2000a {published data only}
- Zhou R, Wu L, Ni A, Xu Z. Observations on the curative effect of combined meridian‐transmitted millimetre wave and Chinese herbs on intermediate and advanced carcinoma of stomach. Shanghai Journal of Acupuncture and Moxibustion 2000;19(2):7‐9. [1005‐0957(2000)‐02‐0007‐03] [Google Scholar]
Zhou 2000b {published data only}
- Zhou J. Clinical report of Huishengtai capsules treating advanced gastric carcinoma. Modern Journal of Integrated Traditional Chinese and Western Medicine 2000;9(6):502‐3. [Google Scholar]
Zhu 1996 {published data only}
- Zhu H, Li Y, Sun Y. Shenlianwendan combining with MF treat advanced gastric carcinoma of 20 cases. Shandong Chinese Medical Journal 1996;15(12):554. [Google Scholar]
Zhu 1999 {published data only}
- Zhu X, Xu J, Huang D, Huang G, Ying X. Clinical and empirical study about Gecko and Radix actinidiae treating gastric carcinoma. China's Naturopathy 1999;3(3):43‐4. [Google Scholar]
Zhu 2004 {published data only}
- Zhu X, Fang M, Wu Y, Tao H, Yang X. Traditional Chinese Medicine treats patients with gastric carcinoma and partial bowel obstruction. Liaoning Journal of Traditional Chinese Medicine 2004;31(12):1011‐2. [1000‐1719(2004)12‐1011‐02] [Google Scholar]
Zhu 2008 {published data only}
- Zhu LY, Tian L. Effect of TCM decoction therapy on quality of life in 38 post‐chemotherapy patients with stomach cancer. Youjiang Medical Journal 2008;36(5):525‐7. [Google Scholar]
Zhu 2009 {published data only}
- Zhu L, Chen YC. The effect of Fuzhengsanjie decoction with OLF therapeutic regimen for late gastric cancer. Yunnan Journal of Traditional Chinese Medicine and Materia Medica 2009;30(6):7‐8. [Google Scholar]
Zhu 2009a {published data only}
- Zhu KW. The effect of self‐made Wei'aishu with chemotherapy for advanced or late gastric cancer in 46 patients. Chinese Community Doctors 2009;11(10):105‐6. [Google Scholar]
Additional references
Bu 2001b
- Bu P, Zhou C, Chen Q. The effects of Recipe of Fuzhenghuayu on inhibiting the metastasis and strengthening immune function of T‐cell in patients with gastric cancer after operation. Journal of Traditional Chinese Medicine 2001;42(4):226. [Google Scholar]
Chen 2001
- Chen R, Su J, Cai K, Fu Y, Li L. The effect of natural antioxidant of Isoverbascoside on inducing the differentiation of gastric cancer cell. Journal of Xiamen University (Natural Science) 2001;40(4):936‐41. [Google Scholar]
Dai 2009
- Dai Z, Gao J, Ji Z, Wang X, Ren H, Liu X, et al. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase‐3. Journal of Ethnopharmacology 2009;123(1):91‐6. [DOI] [PubMed] [Google Scholar]
Duan 2002
- Duan P, He J, Zhang M. The effectiveness of invigorating the spleen and removing toxic substances in the treatment of gastric cancer. Sichuan Traditional Chinese Medicine 2002;20(1):44. [Google Scholar]
Gu 1995
- Gu D, Zhang Y. The research advancement of clinical dispensatories in treatment of gastric cancer. Information on Traditional Chinese Medicine 1995;12(1):8‐10. [Google Scholar]
Guo 1997
- Guo Z, Cheng L. A clinical therapeutic effectiveness analyses of gastric carcinoma. Sichuan Traditional Chinese Medicine 1997;15(3):7‐8. [Google Scholar]
Higgins 2008
- Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Ji 1989
- Ji G, Ji J. The advancement of Chinese medicine in treatment of gastric cancer. Jiangsu Journal of Traditional Chinese Medicine 1989;10(2):40‐2. [Google Scholar]
Lin 2003
- Lin J, Dong H, Oppenheim J, Howard O. Effects of astragali radix on the growth of different cancer cell lines. World Journal of Gastroenterology 2003;9(4):670‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 1996
- Lu W, Sun G, Piao B. The clinical and laboratory research of Yangweikangliu recipe for gastric cancer. Journal of Traditional Chinese Medicine 1996;37(6):350‐2. [Google Scholar]
Lu 2008
- Lu B, Xu L, Yu L, Zhang L. Extract of radix curcumae prevents gastric cancer in rats. Digestion 2008;77(2):87‐91. [DOI] [PubMed] [Google Scholar]
Melchart 2002
- Melchart D, Clemm C, Weber B, Draczynski T, Worku F, Linde K, et al. Polysaccharides isolated from Echinacea purpurea herba cell cultures to counteract undesired effects of chemotherapy‐‐a pilot study. Phytotherapy Research 2002;16(2):138‐42. [DOI] [PubMed] [Google Scholar]
Ning 1985
- Ning C, Wang G, Zhao T, Yu G, Duan F. The effect of Jianpiyishen prescription on toxic‐side effects caused by chemotherapy in late gastric cancer after operation. Chinese Journal of Intergrated Traditional and Western Medicine 1985;5(11):668‐70. [Google Scholar]
Parmar 1998
- Parmar M, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Qiu 1993
- Qiu J. Appraisement of the effect of Traditional Chinese medicine herbs on preventing cancer from metastasis. Journal of Traditional Chinese Medicine 1993;34(9):560. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rui 2008
- Rui D, Xiao C, X Tai, Guan J. Elemene for the treatment of lung cancer. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006054] [DOI] [PubMed] [Google Scholar]
Sa 2003
- Sa R. The retrospect and prospect of combination treatment with Traditional Chinese Medicine and Western medicine for cancer. Bulletin of Chinese Cancer 2003;12(6):324‐6. [Google Scholar]
Shen 2007
- Shen H, Liu Z, Zhang K, Wang W, Guo Q, Yuan S, et al. Effect of Astragulus membranaceus on expression of COX‐1, COX‐2, VEGF, and PEG‐2 in human gastric cancer cell line SGC7901. Tumour 2007;27(3):194‐8. [Google Scholar]
Sun 2002
- Sun W, Zheng X. A clinical report of Aidi injection with chemotherapy for advanced or late gastric cancer. The Thesis Compilation of National Western and Chinese Traditional Medicine Summit Forum of Oncology. Chinese Medical Association, 2002:126‐127. [Google Scholar]
Tang 2004
- Tang C. Gastric Carcinoma. Internal Medicine. 6th Edition. Beijing: The People's Hygiene Publishing House of China, 2004:393‐9. [Google Scholar]
UICC 1997
- Sobin L, Wittekind L. UICC, Manual of Clinical Oncology 1990. TNM Classification of Malignant Tumors. Fifth. New York: Wiley‐Liss, 1997. [Google Scholar]
Wang 2000
- Wang A, Liu B, Zhao Y, Sun G. The relation between the classification of syndrome differentiation on Traditional Chinese Medicine and histopathology classification in moderate‐late stage of gastric carcinoma and the therapeutic evaluation. Chinese Journal of Surgery of Integrated Traditional and Western Medicine 2000;6(3):165‐6. [Google Scholar]
Wu 2001
- Wu M, Yao B. The research advancement of Chinese medicine in treatment of gastric cancer and inducing cellular apoptosis. Journal of Traditional Chinese Medicine 2001;42(12):752‐4. [Google Scholar]
Wu 2008
- Wu T, Munro A, Liu G. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004540] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 1989
- Yang C. The advancement of Chinese medicine and integrated Chinese medicine and western medicine in treatment of gastric cancer. Journal of New Chinese Medicine 1989;21(2):45‐7. [Google Scholar]
Zeng 1986
- Zeng X, Hu P. MFO chemotherapy randomized with addition of Chinese herb medicine in patients with advanced gastric cancer. Chinese Journal of Clinical Oncology 1986;13(3):145‐6. [Google Scholar]
Zhang 2007
- Zhang M, Liu X, Li J, He L, Tripathy D. Chinese medicinal herbs to treat the side‐effects of chemotherapy in breast cancer patients. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004921.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2002
- Zhao P, Tang L, Luo J. The effect of Aidi Injection with chemotherapy for gastric cancer after operation. the Thesis Compilation of National Western and Chinese Traditional Medicine Summit Forum of Oncology. Chinese Medical Association, 2002:244‐5. [Google Scholar]
Zheng 2001
- Zheng Z. The morbidity and mortality of gastric cancer. Gastroenterology. 3rd Edition. Beijing: the People's Hygiene Publishing House of China, 2001:12. [Google Scholar]
Zhou 1999
- Zhou D. The success and future of Chinese medicine integrated western medicine in treatment of malignant tumor. Chinese Journal of Tumour 1999;8(10):455‐7. [Google Scholar]


