Abstract
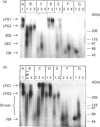
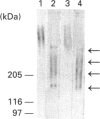
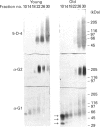
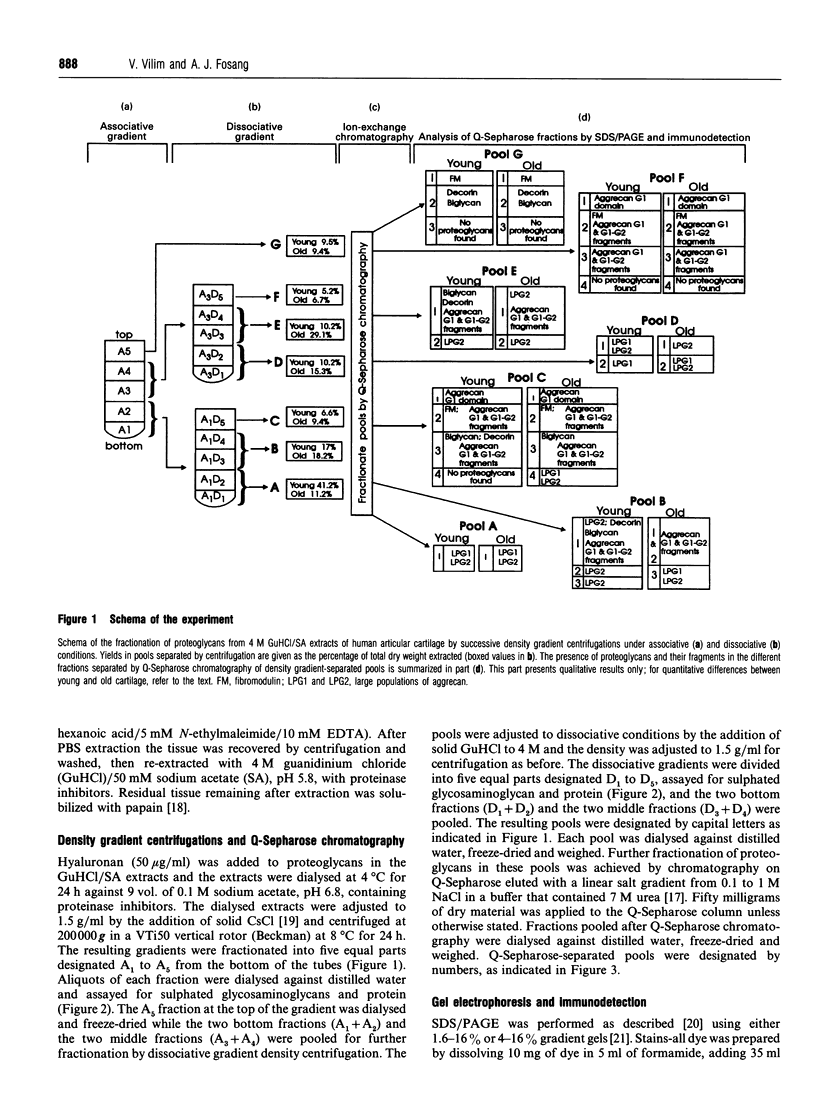
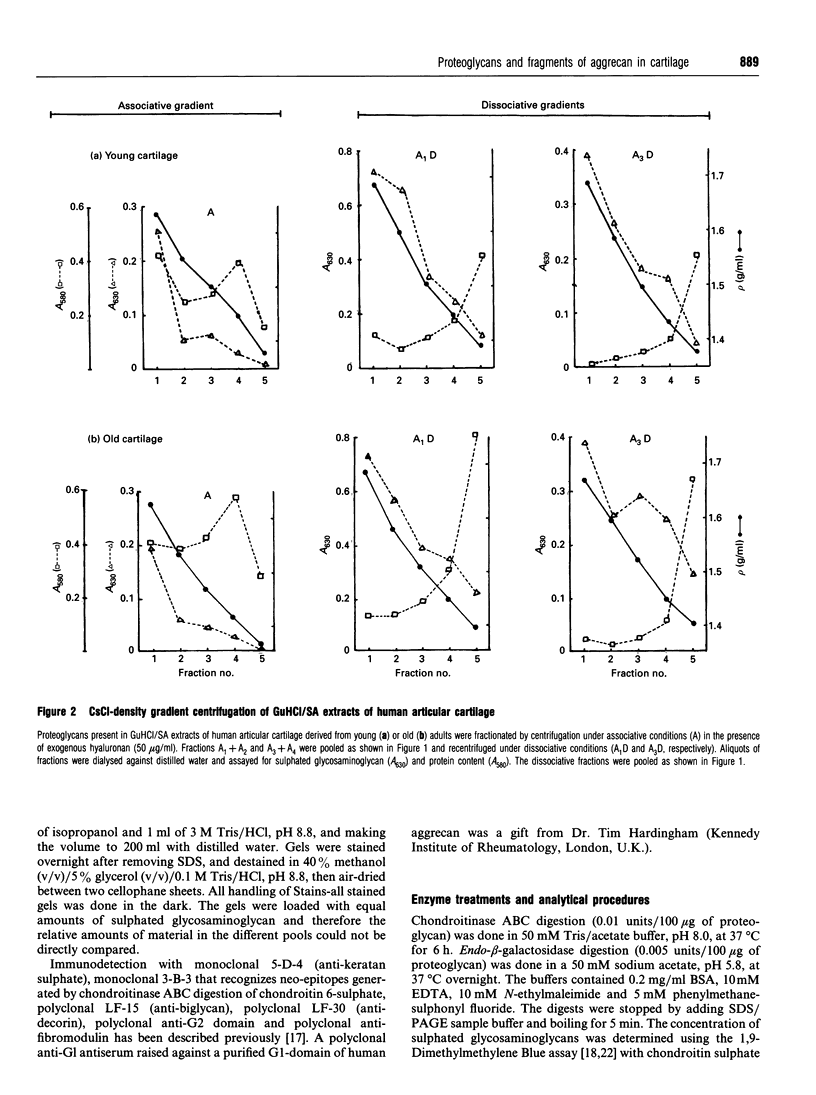
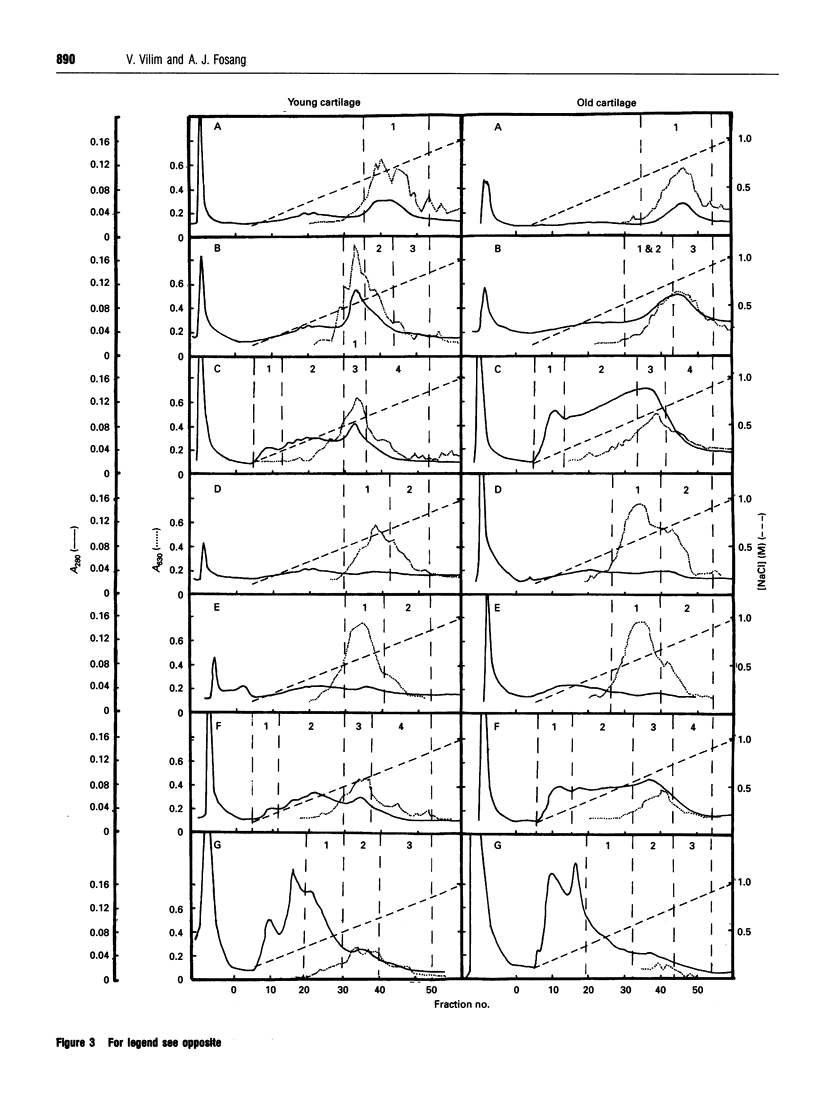
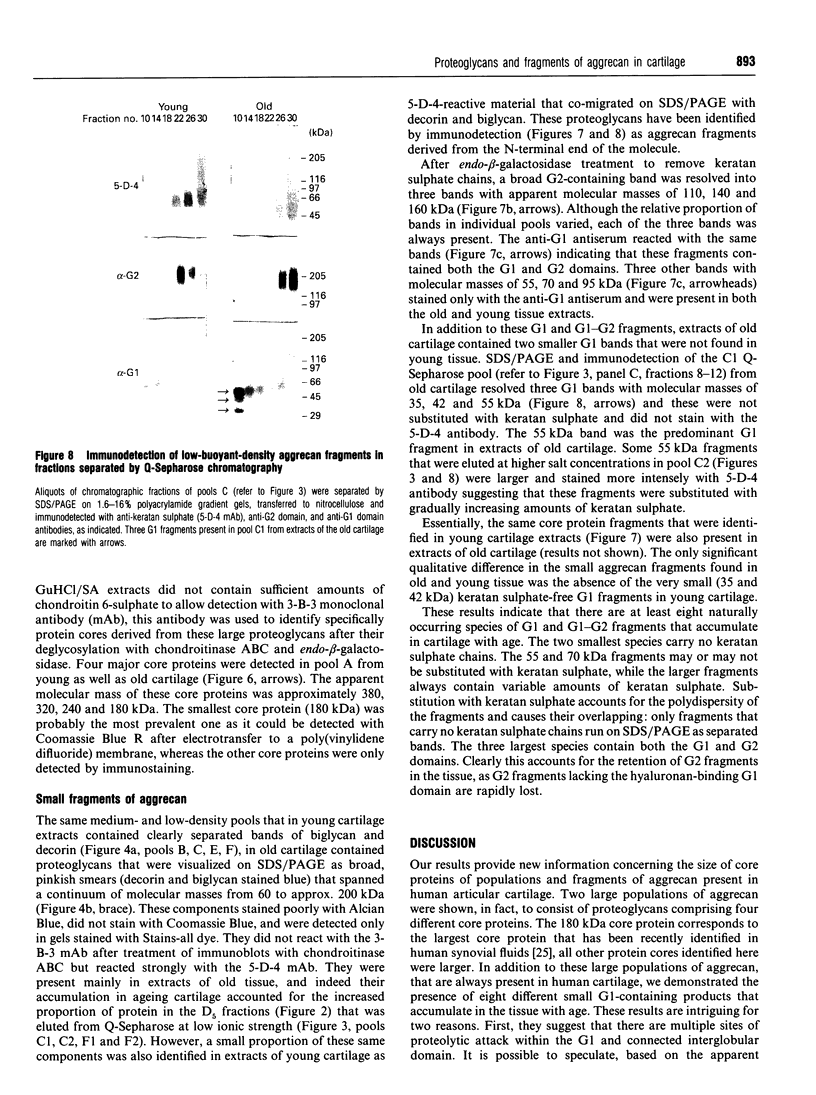
Proteoglycans extracted with 4 M guanidinium chloride from young (mean 20 years) or old (mean 79 years) macroscopically normal human articular cartilage were separated by density gradient centrifugation and Q-Sepharose chromatography and characterized by gradient gel SDS/PAGE and immunodetection before and after removal of glycosaminoglycan chains. The extracts contained two large populations of aggrecan, a population of small N-terminal aggrecan fragments, as well as decorin, biglycan and fibromodulin. The distribution of all these species in density gradient fractions has been determined. The large aggrecan populations comprised four different chondroitin sulphate-bearing core proteins while the population of smaller fragments comprised eight different components. The two smallest fragments (35 and 42 kDa), identified as the first globular domain of aggrecan (N-terminal) (G1) and containing no glycosaminoglycan, were detected only in extracts of old cartilage. A 55 and a 70 kDa fragment of G1 were present in both keratan sulphate-containing and non-keratan sulphate-containing forms. Four other fragments, each containing keratan sulphate epitopes, were identified and these contained either G1 epitopes (one 95 kDa species), or G1 and G2 epitopes (three species). These results have suggested that proteolytic processing at the N-terminus is more extensive than has previously been recognized and raises the possibility that more than one proteinase may be involved in aggrecan degradation in vivo. With the exception of the two smallest G1 fragments, the repertoire of proteoglycan fragments found in young and old human articular cartilage is essentially the same, although the relative abudnance of various species differed. The older tissue contains a larger proportion of C-terminally truncated aggrecan fragments and a significantly decreased content of decorin and biglycan.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss M. T., Ridgway G. D., Ali S. Y. Delayed aggregation of proteoglycans in adult human articular cartilage. Biosci Rep. 1984 Oct;4(10):827–833. doi: 10.1007/BF01138164. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Roughley P. J. The properties of proteoglycan prepared from human articular cartilage by using associative caesium chloride gradients of high and low starting densities. Biochem J. 1985 Nov 15;232(1):111–117. doi: 10.1042/bj2320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidanset D. J., Guidry C., Rosenberg L. C., Choi H. U., Timpl R., Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992 Mar 15;267(8):5250–5256. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992 Jan 15;267(2):1008–1014. [PubMed] [Google Scholar]
- Fosang A. J., Last K., Knäuper V., Neame P. J., Murphy G., Hardingham T. E., Tschesche H., Hamilton J. A. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993 Oct 1;295(Pt 1):273–276. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Last K., Hardingham T. E., Murphy G., Hamilton J. A. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992 Sep 25;267(27):19470–19474. [PubMed] [Google Scholar]
- Hedbom E., Antonsson P., Hjerpe A., Aeschlimann D., Paulsson M., Rosa-Pimentel E., Sommarin Y., Wendel M., Oldberg A., Heinegård D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992 Mar 25;267(9):6132–6136. [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Ilic M. Z., Handley C. J., Robinson H. C., Mok M. T. Mechanism of catabolism of aggrecan by articular cartilage. Arch Biochem Biophys. 1992 Apr;294(1):115–122. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Neame P. J., Sandy J. D. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993 Sep;36(9):1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- Loulakis P., Shrikhande A., Davis G., Maniglia C. A. N-terminal sequence of proteoglycan fragments isolated from medium of interleukin-1-treated articular-cartilage cultures. Putative site(s) of enzymic cleavage. Biochem J. 1992 Jun 1;284(Pt 2):589–593. doi: 10.1042/bj2840589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melching L. I., Roughley P. J. The synthesis of dermatan sulphate proteoglycans by fetal and adult human articular cartilage. Biochem J. 1989 Jul 15;261(2):501–508. doi: 10.1042/bj2610501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. T., Ilic M. Z., Handley C. J., Robinson H. C. Cleavage of proteoglycan aggregate by leucocyte elastase. Arch Biochem Biophys. 1992 Feb 1;292(2):442–447. doi: 10.1016/0003-9861(92)90014-n. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Flannery C. R., Neame P. J., Lohmander L. S. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992 May;89(5):1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Stanescu V., Chaminade F., Muriel M. P. Age-related changes in small proteoglycans of low buoyant density of human articular cartilage. Connect Tissue Res. 1988;17(4):239–252. doi: 10.3109/03008208809017475. [DOI] [PubMed] [Google Scholar]
- Vilim V., Krajickova J. Electrophoretic separation of large proteoglycans in large-pore polyacrylamide gradient gels (1.32-10.0% T) and a one-step procedure for simultaneous staining of proteins and proteoglycans. Anal Biochem. 1991 Aug 15;197(1):34–39. doi: 10.1016/0003-2697(91)90351-s. [DOI] [PubMed] [Google Scholar]
- Vilim V., Krajickova J. Proteoglycans of human articular cartilage. Identification of several populations of large and small proteoglycans and of hyaluronic acid-binding proteins in successive cartilage extracts. Biochem J. 1991 Feb 1;273(Pt 3):579–585. doi: 10.1042/bj2730579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilím V., Fosang A. J. Characterization of proteoglycans isolated from associative extracts of human articular cartilage. Biochem J. 1993 Jul 1;293(Pt 1):165–172. doi: 10.1042/bj2930165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber C., Glant T. T., Roughley P. J., Poole A. R. The identification and characterization of two populations of aggregating proteoglycans of high buoyant density isolated from post-natal human articular cartilages of different ages. Biochem J. 1987 Dec 15;248(3):735–740. doi: 10.1042/bj2480735. [DOI] [PMC free article] [PubMed] [Google Scholar]