Summary:
The profunda artery perforator (PAP) flap, commonly used for small- to medium-sized breast reconstructions, offers easy harvest and inconspicuous donor-site scars. However, its shorter vascular pedicle compared with the deep inferior epigastric perforator flap limits its reach to lateral recipient vessels. This often requires strategic placement of perforators at the flap’s edge to extend reach, potentially causing congestion in the distal part of the flap. To address these challenges, using the posterior accessory saphenous vein (pASV) has proven effective. Using the pASV as a vein graft significantly extends the pedicle length of the PAP flap, enhancing anastomosis success with recipient vessels. Additionally, in cases of flap congestion, the proximal segment of the pASV can be used as an additional venous outflow pathway, while grafting the distal segment further extends its length. This dual approach improves overall flap viability and reduces venous congestion risks. This discussion highlights two cases demonstrating the innovative use of the pASV within the PAP flap. In case 1, the pASV extended the pedicle length, enhancing the flap’s placement flexibility and facilitating anastomosis with thoracodorsal vessels. In case 2, the pASV served as a secondary venous outflow pathway, with the distal segment grafted to extend the proximal portion. This adaptation provided additional venous drainage and effectively managed positioning constraints imposed by recipient vessel locations. These examples illustrate the significant benefits of utilizing the pASV in PAP flap breast reconstructions, offering a novel strategy to improve viability and expand its use in complex scenarios requiring extended vascular reach.
The profunda artery perforator (PAP) flap is often used for small- to medium-sized breast reconstructions due to its relatively easy harvest and less conspicuous donor site scars.1–3 However, compared with the deep inferior epigastric perforator (DIEP) flap, a shorter vascular pedicle is a notable drawback. Although placing the perforators at the edge of the flap can facilitate reaching the recipient vessels, this arrangement may still result in insufficient pedicle length when using lateral vessels such as the thoracodorsal vascular system. Additionally, positioning the perforators at the flap’s edge can cause congestion in the distal part of the flap. However, when elevating the PAP flap in vertical or diagonal orientations,4-6 the posterior accessory saphenous vein (pASV) can be included in the flap, providing additional drainage pathways.5,6 Nonetheless, when the recipient vessels are located laterally, the pASV alone often fails to provide sufficient length to reach the recipient vessels. In this article, we report two cases where the pASV was used as a vein graft to address the inadequacy of vascular length in breast reconstruction with the PAP flap.
CASE REPORTS
Case 1
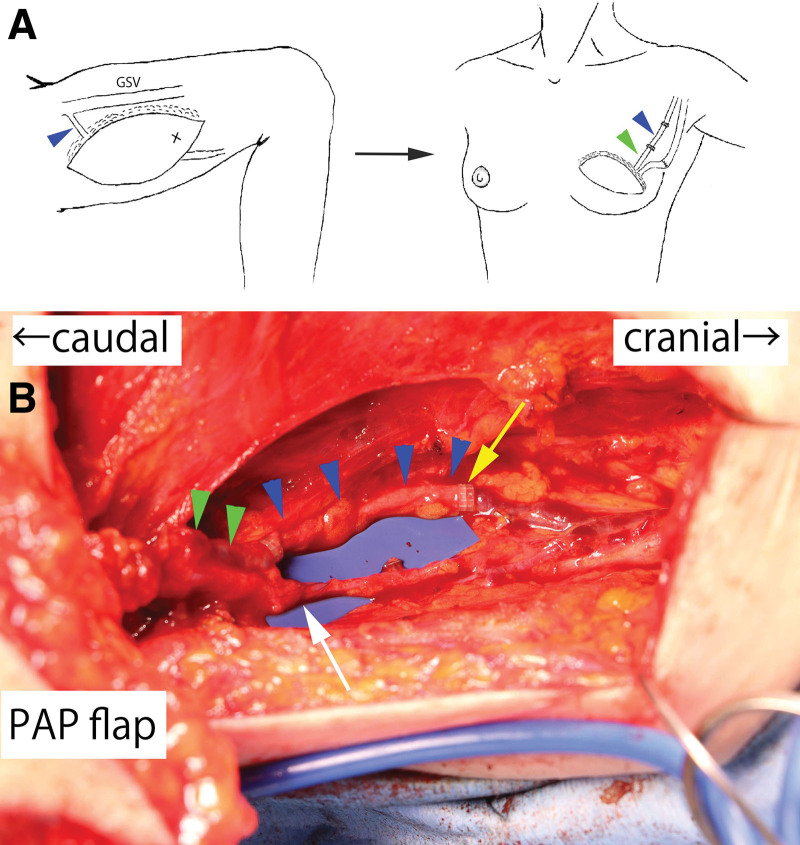
A 48-year-old female patient was planned for nipple-sparing mastectomy and immediate reconstruction using a left-sided PAP flap in a vertical orientation for left breast cancer (flap size: 17 × 7 cm). (See figure, Supplemental Digital Content 1, which displays the flap design in case 1. http://links.lww.com/PRSGO/D478.) The most caudal perforator was dissected to the branching point with the profunda artery and vein, and the flap was elevated with a vascular pedicle length of 8.5 cm. During flap elevation, the pASV was included in the flap by dissecting it to the inflow section of the greater saphenous vein. Given that the breast cancer surgery involved nipple-sparing mastectomy, thoracodorsal vessels were chosen as the recipient vessels. Despite positioning the flap such that the perforators were lateral, the pedicle vessels were insufficient in length. For the artery, the thoracodorsal artery could be anastomosed by dissecting it within the latissimus dorsi muscle, but the diameter of the thoracodorsal vein at the same level was too small for suitable anastomosis. Therefore, the pASV included in the flap was used as a vein graft to extend the pedicle vein, enabling anastomosis to the vein of the serratus anterior branch (Fig. 1). The postoperative course was uneventful, and satisfactory aesthetic results were obtained at 6 months postoperatively (See figure, Supplemental Digital Content 2, which displays the postoperative view at 6 months. http://links.lww.com/PRSGO/D479.)
Fig. 1.
Schematic diagram of case 1 and intraoperative photograph of the vascular anastomoses. A, The pASV (blue arrowhead) was harvested from the PAP flap to the inflow section of the greater saphenous vein, thereby extending the length of the pedicle vein (green arrowhead). GSV, great saphenous vein. B, The pASV graft facilitated the anastomosis to the vein of the serratus anterior branch. Green arrowheads: pedicle vein; blue arrowheads: pASV graft; yellow arrow: anastomosis between the pASV graft and a vein of the serratus anterior branch; white arrow: anastomosis between the pedicle artery and the thoracodorsal artery.
Case 2
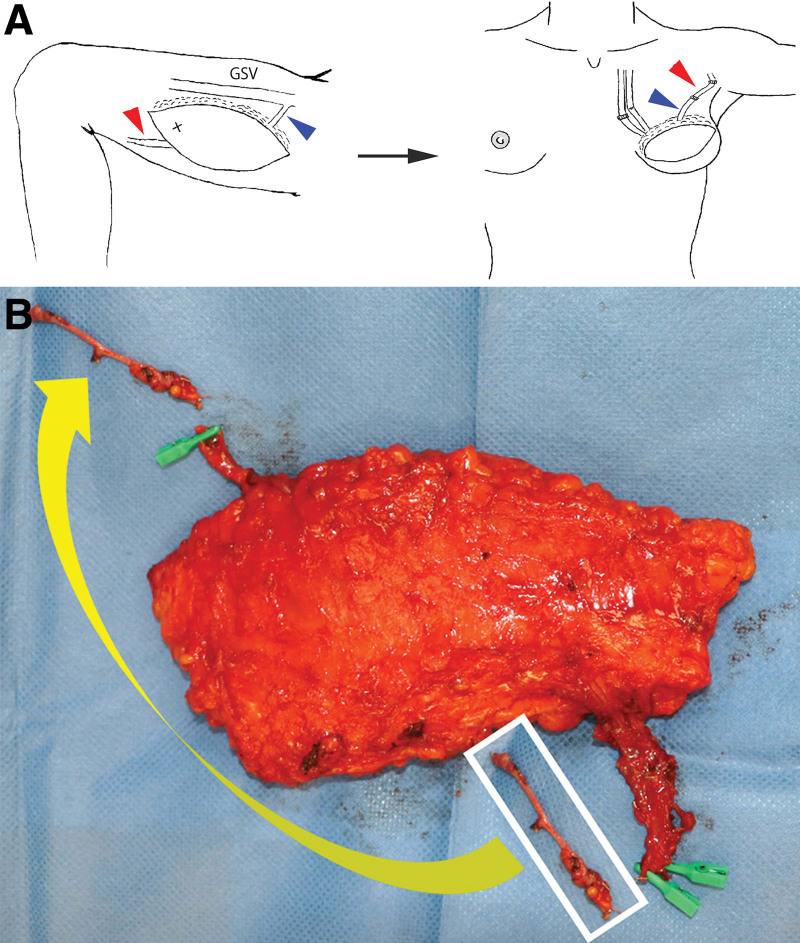
A 41-year-old female patient underwent a two-stage, delayed reconstruction using a right-sided PAP flap in a vertical orientation following left breast cancer surgery (flap size: 18 × 7 cm). The most caudal perforator was dissected to the branching point with the profunda artery, and the flap was elevated with a vascular pedicle length of 9 cm. During flap elevation, the pASV was included in the flap by dissecting it to the inflow section of the greater saphenous vein. While the flap was clamped at the pASV, indocyanine green angiography revealed significant congestion in the proximal third of the flap,7 prompting the use of the pASV as a secondary venous outflow pathway. After anastomosing the pedicle vessels antegrade to the internal mammary artery and vein, an attempt to anastomose the pASV retrograde to the internal mammary vein resulted in significant venous congestion.8 Subsequently, an attempt to anastomose the pASV to the thoracoepigastric vein in the axillary region was made, but the length of the pASV was insufficient. Therefore, a 5 cm segment of the previously ligated and detached distal pASV was harvested and transplanted as a vein graft to the proximal pASV to extend the vessel (Fig. 2). The postoperative course was uneventful, and satisfactory aesthetic outcomes were achieved at 1 year postoperatively.
Fig. 2.
Schematic diagram of case 1 and intraoperative photograph of the PAP flap viewed from the underside. A, To extend the length of the pASV as a second venous outflow pathway (blue arrowhead), 5 cm of the distal pASV (red arrowhead) was harvested and used as a vein graft. GSV, great saphenous vein. B, The distal pASV, harvested as a vein graft, was anastomosed to the proximal pASV, extending the vessel (yellow arrow).
DISCUSSION
The PAP flap has recently become popular for small-sized to medium-sized breast reconstructions.1–3 It is favored for its relatively easy harvest and less noticeable donor site scars, although it has a shorter vascular pedicle compared with the DIEP flap. Colohan et al9 reported an average vascular pedicle length of 16.9 cm for DIEP flaps, whereas Allen et al2 noted an average length of 10.2 cm for PAP flaps. Positioning the perforators at the edge of the flap can facilitate reaching the recipient vessels, but this may still result in inadequate pedicle length when using thoracodorsal vessels as recipients. In such cases, as demonstrated in our case 1, using the pASV as a vein graft can extend the vascular pedicle without requiring additional skin incisions.
Placing perforators at the flap’s edge can cause congestion in the distal part of the flap. However, when elevating the PAP flap in vertical or diagonal orientations, including the pASV in the flap can serve as an additional venous outflow pathway. According to the cadaver study conducted by Karakawa et al,6 the average distance between the proximal thigh crease and the intersection of the anterior edge of the gracilis muscle with the accessory saphenous vein was 7.7 ± 2.5 cm. By referencing these data along with preoperative ultrasound examination, the pASV can be easily identified and preserved at the anterior edge of the PAP flap during elevation.
Iida et al5 reported eight cases of reconstruction using the PAP flap with the pASV included (seven cases in head and neck reconstructions, one in breast reconstruction), and in the breast reconstruction case, the included pASV was not anastomosed. Their clinical report suggests that the average vascular length of the included pASV was 8.6 cm, suggesting that, like the PAP vessels, the pASV often lacks sufficient length to reach the recipient vessels. Karakawa et al reported a case where the pASV included in the PAP flap served as a second venous pathway in breast reconstruction, where the pASV was anastomosed to the internal mammary vein.6 However, when recipient vessels need to be located laterally, the length of the pASV often proves insufficient. In such situations, as in our case 2, harvesting and using the pASV from the distal end of the flap as a venous graft can extend the available length of the pASV. Although the distal end of the pASV may have a smaller diameter when used as a vein graft, in our case 2, a 2 mm vascular coupler was successfully used for the distal anastomosis of the pASV graft.
CONCLUSIONS
The pASV can serve as a secondary venous outflow pathway in breast reconstruction using the PAP flap, and can also be used as a vein graft to resolve various issues caused by insufficient flap vascular length. When elevating PAP flaps in vertical or diagonal orientations, it is advisable to include the pASV in the flap, having confirmed its course with preoperative ultrasound, to prepare for potential complications during unexpected vascular anastomoses.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
All procedures conformed to the principles set forth in the Declaration of Helsinki. This study was approved by the Ethics Committee of Kindai University.
Supplementary Material
Footnotes
Published online 4 September 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. 2012;129:16e–23e. [DOI] [PubMed] [Google Scholar]
- 2.Allen RJ, Jr, Lee ZH, Mayo JL, et al. The profunda artery perforator flap experience for breast reconstruction. Plast Reconstr Surg. 2016;138:968–975. [DOI] [PubMed] [Google Scholar]
- 3.Cohen Z, Azoury SC, Matros E, et al. Modern approaches to alternative flap-based breast reconstruction: profunda artery perforator flap. Clin Plast Surg. 2023;50:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen Z, Azoury SC, Nelson JA, et al. The preferred design of the profunda artery perforator flap for autologous breast reconstruction: transverse or diagonal? Plast Reconstr Surg Glob Open. 2023;11:e5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iida T, Yoshimatsu H, Karakawa R, et al. Additional venous anastomosis in free profunda artery perforator flap transfer using the posterior accessory saphenous vein. J Plast Reconstr Aesthet Surg. 2019;72:1936–1941. [DOI] [PubMed] [Google Scholar]
- 6.Karakawa R, Yoshimatsu H, Fuse Y, et al. The correlation of the perforators and the accessory saphenous vein in a profunda femoris artery perforator flap for additional venous anastomosis: a cadaveric study and clinical application. Microsurgery. 2020;40:200–206. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimatsu H, Karakawa R, Scaglioni MF, et al. Application of intraoperative indocyanine green angiography for detecting flap congestion in the use of free deep inferior epigastric perforator flaps for breast reconstruction. Microsurgery. 2021;41:522–526. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara J, Satake T, Muto M, et al. Dynamic blood flow to the retrograde limb of the internal mammary vein in breast reconstruction with free flap. Microsurgery. 2015;35:622–626. [DOI] [PubMed] [Google Scholar]
- 9.Colohan S, Maia M, Langevin CJ, et al. The short- and ultrashort-pedicle deep inferior epigastric artery perforator flap in breast reconstruction. Plast Reconstr Surg. 2012;129:331–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.