Abstract
Introduction:
Sarcomatoid renal cell carcinoma (SRCC) is clinically rare, accounting for ~1.0–1.5% of renal parenchymal tumors. Although the concept of SRCC was proposed in 1968, the molecular mechanisms and immunological characteristics of sarcomatoid changes remain unclear. In the era of targeted therapy, the overall survival (OS) of patients with SRCC is typically less than 12 months.
Case presentation:
This article reports a case of SRCC in an 81-year-old male. Progression-free survival (PFS) was as long as 25 months and OS was 30 months after immunotherapy and the effect was significant. This is the first report of successful use toripalimab in the treatment of SRCC.
Clinical discussion:
SRCC is a rare type of renal cancer with no obvious specific clinical manifestations or imaging findings, and the diagnosis of the disease is based on pathological examinations. SRCC has a high degree of malignancy, progresses rapidly, and has a poor prognosis. The effect of traditional treatment is limited, and immune checkpoint inhibitors may have therapeutic potential.
Conclusions:
Toripalimab may be effective and further exploration is anticipated to advance a new period of SRCC.
Keywords: case report, immune checkpoint inhibitors, immunotherapy, sarcomatoid renal cell carcinoma, toripalimab
Introduction
Highlights
Sarcomatoid renal cell carcinoma (SRCC) is a rare entity.
To accurately diagnose the condition and give the patient a fair prognosis, a multidisciplinary approach involving oncologists, pathologists, and internists is advised.
The traditional treatment of SRCC has limited efficacy, while immune checkpoint inhibitors may have therapeutic potential.
Sarcomatoid renal cell carcinoma (SRCC) is the most fatal type of renal cell carcinoma (RCC) and is histologically characterized by the presence of spindle-shaped mesenchymal-like cells in any RCC subtype. Sarcomatoid changes indicate an increased frequency of aggressive behavior of the tumor, including rapid progression and poor prognosis. The natural history and prognosis of SRCCs are poor, as ~60–80% of patients present with advanced or late-stage disease1. Median overall survival (OS) is ~6–13 months, and a higher percentage of sarcomatoid dedifferentiation on histology has been reported to confer a worse prognosis2–4. Kawakami et al.5 proved that SRCC showed higher PD-L1 expression and higher PD-1- and CD8-positive cell density; the results indicate a notable immunosuppressive environment in SRCC and suggest PD-1/PD-L1 blockade therapy as a potential therapeutic approach for SRCC. Toripalimab is a humanized recombinant anti-PD-1 IgG4 antibody that selectively blocks the interaction of PD-1 with its ligands, PD-L1 and PD-L2, and promotes antigen-specific T cell activation. The present report describes a case of SRCC with significant benefits after immunotherapy. Immunotherapy improved the prognosis of this patient, and immune checkpoint inhibitors (ICIs) may impact SRCC management in the future. This case report is reported according to Surgical CAse REport (SCARE) guidelines6.
Case report
An 81-year-old male patient with a medical history of hypertension, benign prostatic hyperplasia, and coronary heart disease was admitted to a hospital in another province due to the discovery of a soft tissue mass in the left renal pelvis during B-ultrasonography for one week after a laparoscopic total length left nephroureterectomy performed in May 2019. There was no history of cancer in the family. Postoperative pathological immunohistochemical diagnosis of sarcomatoid carcinoma indicated the following: CK, vimentin, CK7, and GATA-3 were partially positive; CK20, uroplakin II, and P53 were negative; and Ki-67 (localized, ~50%+). Subsequently, postoperative intravesical instillation with mitomycin was performed four times. No further antitumor therapy was given. Re-examination by positron emission tomography/computed tomography (PET/CT) in November 2019 showed a soft tissue mass in the left kidney and ureter, and tumor recurrence was suspected. In addition, soft tissue thickening of the posterior and right sidewalls of the nasopharyngeal roof was considered a nasopharyngeal carcinoma. Multiple lymph node metastases were observed in the left supraclavicular area, right behind the diaphragmatic angle and abdomen, and adjacent to the common iliac vessels. Nasopharyngeal laryngoscopy revealed squamous cell carcinoma: immunohistochemistry results indicated AE1/AE3, CK5/6, P63, P40, and epidermal growth factor receptor (EGFR) were positive (3+); P16, vascular endothelial growth factor (VEGF), and Epstein-Barr encoding region were negative; and Ki-67 (~30%+). A biopsy of the left supraclavicular lymph node revealed a malignant tumor, and renal tumor metastasis was considered. Due to intermittent pain on the left side of the abdomen, hearing loss, and nasal congestion during the treatment period in our hospital from February 2020 to March 2022, the patient received toripalimab successfully (36 times) but suspended due to elevated myocardial enzymes and brain natriuretic peptide, and finally passed away in August 2022. Notably, during periodic review, imaging indicated stable disease (SD) in renal tumors (Fig. 1), partial response (PR) in nasopharyngeal tumor (Fig. 2), and the progression-free survival (PFS) was up to 25 months, and overall survival (OS) was up to 30 months.
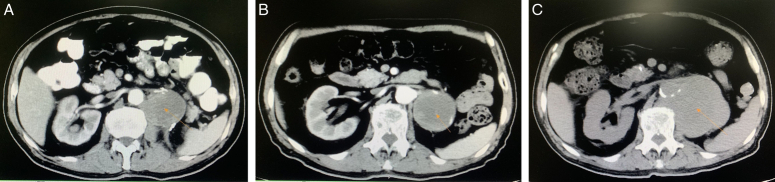
Figure 1.
Dynamic changes in computed tomography imaging of the renal tumor during treatment: (A) before immunotherapy (2020-02-15, 4.6×4.5 cm); (B) after four immunotherapy treatments [2020-05-11, 5.5×5.4 cm, indicating stable disease (SD)]; (C) after 36 immunotherapy treatments [2022-03-13, 8.4×8.0 cm, indicating progressive disease (PD)].
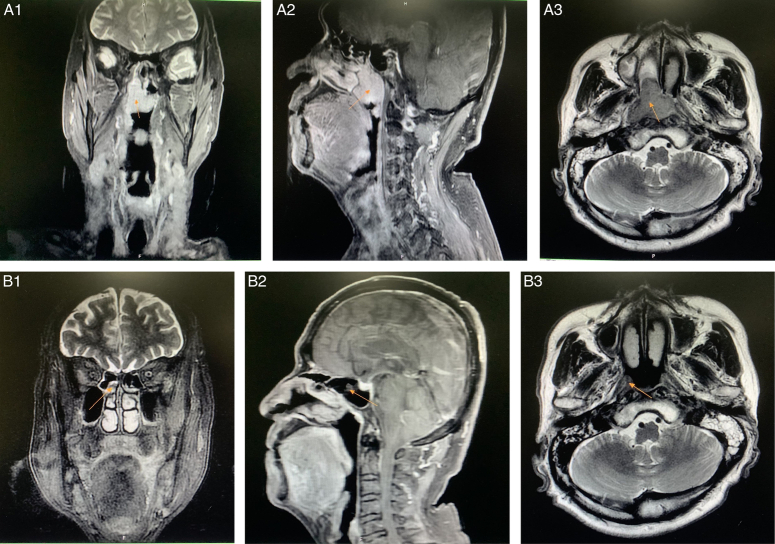
Figure 2.
Dynamic changes in MRI of the nasopharynx during treatment: (A1/A2/A3) before immunotherapy (2020-02-15, 3.2×3.1×3.7 cm) and (B1/B2/B3) after four immunotherapy treatments [2020-05-10, 0.6×0.8×2.0 cm, indicating partial response (PR)].
Discussion
In 1968, Farrow et al.7 found a type of renal cancer with a mixture of pleomorphic spindle cells and giant cells under the microscope, which was similar to sarcoma, and named it SRCC. Later studies found that sarcomatoid components can be found in the traditional histological types of renal cancer. Therefore, the American Joint Committee on Cancer removed SRCC from the histological types of renal cancer as a separate subtype in 1997, and it is now regarded as a special pathological feature of renal cancer. Only a proportion of sarcomatoid components in each subtype of tumor tissue has been described, and almost all sarcomatoid components are unclassified kidney cancers. Compared with other subtypes of RCC, SRCC progresses rapidly and has poor prognosis; the higher the proportion of sarcomatoid dedifferentiation, the worse the prognosis1,8.
Clinical manifestations of SRCC
SRCC is more common in middle-aged and elderly people (median age of onset: 60 years) than other age groups and is slightly more common in men than in women (1.6:1). It is often unilateral. The clinical manifestations of SRCC are closely related to the clinical staging at the time of consultation. It is often asymptomatic in its early stages and not easily detected. However, 90% of patients are mostly in the late stage at the time of consultation, with clinical symptoms such as abdominal pain on the affected side, waist mass, and hematuria. The most common metastatic sites of SRCC are the lungs, lymph nodes, bones, liver, and brain. For every 10% increase in the proportion of sarcomatoid dedifferentiation compared with non-SRCC, the risk of death increases by ~6%. Most patients with a survival period of more than 1 year are in the early stages of the disease (T1 and T2 stages) when they are diagnosed, and 60–80% of patients with SRCC have lymphatic invasion and distant metastasis at the time of diagnosis. The median OS (mOS) was 6–13 months, and the median PFS (mPFS) was 3.5–5.8 months9. The PFS and OS of this patient were as long as 25 and 30 months after immunotherapy, and the effect was significant.
SRCC imaging
There is no obvious capsule formation in the tumor during the plain CT scan, but the tumor shows infiltrative growth with unclear boundaries. Cystic degeneration and tumor necrosis are common in the inner and central regions of tumors, showing the appearance of cystic and solid tumors. Enhanced CT shows heterogeneous enhancement of the tumor, which is lower than that of the normal renal cortex. The larger the diameter of the tumor, the greater the probability of lesion necrosis. Necrosis is more uneven, mainly due to ischemia caused by the extrusion and rupture of blood vessels; however, regardless of the reason, tumor blood vessels can still cater to a small part of the cancer nest. Therefore, necrosis is incomplete and may result in ‘necrotic intra-enhancing foci’ seen on imaging. The sensitivity and accuracy of MRI in the diagnosis of SRCC are similar to those of CT, but MRI is superior to CT in showing involvement of the renal vein or inferior vena cava, invasion of surrounding organs, and differentiation from benign tumors or cystic masses. The enhanced CT of the case showed cystic low-density foci with enhanced edges, uniform inner density, and calcification. SRCC lacks specific imaging manifestations and cannot be correctly diagnosed before surgery; diagnosis depends on postoperative pathological and immunohistochemical examinations.
Pathological features of SRCC
SRCC can originate from various RCC subtypes, such as clear cell carcinoma, papillary RCC, and chromophobe RCC. SRCC is dominated by tumor necrosis and cystic degeneration, and its diagnosis depends on the presence of cord-like spindle cells in the sarcomatoid component area10,11. Of note, SRCC is derived from epithelial tissue, but there are two types of epithelial and mesenchymal differentiation in its morphology. Sarcomatoid carcinomas express both epithelial markers, such as CK18, CK7, EMA, and mesenchymal markers (vimentin, S-100, etc.)12. The sarcomatoid component of carcinosarcoma only expresses mesenchymal markers. Immunohistochemistry results of the this patient indicated: AE1/AE3, CK5/6, P63, P40, and EGFR were positive (3+); P16, VEGF, and Epstein-Barr encoding region were negative; and Ki-67 (~30%+).
Treatment of SRCC
The results of some studies showed that surgery1,3,13,14, chemotherapy15, or targeted therapy16–19 were not effective in patients with advanced SRCC. Approximately 77–80% of patients who receive nephrectomy with curative intent for localized SRCC recur within 5–26 months3,13. mOS was not more than 1 year in patients who underwent cytoreductive nephrectomy1,14. A prospective phase II clinical trial14 evaluated the efficacy of a combination of doxorubicin and gemcitabine in patients with previously untreated SRCCs, mOS, and mPFS at 8.8 and 3.5 months, respectively. Keskin et al.16 noted a 12-month OS benefit in patients with SRCC treated in the targeted therapy era, which assessed sunitinib, sorafenib, bevacizumab, etc. In a larger retrospective series17, VEGF inhibitor therapy in 230 patients with metastatic SRCCs, objective response rate (ORR) was 20%, mPFS and mOS were 4.5 and 10.4 months. A phase II trial18 with metastatic SRCCs was conducted to explore the use of combination chemotherapy with targeted therapy agents, ORR was 20%, mPFS and mOS were 5.5 and 12 months. Another trial19 presented that mPFS and mOS were 5.29 and 9.43 months for sunitinib plus gemcitabine, and 2.99 and 7.59 months for sunitinib.
Zhao et al.20 collected 59 patients diagnosed with SRCC between 2012 and 2022, the positive expression of PD-1 and PD-L1 was 57.6% and 62.7%, respectively. OS was shorter in the subgroup of patients with PD-L1-positive SRCC compared with the PD-L1-negative subgroup. Toripalimab, a humanized anti-programmed cell death protein 1 (PD-1) IgG4κ antibody21, is approved in China for the treatment of six cancer indications, including melanoma22, urothelial cancer23, non-small-cell lung cancer24, and esophageal squamous cell cancer25, among others. In a phase I trial26, single-agent toripalimab showed promising clinical activities for patients with previously treated advanced RCC, with an ORR of 33.3% and a disease control rate (DCR) of 50%. To our knowledge, this is the first case report of the use of toripalimab for the treatment of SRCC. Other immunotherapy trials related to SRCC, such as the Checkmate-214 and 016 trials27–29, compared nivolumab combined with ipilimumab and sunitinib for the first-line treatment of advanced intermediate and high-risk renal cancer. Patients with SRCCs treated with ipilimumab plus nivolumab had improved mPFS (26.5 vs. 5.1 months), complete response rates (CRR) (18.9% vs. 3.1%), partial response rates (PRR) (41.9% vs. 20.0%) and mOS (not reached vs. 14.2 months) compared with those treated with sunitinib. Therefore, it is recommended by major guidelines as the first-line treatment option for SRCC. The KEYNOTE-426 study30–32 compared pembrolizumab combined with axitinib and sunitinib as first-line treatment for advanced RCC. The results showed that the ORR (58.8% vs. 31.5%), complete CRR (11.8% vs. 0), and 1-year tumor PFS (57% vs. 26%) in the combination group were improved compared with those in the control group. Phase III clinical trials support its use as a first-line treatment option for SRCC. The AVELIN Renal 101 study33 compared the efficacy of avelumab combined with axitinib and sunitinib in the treatment of SRCC, and the results showed that for PD-L1-positive patients, the combination group could significantly improve the mPFS and ORR compared with the control group (13.8 vs. 7.2 months and 46.8% vs. 21.3%, respectively)34. The phase III clinical trial, IMmotion151 study35–37, compared atezolizumab combined with bevacizumab and sunitinib as first-line treatment for SRCC. The ORR of the combination group and the control group was 49% and 14%, and the mPFS was 8.3 and 5.3 months, respectively; these results support its use as a first-line treatment option for patients with advanced SRCC. In conclusion, the combination of ICIs and targeted drugs prolong OS in patients with advanced SRCC compared with targeted therapy. The patient was diagnosed as having SRCC with multiple lymph node metastases. After toripalimab immunotherapy, the PFS and OS were 25 and 30 months, respectively, and its curative effect was significant.
Conclusion
SRCC is a rare entity. The effect of traditional treatment is limited, and ICIs may have therapeutic potential. Importantly, our patient obtained PFS for up to 25 months while OS for up to 30 months through the application of ICIs. However, because this is a single case report, it is necessary to further expand the sample size in clinical practice to confirm its therapeutic value.
Ethical approval
Not required.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
This study did not receive any sources of funding.
Author contribution
H.S. and G.S.: drafted the manuscript and the collation of the case; X.M.: critically revised the paper; X.Y. and C.Y.: participated in the collection and sorting of images and the format editing of the article. All authors contributed toward data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
Not required.
Guarantor
Hui Su, the corresponding author.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Hui Su, upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Hui Su, Email: shhappy065010733@126.com.
Chao Yu, Email: yuchao-111@163.com.
Xuezhen Ma, Email: 86485318@qq.com.
Xiao Yu, Email: shlcsrmyy@126.com.
Guiming Sun, Email: yclcsrmyy@126.com.
References
- 1.Alevizakos M, Gaitanidis A, Nasioudis D, et al. Sarcomatoid renal cell carcinoma: population-based study of 879 patients. Clin Genitourin Cancer 2019;17:e447–e453. [DOI] [PubMed] [Google Scholar]
- 2.Kim T, Zargar-Shoshtari A, Dhillon J, et al. Using percentage of sarcomatoid differentiation as a prognostic factor in renal cell carcinoma. Clin Genitourin Cancer 2015;13:225–230. [DOI] [PubMed] [Google Scholar]
- 3.Zhang BY, Thompson RH, Lohse CM, et al. A novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU Int 2015;115:405–411. [DOI] [PubMed] [Google Scholar]
- 4.Adibi M, Thomas AZ, Borregales LD, et al. Percentage of sarcomatoid component as a prognostic indicator for survival in renal cell carcinoma with sarcomatoid dedifferentiation. Urol Oncol 2015;33:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami F, Sircar K, Rodriguez-Canales J, et al. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer 2017;13:4823–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohrabi C, Mathew G, Maria N, et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl 2023;109:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrow GM, Harrison EG, Jr, Utz DC, et al. Sarcomas and sarcomatoid and mixed malignant tumors of the kidney in adults. I. Cancer 1968;22:545–550. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Wu B, Zha Z, et al. The prognostic value and clinicopathological features of sarcomatoid differentiation in patients with renal cell carcinoma: a systematic review and meta-analysis. Cancer Manag Res 2018;10:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum KA, Gupta S, Tickoo SK, et al. Sarcomatoid renal cell carcinoma: biology, natural history and management. Nat Rev Urol 2020;17:659–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanas IO, He H, Grossmann P, et al. Low-grade spindle cell proliferation in clear cell renal cell carcinoma is unlikely to be an initial step in sarcomatoid differentiation. Histopathology 2018;72:804–813. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JK, Agarwal N, Brugarolas J, et al. Checking the hippo in sarcomatoid renal cell carcinoma with immunotherapy. Clin Cancer Res 2021;27:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalova K, Steiner P, Alaghehbandan R, et al. Papillary renal cell carcinoma with cytologic and molecular genetic features overlapping with renal oncocytoma: analysis of 10 cases. Ann Diagn Pathol 2018;35:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Merrill MM, Wood CG, Tannir NM, et al. Clinically nonmetastatic renal cell carcinoma with sarcomatoid dedifferentiation: natural history and outcomes after surgical resection with curative intent. Urol Oncol 2015;33:166.e121–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014;66:704–710. [DOI] [PubMed] [Google Scholar]
- 15.Haas NB, Lin X, Manola J, et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol 2012;29:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keskin SK, Msaouel X, Hess KR, et al. Outcomes of patients with renal cell carcinoma and sarcomatoid dedifferentiation treated with nephrectomy and systemic therapies: comparison between the cytokine and targeted therapy eras. J Urol 2017;198:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyriakopoulos CE, Chittoria N, Choueiri TK, et al. Outcome of patients with metastatic sarcomatoid renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clin Genitourin Cancer 2015;13:e79–e85. [DOI] [PubMed] [Google Scholar]
- 18.Maiti A, Nemati-Shafaee N, Msaouel P, et al. Phase 2 trial of capecitabine, gemcitabine, and bevacizumab in sarcomatoid renal-cell carcinoma. Clin Genitourin Cancer 2017 Aug 10:S1558-7673(17)30238-0 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 19.Haas NB, Puligandla M, McDermott DF, et al. ECOG 1808: Randomized phase II trial of sunitinib with or without gemcitabine in advanced kidney cancer with sarcomatoid features. J Clin Oncol 2016;34:4511. [Google Scholar]
- 20.Zhao Y, Shi Z, Xie Y, et al. The association between PD-1/PD-L1 expression an clinicopathological features in sarcomatoid renal cell carcinoma. Asian J Surg 2023;7:S1015–S9584. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Hao B, Geng Z, et al. Toripalimab: the first domestic anti-tumor PD-1 antibody in China. Front Immunol 2022;12:730666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang B, Chi Z, Chen Y, et al. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter phase II trial. Clin Cancer Res 2020;26:4250–4259. [DOI] [PubMed] [Google Scholar]
- 23.Sheng X, Chen H, Hu B, et al. Safety, efficacy, and biomarker analysis of toripalimab in patients with previously treated advanced urothelial carcinoma: results from a multicenter phase II trial POLARIS-03. Clin Cancer Res 2022;28:489–497. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Wu L, Li B, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non–small-cell lung cancer: a multicenter randomized phase III trial (CHOICE-01). J Clin Oncol 2023;41:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H-X, Pan Y-Q, He Y, et al. Clinical benefit of first-line programmed death-1 antibody plus chemotherapy in low programmed cell death ligand 1–expressing esophageal squamous cell carcinoma: a post hoc analysis of JUPITER-06 and meta-analysis. J Clin Oncol 2023;41:1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang B, Yan X, Sheng X, et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol 2019;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol 2017;35:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tannir NM, Signoretti S, Choueiri TK, et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res 2021;27:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 31.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J Clin Oncol 2019;37:4500. [Google Scholar]
- 32.Powles T, Plimack ER, Soulieres D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomized, open-label, phase 3 trial. Lancet Oncol 2020;21:1563–1573. [DOI] [PubMed] [Google Scholar]
- 33.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choueiri TK, Larkin J, Pal SK, et al. Efficacy and biomarker analysis of patients (pts) with advanced renal cell carcinoma (aRCC) with sarcomatoid histology (SRCC): subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab plus axitinib (A + Ax) vs sunitinib (S). Ann Oncol 2019;30:361.30715160 [Google Scholar]
- 35.McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018;24:749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393:2404–2415. [DOI] [PubMed] [Google Scholar]
- 37.Rini BI, Motzer RJ, Powles T, et al. Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: a prespecified subgroup analysis of the IMmotion151 clinical trial. Eur Urol 2021;79:659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Hui Su, upon reasonable request.