Abstract
Robotic surgery, known for its minimally invasive techniques and computer-controlled robotic arms, has revolutionized modern medicine by providing improved dexterity, visualization, and tremor reduction compared to traditional methods. The integration of artificial intelligence (AI) into robotic surgery has further advanced surgical precision, efficiency, and accessibility. This paper examines the current landscape of AI-driven robotic surgical systems, detailing their benefits, limitations, and future prospects. Initially, AI applications in robotic surgery focused on automating tasks like suturing and tissue dissection to enhance consistency and reduce surgeon workload. Present AI-driven systems incorporate functionalities such as image recognition, motion control, and haptic feedback, allowing real-time analysis of surgical field images and optimizing instrument movements for surgeons. The advantages of AI integration include enhanced precision, reduced surgeon fatigue, and improved safety. However, challenges such as high development costs, reliance on data quality, and ethical concerns about autonomy and liability hinder widespread adoption. Regulatory hurdles and workflow integration also present obstacles. Future directions for AI integration in robotic surgery include enhancing autonomy, personalizing surgical approaches, and refining surgical training through AI-powered simulations and virtual reality. Overall, AI integration holds promise for advancing surgical care, with potential benefits including improved patient outcomes and increased access to specialized expertise. Addressing challenges and promoting responsible adoption are essential for realizing the full potential of AI-driven robotic surgery.
Keywords: artificial intelligence, computers, haptic technology, robotic surgical procedures, surgeons
Introduction
Highlights
Robotic surgery, known for its minimally invasive techniques and computer-controlled robotic arms, has revolutionized modern medicine by providing improved dexterity, visualization, and tremor reduction compared to traditional methods.
AI applications in robotic surgery focused on automating tasks like suturing and tissue dissection to enhance consistency and reduce surgeon workload.
AI integration holds promise for advancing surgical care, with potential benefits including improved patient outcomes and increased access to specialized expertise.
Robotic surgery, a minimally invasive surgical technique utilizing computer-controlled robotic arms, has revolutionized modern medicine. Compared to traditional laparoscopic surgery, it offers enhanced dexterity, improved visualization, and reduced tremors, leading to several benefits for patients. These include smaller incisions, lesser blood loss, faster recovery times and reduced pain1.
Artificial intelligence (AI) encompasses a range of intelligent technologies that can learn, reason, and make decisions without explicit programming. In the medical field, AI is finding increasing applications in various areas, including medical imaging analysis and diagnosis2, drug discovery and development3 and robot-assisted surgery4.
The integration of AI into robotic surgery holds immense promise for further enhancing its precision, efficiency, and accessibility. This paper explores the current state of AI-driven robotic surgical systems, their advantages and limitations, and future directions for this transformative technology.
The development of robotic surgery can be traced back to the 1980s with the introduction of the PUMA robot5. Early robotic surgical systems were primarily used for telemanipulation, allowing surgeons to operate remotely. Subsequent advancements led to the creation of more sophisticated robotic arms with improved dexterity and control. The landmark FDA approval of the da Vinci Surgical System in 2000 marked a significant milestone in the field6.
The application of AI in healthcare has witnessed significant growth in recent years, fueled by advancements in machine learning algorithms and the availability of vast amounts of medical data. Early applications focused on tasks like analyzing medical images for cancer detection or predicting patient outcomes7–9.
The initial integration of AI in robotic surgery focused on automating specific surgical tasks, such as suturing or tissue dissection. These applications aimed to improve consistency and reduce surgeon workload4,10.
Current AI-driven robotic surgical systems incorporate various functionalities, including image recognition and segmentation: AI algorithms can analyze surgical field images in real-time to identify critical structures, blood vessels, and tumors, aiding surgeons in decision-making11,12.
With motion control and path planning: AI can assist in planning and optimizing surgical instrument movements, leading to smoother and more precise procedures13. With haptic feedback: AI can enhance the sense of touch experienced by the surgeon through the robotic interface, providing valuable feedback on tissue texture and resistance14,15.
Methods
A comprehensive literature search for this narrative review was conducted using the PubMed, Embase and Google Scholar databases, focusing on peer-reviewed publications from January 2010 till May 2024 to ensure the inclusion of the latest developments in the field. The search employed the following terms: artificial intelligence and related terms, machine learning and related terms, and robotic or robot-assisted surgery and related terms. The selection process followed the PRISMA guidelines, as illustrated in Figure. Initially, 483 unique records were identified. After screening, 457 full-text articles were evaluated for their relevance, and 103 were chosen as representative of the most recent advancements in the field to be included in this narrative review. The search terms were reviewed and examined by two authors. Any disagreements were resolved through the involvement of an independent third author. The study methodology is illustrated in Figure 1.
Figure 1.
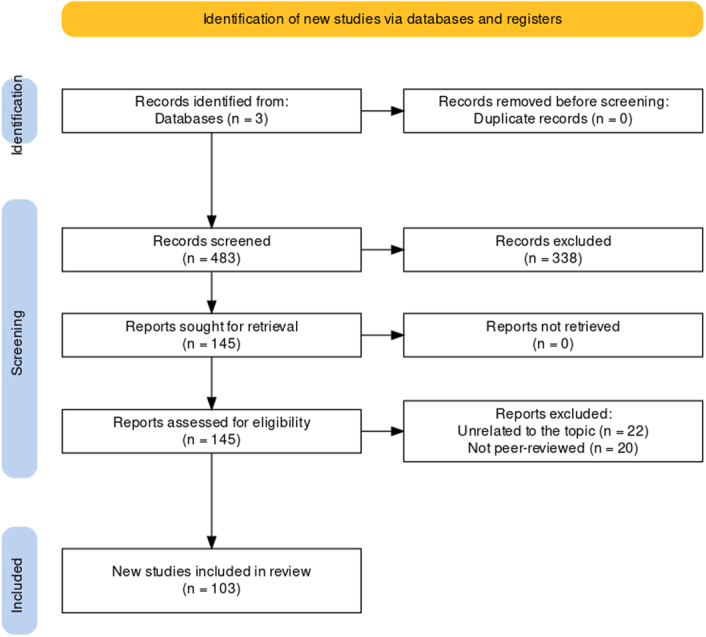
Flowchart of study methodology.
Review
The advancement of autonomous control in surgical robotic platforms holds promise for achieving greater precision, intelligent maneuvers, and avoidance of tissue damage. Although many autonomous robotic systems remain experimental, some have already made their way into clinical practice. Ongoing research aims to develop fully autonomous surgical systems capable of performing complex tasks on deformable soft tissues, such as suturing and intestinal anastomosis, within open surgical settings. Initial findings suggest that supervised autonomous procedures can surpass both expert surgeon-performed surgeries and robot-assisted approaches in terms of effectiveness and consistency. These strides in autonomous robotic surgery have the potential to enhance surgical outcomes and expand access to optimized techniques16,17.
Despite initial skepticism among some surgeons, advances in AI and robotics are paving the way for increased autonomy in surgical procedures. While the absence of haptics has traditionally hindered the widespread adoption of robotic surgery, there is growing recognition within the surgical community of the true potential of robotics. Consequently, the integration of AI is becoming increasingly crucial, offering new avenues for enhancing surgical precision and outcomes17–19.
The levels of autonomy in robotic surgery offer a progressive framework for understanding the evolving capabilities of surgical robots. As per Yang et al.’s20 classification of the level of autonomy shown in the figure; At Level 0, exemplified by the da Vinci system, surgeons directly control robotic movements without any assistance or constraints21. Moving up to Level 1, robots begin to assist surgeons by providing virtual fixtures or active limitations to guide their actions, facilitated by technologies like tissue interface sensing and eye tracking21.
Level 2 autonomy grants robots the ability to execute specific surgical tasks based on physician-provided guidelines, with control shifting from human operators to machines during task execution21. Examples include autonomous algorithms for tasks like tip retroflection in magnetic colonoscopy and tissue retraction systems using visual markers and fuzzy logic19,22.
Advancements to Level 3 introduce perceptual abilities to robots, enabling them to plan and execute tasks independently within the surgical setting. Examples include flexible endoscopic robots navigating unstructured environments autonomously during procedures like colonoscopy17,22,23.
Level 4 autonomy represents a significant leap, where robots interpret preoperative and intraoperative data to create intervention plans, execute actions, and adapt in real-time. While specific examples are limited, potential applications include intelligent tissue removal in cancer surgery, aiming to minimize damage to healthy tissue while targeting cancerous areas21.
Level 5 autonomy, where robots perform surgery without human intervention, remains aspirational and has not yet been achieved. However, advancements across lower autonomy levels indicate a promising trajectory toward more sophisticated and independent robotic surgical systems21.
The levels of surgical autonomy are illustrated in Figure 2.
Figure 2.
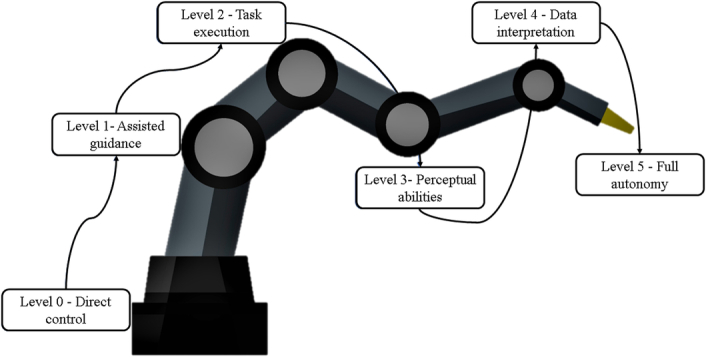
Levels of robotic autonomy.
Several examples illustrate the current state of autonomous robotic surgery interventions, particularly at level 3. One notable system is the Smart Tissue Autonomous Robot (STAR), designed by Axel Kriger, which has demonstrated the ability to match or even surpass human surgeons in bowel anastomosis24. STAR operates autonomously with human approval of the surgical plan, exhibiting remarkable efficacy in reducing errors and achieving smoother tissue reconstruction. The system assesses tissue thickness and structure to devise a suture insertion plan, then proceeds to sew autonomously after receiving human confirmation24. Continuous communication with the surgeon ensures adaptation to tissue deformation or unexpected changes throughout the procedure24.
While advancements in autonomous suturing are promising, they are currently limited to anatomical phantoms or ex-vivo models, with full autonomy in suturing still a distant prospect21. Another autonomous system, TSolution One, specializes in bone carving according to a pre-established plan, particularly in hip and knee replacement surgeries25. Although effective in bone drilling, its inability to differentiate between tissue types necessitates manual relocation of soft tissues to prevent damage. However, long-term data on survival and outcomes are lacking, impeding the assessment of its cost-effectiveness25.
Veebot represents another automated system specifically for blood sample collection. Employing infrared light and ultrasonography, Veebot identifies suitable veins for blood collection with a success rate comparable to human performance26,27. Similarly, the ARTAS system, a robotic graft harvesting device, enhances hair restoration surgery through follicular unit extraction, leveraging robotic precision for optimal harvesting28.
The CyberKnife robot showcases advanced autonomous capabilities in performing radiosurgery for brain and spine malignancies under human supervision. Utilizing stereotactic principles and real-time imaging, the system continuously adjusts for minor patient posture variations during treatment, ensuring precise and effective radiation delivery29.
These examples underscore the evolving landscape of autonomous robotic surgery, highlighting both achievements and ongoing challenges in integrating artificial intelligence into surgical practice.
Several AI algorithms are being explored for use in robotic surgery, including:
Deep learning: This technique is particularly adept at image recognition and can be used to identify anatomical structures, predict bleeding risks, and even guide surgical instrument trajectories30–34.
Reinforcement learning: This approach allows AI systems to learn through trial and error, potentially enabling them to perform complex surgical tasks autonomously in the future35.
The integration of AI in robotic surgery is a rapidly evolving field with numerous ongoing advancements. Here are some compelling case studies and examples across different surgical specialties.
Minimally invasive cardiac surgery
AI-assisted robotic coronary artery bypass grafting (CABG): Studies are demonstrating the potential of AI to improve outcomes in minimally invasive cardiac surgery. A multicenter, retrospective study published in the Journal of Thoracic and Cardiovascular Surgery (JTCVS) by Cuartas et al. and Cao et al. 36,37 explored the use of AI-assisted robotic minimally invasive CABG. The results showed promising outcomes, with shorter operative times, reduced blood loss, and fewer complications compared to traditional techniques.
Research is ongoing to leverage AI for real-time risk stratification during cardiac surgery. This could involve analyzing various physiological parameters to predict potential complications and guide surgeons in making informed decisions. Bonatti et al. and Ralf et al. 38,39 highlight the potential applications of AI in this area.
Neurological procedures
AI-powered Image Guidance for Brain Tumor Resection: Multiple studies40–42 showcased the potential of AI for assisting in complex brain tumor surgeries. The study described a case where AI-powered image guidance helped surgeons achieve a more complete tumor resection during delicate brain surgery, potentially leading to improved patient outcomes.
AI is showing promise in assisting with delicate neurological procedures like brain tumor removal. A recent article in Nature Medicine highlighted a case where AI-powered image guidance helped surgeons achieve a more complete tumor resection during a complex brain surgery40–42
AI algorithms are being explored for pre-surgical planning and intraoperative navigation in neurological procedures. This could involve creating 3D models of the brain based on patient scans and utilizing AI for real-time visualization of critical structures during surgery.
Orthopedic surgery
In orthopedic surgery, the initial application of AI concentrated on hip and knee procedures, employing data from the preoperative, intraoperative, and postoperative phases. The adoption of AI in shoulder surgery is more recent, with a growing number of reports but limited comprehensive studies43.
Patient-specific instrumentation (PSI), which has been developed over an extended period and is now commonly used in shoulder surgeries like shoulder arthroplasty, contributes to successful outcomes by ensuring precise implant placement. Properly securing and aligning the glenoid component presents a major challenge in total shoulder arthroplasty (TSA), and PSI supports surgeons by facilitating preoperative planning. A meta-analysis of 12 studies involving 227 participants revealed that PSI greatly enhanced glenoid positioning and decreased component malpositioning from 68.6 to 15.3% compared to traditional methods44.
Concurrently, robotic-assisted surgery has advanced significantly, especially in total knee arthroplasty (TKA) and total hip arthroplasty (THA). Platforms like the MAKO robotic arm-assisted system improve preoperative evaluations through CT scans, offering a 3D perspective of the joint that aids in accurate implant placement and virtual adjustments for balanced knee ligaments45. Research indicates that the MAKO system enhances the precision of component positioning, reduces postoperative pain and hospital stays, and improves functional outcomes45,46. For THA, the MAKO system has shown superior results with comparable complication rates to conventional methods, and patients report better outcomes and fewer instances of implant malpositioning47,48.
In shoulder surgery, AI is also progressing in the diagnosis and treatment of conditions such as rotator cuff tears (RCT). Traditionally, orthopedists diagnose RCT by interpreting MRI data, but deep learning systems using 3D convolutional neural networks (CNN) have been created for automated, accurate diagnosis. These systems can identify RCT, determine tear sizes, and visualize tear locations. Additionally, AI algorithms can assess muscle atrophy to predict the reparability of extensive RCTs, improving diagnostic efficiency and objectivity by measuring factors like the supraspinatus muscle occupation ratio49,50.
Research suggests AI can improve implant positioning and potentially reduce long-term complications in orthopedic surgery. Batailler et al. and Kayani et al. 51,52 investigated AI-assisted robotic TKA. The results demonstrated improved component positioning and alignment compared to conventional techniques, potentially leading to better implant longevity and patient outcomes.
AI-powered robotic systems are being utilized for complex orthopedic surgeries like hip and knee replacements. Research published in The Bone & Joint Journal found that AI-assisted robotic surgery for total knee arthroplasty resulted in improved implant positioning and potentially reduced long-term complications51–53.
The integration of AI and robotic technologies in orthopedic surgery is revolutionizing preoperative planning, intraoperative accuracy, and postoperative results across a range of surgeries, including those for the hip, knee, and shoulder.
AI has the potential to personalize surgical approaches in orthopedics based on factors like patient anatomy and medical history. This could involve optimizing implant selection and surgical techniques for each individual patient.
Urology
Robotic-assisted laparoscopic prostatectomy for prostate cancer with AI for improved tissue identification and nerve sparing54. The adoption of minimally invasive robotic surgery has seen a notable increase, especially for significant uro-oncological procedures. This innovation has profoundly transformed the surgical landscape, representing a major advancement toward the most effective and least invasive treatment options for patients. Modern robotic surgery systems typically employ a “master–slave” model, where surgeons control robotic arms remotely from an advanced console. This collaboration between human expertise and machine precision allows for meticulous monitoring and enhancement of surgical actions through AI55.
Machine learning (ML) has been extensively applied across various medical domains, improving disease diagnosis accuracy, aiding therapy selection, facilitating patient monitoring, and assisting in primary prevention risk assessment. ML techniques are crucial for enhancing surgical systems, particularly through the automated analysis of patient imaging and precise tracking of surgical anatomy and instruments during the perioperative period55,56.
Although no surgical system can yet perform operations entirely independently, robots have shown promising results in tasks such as anatomic tracking, suturing, and biopsy sampling57. The field of urology has seen an increase in the use of semi-autonomous surgical systems like Aquablation, with studies demonstrating the benefits of robotic assistance in therapeutic procedures58. Advances in selecting surgical candidates and the development of automated surgical robotic systems could significantly improve surgical precision and patient outcomes55.
Robotic surgery leverages advanced 3D visualization to augment the surgeon’s skills and accuracy. However, the absence of haptic feedback can negatively impact surgical outcomes59. Visual cues alone govern actions like dissection, pressure application, and tissue response evaluation, which can lead to issues such as excessive force on delicate tissues or insufficient force during knot-tying. For instance, excessive force during robotic radical prostatectomy (RARP) can damage neurovascular bundles, causing neuropraxia and delaying the recovery of sexual function, while insufficient force may lead to poor suture retention55,59. To address these issues, Dai et al. 59 developed an advanced warning system to detect suture breakage, incorporating biaxial shear detection and haptic feedback to alert the surgeon before a potential suture rupture. This system, integrated with the Da Vinci surgical system, provides vibrotactile feedback as suture tension approaches its limit, leading to a significant reduction in suture breakage and knot slippage and improved task consistency among inexperienced surgeons59.
Additionally, Piana et al. 60 demonstrated the use of three-dimensional augmented reality (AR) guidance during kidney transplantation (KT) to enhance surgical navigation and safety for patients with atheromatic vascular disease. This technology, which does not require haptic input, utilizes high-accuracy CT scan imaging to create 3D virtual models that are overlaid onto the vasculature during robot-assisted kidney transplantation (RAKT) using the Da Vinci console60.
Computer vision (CV), a subset of machine learning that focuses on image analysis, also holds promise for improving the diagnosis and identification of urologic conditions61. For example, CV algorithms applied to CT abdominal imaging data can accurately locate kidney stones, thanks to advanced image signal processing that allows the algorithms to discern even the smallest visual differences between abnormal and healthy anatomical structures55,61,62
Gastrointestinal surgery
Robotic-assisted minimally invasive colorectal surgery with AI is being studied for enhanced visualization and improved surgical precision63.
Robotic systems have shown significant effectiveness in colorectal cancer surgeries, particularly in complex procedures such as total mesorectal excision and complete mesocolon excision1,64. The robotic platform aids surgeons in performing vascular dissection, intracorporeal anastomoses, and lymphadenectomy, especially in anatomically challenging areas like those near critical vascular structures or the lateral pelvic walls64. Many medical centers now standardize the use of robotic assistance for rectal resections, reflecting the increased success and advantages of robotic surgery in these technically demanding colorectal procedures1,65.
One major concern regarding the widespread adoption of robotic assistance in colorectal surgery has been the high cost. Nevertheless, substantial evidence consistently demonstrates the undeniable benefits of robotic surgery, particularly in left colectomies and various rectal procedures, often surpassing the capabilities of advanced 3D laparoscopic systems15,66. Robotic-assisted surgery can overcome the limitations of traditional laparoscopy, offering advantages such as reduced blood loss, shorter hospital stays, faster restoration of bowel function, favorable oncological outcomes, and a lower conversion rate to open surgery1,66. A meta-analysis by Trastulli et al. 67 confirmed that robotic colorectal surgeries result in fewer perioperative complications and surgical site infections compared to laparoscopic procedures67.
A promising innovation in robotic rectal resections is the integration of Firefly technology, which is particularly beneficial during the low ligation of the inferior mesenteric artery (IMA) pedicle68. The precision offered by robots in retroperitoneal and pelvic dissection is crucial for accurate lymphadenectomy around the IMA68.
The use of robotics in bariatric surgery has been advancing since Cadiere et al. 69 first reported a case in 199969. Roux-en-Y gastric bypass is widely regarded as the most effective surgical procedure for severe obesity, and robotic surgery has emerged as a promising technology to enhance this procedure due to its documented advantages70. It is the most extensively studied robotic bariatric procedure70. Sleeve gastrectomy is also gaining popularity due to its low risk of complications, excellent outcomes, and perceived technical simplicity71. However, it involves specific challenges such as the risk of leakage along the staple line and the need for precise dissection in the left crus and hiatus area to mobilize the fundus71. Robotic surgery offers advantages over laparoscopic surgery, including endo-wrist capabilities that facilitate precise dissection and suturing of the staple line71. A systematic review by Cirocchi et al. 72 indicated that robotic bariatric surgery is increasingly used not only in redo cases but also in primary procedures, such as creating intracorporeal anastomoses during Roux-en-Y gastric bypass or managing complex resections in sleeve gastrectomy72. Robotic technology also improves the efficiency of closing enterotomies or gastrotomies, even when stapling is used for anastomoses72.
In pancreatic surgery, a study of 250 robotic pancreatic resections showed that robotic-assisted surgery is feasible for both oncologic and benign conditions, with a low conversion rate to open surgery73. However, it is crucial to remember that robotic technology is a tool that ultimately relies on the surgeon’s expertise74. The robotic platform enables surgeons to overcome the limitations of laparoscopy, especially in procedures like D2 lymphadenectomy75. The utility of the surgical robot is evident in tasks such as performing robotic-sewn anastomoses and navigating difficult dissections near the gastroesophageal junction and pyloric region, which is particularly advantageous in total gastrectomies76.
A study by Oliveira et al. 77 in head and neck surgery also demonstrated AI-powered robotic systems for greater dexterity and potentially reduced postoperative complications in head and neck surgery77.
These examples showcase the diverse applications of AI in robotic surgery and its potential to revolutionize surgical care across various disciplines. As research and development continue, we can expect even more advancements in this exciting field.
Advantages and limitations of AI integration in robotic surgeries
Advantages
Enhanced precision and accuracy: AI can assist surgeons in achieving greater precision during delicate procedures, potentially leading to improved surgical outcomes78.
Reduced surgeon fatigue: AI can automate repetitive tasks, minimizing surgeon fatigue and potentially improving focus during critical aspects of the surgery79.
Improved safety: AI-driven systems can provide real-time feedback on potential complications, such as bleeding or instrument clashes, aiding in preventing surgical errors80,81.
Some AI features with their benefits are summarized in Table 1.
Table 1.
AI features relevant to robotic surgery.
| Feature | Description | Potential benefits |
|---|---|---|
| Enhanced precision with motion prediction82 | AI can analyze a surgeon’s movements and predict their next actions, allowing robotic instruments to anticipate and follow seamlessly | - Minimized reaction times - Improved surgical precision - Reduced tissue damage |
| Intraoperative tissue recognition with AI-powered vision83 | AI algorithms can analyze real-time surgical video to identify critical anatomical structures and potential complications | - Improved surgical navigation - Reduced risk of accidental injury |
| Automated suturing and knot-tying35 | Advancements in AI and robotics are leading to the development of automated suturing and knot-tying systems | - Reduced surgeon fatigue - Improved consistency of suturing - Potentially shorter surgical times |
| Personalized surgical planning with AI78 | AI can analyze patient data (scans, medical history) to generate individualized surgical plans and predict potential outcomes | - Optimized surgical approaches - Improved patient selection for robotic surgery - Potentially better long-term results |
AI, artificial intelligence.
Limitations
High development and implementation costs: The development and implementation of AI-driven robotic surgical systems are expensive, including the initial purchase cost, ongoing maintenance, and infrastructure upgrades. This can limit their accessibility, particularly for smaller hospitals and healthcare institutions in resource-constrained settings84,85.
Reliance on data quality: The effectiveness of AI algorithms heavily depends on the quality and quantity of training data. Biases in training data can lead to biased decision-making by the AI system, potentially exacerbating existing healthcare disparities86.
Ethical considerations: The increasing autonomy of AI in surgery raises ethical concerns regarding responsibility and liability in case of adverse events. Clear guidelines and regulations are needed to ensure patient safety and address medico-legal issues87.
Challenges faced in implementing AI in robotic surgeries
Regulatory hurdles: Obtaining regulatory approvals for AI-driven robotic surgical systems can be a complex and time-consuming process, hindering their wider adoption88,89. Regulatory bodies need to establish clear guidelines for evaluating the safety and efficacy of these systems while fostering innovation.
Integration with existing workflows: Integrating AI-powered robotic systems into existing surgical workflows can be challenging. This may require changes in surgical team dynamics and necessitate additional training for surgeons and surgical staff to adapt to the new technology90,91.
Cybersecurity concerns: The increasing reliance on AI systems in surgery raises cybersecurity concerns regarding potential hacking or malfunctions that could compromise patient safety. Robust security measures are essential to ensure the integrity and reliability of these systems92,93.
Future directions
The integration of AI in robotic surgery is a rapidly evolving field with immense potential to transform surgical care. Here are some exciting future directions to consider:
Enhanced autonomy: Advancements in AI could lead to the development of more autonomous robotic surgical systems, potentially enabling surgeons to perform complex procedures remotely or with minimal assistance94,95. However, careful consideration of ethical implications and ensuring surgeon oversight remain crucial.
Personalized surgery: AI can be used to analyze patient data and tailor surgical approaches to individual needs, leading to more personalized and effective treatments96,97. This could involve factors like patient anatomy, medical history, and genetic variations.
Improved surgical training: AI-powered simulations can provide surgeons with realistic training environments to practice complex procedures and refine their skills, potentially leading to improved surgical outcomes98,99. Virtual reality (VR) integrated with AI could further enhance the training experience.
Robotic-assisted technologies have fundamentally transformed how various tasks are executed, with one of the most significant advancements being the integration of artificial intelligence (AI)100. The incorporation of AI algorithms enhances the capabilities of robotic systems by enabling them to learn, adapt, and make decisions in real-time100. This enhancement allows robots to perform complex tasks with increased efficiency and precision. Machine learning algorithms also improve human-robot interaction, making robots more intuitive and responsive to user needs. Despite these advancements, there is currently no evidence that AI can independently recognize the critical tasks in robotic-assisted surgeries that determine patient outcomes. Therefore, extensive studies on large data sets and external validation are required to verify the efficacy of AI algorithms in robotic-assisted surgeries100.
The increasing autonomy in robotic surgery has the potential to standardize surgical outcomes, making them less dependent on the surgeon’s training, experience, and daily performance variations24. A survival study indicated that a developed robotic system could match the performance of an expert surgeon24. However, robotic-assisted surgeries have yet to be thoroughly explored in emergency settings, though initial experiences have been documented in the literature101. Two promising areas of ongoing research in this field are micro-robotics and telesurgery102,103.
Micro-robotics research includes the development of portable capsule endoscopes for various diagnostic tasks, surgical applications, and targeted drug delivery102,103. These microrobots, which are millimeter-sized, can be guided by extracorporeal magnets to perform specific functions, such as applying a nitinol clip to stop chronic bleeding during a biopsy in porcine models102,103. Research in micro-robotics focuses on four key areas: miniaturized functionality, contained propulsion, consistent visualization, and precise telemanipulation103.
The integration of AI in robotic-assisted technologies is advancing the field by improving the efficiency, accuracy, and user interaction of robotic systems. However, further research is necessary to validate the independent effectiveness of AI in critical surgical tasks. Increased autonomy in robotic surgery could lead to more standardized outcomes, and emerging areas like micro-robotics and telesurgery hold significant promise for future applications.
Conclusion
The integration of AI into robotic surgery holds immense promise for revolutionizing surgical care. By enhancing precision, efficiency, and accessibility, AI has the potential to improve patient outcomes, reduce complications, and democratize access to specialized surgical expertise. However, addressing challenges related to cost, data quality, ethical considerations, and regulatory hurdles is crucial for the responsible and widespread adoption of this transformative technology. As AI continues to evolve, the future of robotic surgery is poised to become even more remarkable, paving the way for a new era of personalized, precise, and patient-centered surgical care.
Ethical approval
Ethics approval was not required for this Review.
Consent
Informed consent was not required for this Review.
Source of funding
No funding was received in any shape or form for this paper.
Author contribution
All authors fulfill the ICMJE criteria for authorship. All authors reviewed the final version of the manuscript and approved it for submission and publication.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
This review has been registered in Open Science framework online and can be accessed from: https://doi.org/10.17605/OSF.IO/NJYBU.
Guarantor
Hassan Mumtaz.
Data availability statement
Data are available upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Muhammad Iftikhar, Email: muhammadiftikhar271@gmail.com.
Muhammad Saqib, Email: muhammadsaqib.drkmc@gmail.com.
Muhammad Zareen, Email: muhammad.zarin@kmc.edu.pk.
Hassan Mumtaz, Email: hassanmumtaz.dr@gmail.com.
References
- 1.Fairag M, Almahdi RH, Siddiqi AA, et al. Robotic revolution in surgery: diverse applications across specialties and future prospects review article. Cureus 2024;16:e52148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.B.J. C. “artificial intelligence” [Internet]. [Available from: https://www.britannica.com/technology/artificial-intelligence
- 3.Paul D, Sanap G, Shenoy S, et al. Artificial intelligence in drug discovery and development. Drug Discov Today 2021;26:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knudsen JE, Ghaffar U, Ma R, et al. Clinical applications of artificial intelligence in robotic surgery. J Robot Surg 2024;18:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theodore N, Arnold PM, Mehta AI. Introduction: the rise of the robots in spinal surgery. Neurosurg Focus 2018;45(VideoSuppl1):Intro. doi: 10.3171/2018.7.FocusVid.Intro [DOI] [PubMed] [Google Scholar]
- 6.Da Vinci Robotic System: Food and Drug Administration; [Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf20/K202834.pdf.
- 7.Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 2019;69:127–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midthun DE. Early diagnosis of lung cancer. F1000Prime Rep 2013;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaffie A, Soliman A, Ghazal M, Taher F, Dunlap N, Wang B, et al. A new framework for incorporating appearance and shape features of lung nodules for precise diagnosis of lung cancer IEEE International Conference on Image Processing (ICIP); 2017. [Google Scholar]
- 10.Mansour M, Cumak EN, Kutlu M, et al. Deep learning based suture training system. Surg Open Sci 2023;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan Yimeng L, Alyssa Imperatore Z, Lauryn U, et al. Artificial Intelligence in Surgery, Surgical Subspecialties, and Related Disciplines In: Stanislaw PS, editor. Artificial Intelligence in Medicine and Surgery. Rijeka: IntechOpen; 2023. [Google Scholar]
- 12.Pierson HA. Deep Learning in Robotics: A Review of Recent Research [Internet]. [Available from: https://arxiv.org/pdf/1707.07217
- 13.Liu J, Dong X, Yang Y, et al. Trajectory tracking control for uncertain robot manipulators with repetitive motions in task space. Math Problems Eng 2021;2021:8838927. [Google Scholar]
- 14.Okamura AM. Haptic feedback in robot-assisted minimally invasive surgery. Curr Opin Urol 2009;19:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergholz M, Ferle M, Weber BM. The benefits of haptic feedback in robot assisted surgery and their moderators: a meta-analysis. Sci Rep 2023;13:19215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shademan A, Decker RS, Opfermann JD, et al. Supervised autonomous robotic soft tissue surgery. Sci Transl Med 2016;8:337ra64. [DOI] [PubMed] [Google Scholar]
- 17.Rivero-Moreno Y, Rodriguez M, Losada-Muñoz P, et al. Autonomous robotic surgery: has the future arrived? Cureus 2024;16:e52243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumbs AA, Frigerio I, Spolverato G, et al. Artificial intelligence surgery: how do we get to autonomous actions in surgery? Sensors 2021;21:5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slawinski PR, Taddese AZ, Musto KB, et al. Autonomous retroflexion of a magnetic flexible endoscope. IEEE Robot Automat Lett 2017;2:1352–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang GZ, Cambias J, Cleary K, et al. Medical robotics—regulatory, ethical, and legal considerations for increasing levels of autonomy. Sci Robot 2017;2:eaam8638. [DOI] [PubMed] [Google Scholar]
- 21.Attanasio A Scaglioni B De Momi E, et al. , Valdastri PJARoC, Robotics, Systems A. Autonomy in surgical robotics. 2021;4:651-79.
- 22.Rajnai Z, Kocsis I. Assessing industry 40 readiness of enterprises. IEEE 16th World Symposium on Applied Machine Intelligence and Informatics (SAMI); 2018:225–230. [Google Scholar]
- 23.Martin JW, Slawinski PR, Scaglioni B, et al. 382 Assistive-autonomy in colonoscopy: propulsion of a magnetic flexible endoscope 2019;89:AB76–AB77. [Google Scholar]
- 24.Saeidi H, Opfermann JD, Kam M, et al. Autonomous robotic laparoscopic surgery for intestinal anastomosis. Sci Robot 2022;7:eabj2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liow MHL, Chin PL, Pang HN, et al. THINK surgical TSolution-One(®) (Robodoc) total knee arthroplasty. Sicot-j 2017;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi CQJIS. IEEE Spectrum. 2020;21.
- 27.Strickland E. IEEE Spectrum. Retrieved from Facebook Announces” Typing-by-Brain” Project; 2017: http://spectrum
- 28.Rose PT, Nusbaum B. Robotic hair restoration. Dermatol Clin 2014;32:97–107. [DOI] [PubMed] [Google Scholar]
- 29.Kilby W, Dooley JR, Kuduvalli G, et al. The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat 2010;9:433–452. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Jiang Y, Zhang Y, et al. Medical image analysis using deep learning algorithms. Front Public Health 2023;11:1273253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madani A, Namazi B, Altieri MS, et al. Artificial intelligence for intraoperative guidance: using semantic segmentation to identify surgical anatomy during laparoscopic cholecystectomy. Ann Surg 2022;276:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalid S, Goldenberg M, Grantcharov T, et al. Evaluation of deep learning models for identifying surgical actions and measuring performance. JAMA Netw Open 2020;3:e201664–e201664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen JS, Laursen MS, Rajeeth Savarimuthu T, et al. Deep learning detects and visualizes bleeding events in electronic health records. Res Pract Thromb Haemostasis 2021;5:e12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pangal DJ, Kugener G, Zhu Y, et al. Expert surgeons and deep learning models can predict the outcome of surgical hemorrhage from 1 min of video. Sci Rep 2022;12:8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.https://arxiv.org/pdf/2309.00773 Ren CQaH. Deep Reinforcement Learning in Surgical Robotics: Enhancing the Automation Level [Available from:
- 36.Marin-Cuartas M, Sá MP, Torregrossa G, et al. Minimally invasive coronary artery surgery: Robotic and nonrobotic minimally invasive direct coronary artery bypass techniques. JTCVS Tech 2021;10:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao C, Indraratna P, Doyle M, et al. A systematic review on robotic coronary artery bypass graft surgery. 2016;5:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonatti J, Wallner S, Crailsheim I, et al. Minimally invasive and robotic coronary artery bypass grafting—a 25-year review. J Thorac Dis 2020;13:1922–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sulague RM Beloy FJ Medina JR, et al. Artificial Intelligence in Cardiac Surgery: A Systematic Review. [DOI] [PubMed]
- 40.Tangsrivimol JA, Schonfeld E, Zhang M, et al. Artificial intelligence in neurosurgery: a state-of-the-art review from past to future. Diagnostics (Basel, Switzerland) 2023;13:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams S, Layard Horsfall H, Funnell JP, et al. Artificial intelligence in brain tumour surgery-an emerging paradigm. Cancers 2021;13:5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Meng Y, Qiu K, et al. Applications of artificial intelligence based on medical imaging in glioma: current state and future challenges. 2022;12:892056. doi: 10.3389/fonc.2022.892056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee K-S, Jung SH, Kim D-H, et al. Artificial intelligence- and computer-assisted navigation for shoulder surgery 2024;32:10225536241243166. [DOI] [PubMed] [Google Scholar]
- 44.Villatte G, Muller AS, Pereira B, et al. Use of Patient-Specific Instrumentation (PSI) for glenoid component positioning in shoulder arthroplasty. A systematic review and meta-analysis. PLoS ONE 2018;13:e0201759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roche M. The MAKO robotic-arm knee arthroplasty system. Arch Orthop Trauma Surg 2021;141:2043–2047. [DOI] [PubMed] [Google Scholar]
- 46.Batailler C, Fernandez A, Swan J, et al. MAKO CT-based robotic arm-assisted system is a reliable procedure for total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 2021;29:3585–3598. [DOI] [PubMed] [Google Scholar]
- 47.St Mart JP, Goh EL, Shah Z. Robotics in total hip arthroplasty: a review of the evolution, application and evidence base. EFORT Open Rev 2020;5:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Domb BG, Chen JW, Lall AC, et al. Minimum 5-year outcomes of robotic-assisted primary total hip arthroplasty with a nested comparison against manual primary total hip arthroplasty: a propensity score-matched study. J Am Acad Orthop Surg 2020;28:847–856. [DOI] [PubMed] [Google Scholar]
- 49.Shim E, Kim JY, Yoon JP, et al. Author Correction: Automated rotator cuff tear classification using 3D convolutional neural network. Sci Rep 2021;11:15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JY, Ro K, You S, et al. Development of an automatic muscle atrophy measuring algorithm to calculate the ratio of supraspinatus in supraspinous fossa using deep learning. Comput Methods Programs Biomed 2019;182:105063. [DOI] [PubMed] [Google Scholar]
- 51.Batailler C, Shatrov J, Sappey-Marinier E, et al. Artificial intelligence in knee arthroplasty: current concept of the available clinical applications. Arthroplasty (London, England) 2022;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kayani B, Haddad FS. Robotic total knee arthroplasty: clinical outcomes and directions for future research. Bone Joint Res 2019;8:438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisacek-Kiosoglous AB, Powling AS, Fontalis A, et al. Artificial intelligence in orthopaedic surgery. 2023;12:447–454. doi: 10.1302/2046-3758.127.Bjr-2023-0111.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar A, Patel VR, Panaiyadiyan S, et al. Nerve-sparing robot-assisted radical prostatectomy: current perspectives. Asian J Urol 2021;8:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellos T, Manolitsis I, Katsimperis S, et al. Artificial intelligence in urologic robotic oncologic surgery: a narrative review. Cancers 2024;16:1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yildirim M, Bingol H, Cengil E, et al. Automatic classification of particles in the urine sediment test with the developed artificial intelligence-based hybrid model. Diagnostics (Basel, Switzerland) 2023;13:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moustris GP, Hiridis SC, Deliparaschos KM, et al. Evolution of autonomous and semi-autonomous robotic surgical systems: a review of the literature. Int J Med Robot + Computer Assist Surg 2011;7:375–392. [DOI] [PubMed] [Google Scholar]
- 58.Roehrborn CG, Teplitsky S, Das AK. Aquablation of the prostate: a review and update. Can J Urol 2019;26(4 Suppl 1):20–24. [PubMed] [Google Scholar]
- 59.Dai Y, Abiri A, Pensa J, et al. Biaxial sensing suture breakage warning system for robotic surgery. Biomed Microdevices 2019;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piana A, Gallioli A, Amparore D, et al. Three-dimensional augmented reality-guided robotic-assisted kidney transplantation: breaking the limit of atheromatic plaques. Eur Urol 2022;82:419–426. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Wang S, Turkbey EB, et al. Computer-aided detection of renal calculi from noncontrast CT images using TV-flow and MSER features. Med Phys 2015;42:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Wang S, Linguraru MG, et al. Computer-aided detection of exophytic renal lesions on non-contrast CT images. Med Image Anal 2015;19:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erozkan K, Gorgun E. Robotic colorectal surgery and future directions. Am J Surg 2024;230:91–98. [DOI] [PubMed] [Google Scholar]
- 64.Gómez Ruiz M, Lainez Escribano M, Cagigas Fernández C, et al. Robotic surgery for colorectal cancer. Ann Gastroenterol Surg 2020;4:646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerrieri M, Campagnacci R, Sperti P, et al. Totally robotic vs 3D laparoscopic colectomy: a single centers preliminary experience. World J Gastroenterol 2015;21:13152–13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weaver A, Steele S. Robotics in colorectal surgery. F1000Res 2016;5:2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trastulli S, Cirocchi R, Desiderio J, et al. Robotic versus laparoscopic approach in colonic resections for cancer and benign diseases: systematic review and meta-analysis. PLoS ONE 2015;10:e0134062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bae SU, Min BS, Kim NK. Robotic low ligation of the inferior mesenteric artery for rectal cancer using the firefly technique. Yonsei Med J 2015;56:1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cadiere GB, Himpens J, Vertruyen M, et al. The world’s first obesity surgery performed by a surgeon at a distance. Obes Surg 1999;9:206–209. [DOI] [PubMed] [Google Scholar]
- 70.Kersebaum JN, Möller T, von Schönfels W, et al. Robotic Roux-en-Y gastric bypass procedure guide. JSLS 2020;24:e2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bindal V, Bhatia P, Dudeja U, et al. Review of contemporary role of robotics in bariatric surgery. J Minim Access Surg 2015;11:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cirocchi R, Boselli C, Santoro A, et al. Current status of robotic bariatric surgery: a systematic review. BMC Surg 2013;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zureikat AH, Moser AJ, Boone BA, et al. 3rd. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554–559; discussion 9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wayne M, Steele J, Iskandar M, et al. Robotic pancreatic surgery is no substitute for experience and clinical judgment: an initial experience and literature review. World J Surg Oncol 2013;11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caruso S, Patriti A, Roviello F, et al. Laparoscopic and robot-assisted gastrectomy for gastric cancer: current considerations. World J Gastroenterol 2016;22:5694–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baek SJ, Lee DW, Park SS, et al. Current status of robot-assisted gastric surgery. World J Gastrointest Oncol 2011;3:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliveira CM, Nguyen HT, Ferraz AR, et al. Robotic surgery in otolaryngology and head and neck surgery: a review. Minim Invasive Surg 2012;2012:286563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takeuchi M, Kitagawa Y. Artificial intelligence and surgery. Ann Gastroenterol Surg 2024;8:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rasouli JJ, Shao J, Neifert S, et al. Artificial intelligence and robotics in spine surgery. Glob Spine J 2021;11:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choudhury A, Asan O. Role of artificial intelligence in patient safety outcomes: systematic literature review. JMIR Med Inform 2020;8:e18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rus G, Andras I, Vaida C, et al. Artificial intelligence-based hazard detection in robotic-assisted single-incision oncologic surgery. Cancers 2023;15:3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sone K, Tanimoto S, Toyohara Y, et al. Evolution of a surgical system using deep learning in minimally invasive surgery (Review). Biomed Rep 2023;19:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Liu MB, Luis C. Garcia-Peraza-Herrera, Tom Vercauteren, Prokar Dasgupta, Alejandro Granados, and Sebastien Ourselin LoViT: Long Video Transformer for Surgical Phase Recognition [Available from: https://arxiv.org/pdf/2305.08989
- 84.Shen C, Gu D, Klein R, et al. Factors associated with hospital decisions to purchase robotic surgical systems. MDM Policy Pract 2020;5:2381468320904364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng Y, Liu Y, Lai S, et al. Global trends and prospects in health economics of robotic surgery: a bibliometric analysis. Int J Surg 2023;109:3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nazer LH, Zatarah R, Waldrip S, et al. Bias in artificial intelligence algorithms and recommendations for mitigation. PLOS Digit Health 2023;2:e0000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pressman SM, Borna S, Gomez-Cabello CA, et al. AI and ethics: a systematic review of the ethical considerations of large language model use in surgery research. Healthcare (Basel, Switzerland) 2024;12:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mennella C, Maniscalco U, De Pietro G, et al. Ethical and regulatory challenges of AI technologies in healthcare: a narrative review. Heliyon 2024;10:e26297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farhud DD, Zokaei S. Ethical issues of artificial intelligence in medicine and healthcare. Iran J Public Health 2021;50:i–v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehta A, Cheng Ng J, Andrew Awuah W, et al. Embracing robotic surgery in low- and middle-income countries: potential benefits, challenges, and scope in the future. Ann Med Surg 2022;84:104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elendu C, Amaechi DC, Elendu TC, et al. Ethical implications of AI and robotics in healthcare: a review. Medicine 2023;102:e36671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jada I, Mayayise TO. The impact of artificial intelligence on organisational cyber security: an outcome of a systematic literature review. Data Inform Manag 2024;8:100063. [Google Scholar]
- 93.Gordon WJ, Ikoma N, Lyu H, et al. Protecting procedural care-cybersecurity considerations for robotic surgery. NPJ Digit Med 2022;5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fiorini P, Goldberg KY, Liu Y, et al. Concepts and trends n autonomy for robot-assisted surgery. Proc IEEE Inst Electric Electr Eng 2022;110:993–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reddy K, Gharde P, Tayade H, et al. Advancements in robotic surgery: a comprehensive overview of current utilizations and upcoming frontiers. Cureus 2023;15:e50415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rezayi S, Niakan Kalhori S R, Saeedi S. Effectiveness of artificial intelligence for personalized medicine in neoplasms: a systematic review. Biomed Res Int 2022;2022:7842566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khansari N. The Impact of Artificial Intelligence on Personalized Medicine. 2024.
- 98.Varas J, Coronel BV, Villagrán I, et al. Innovations in surgical training: exploring the role of artificial intelligence and large language models (LLM). Revista do Colegio Brasileiro de Cirurgioes 2023;50:e20233605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rogers MP, DeSantis AJ, Janjua H, et al. The future surgical training paradigm: virtual reality and machine learning in surgical education. Surgery 2021;169:1250–1252. [DOI] [PubMed] [Google Scholar]
- 100.Moglia A, Georgiou K, Georgiou E, et al. A systematic review on artificial intelligence in robot-assisted surgery. Int J Surg 2021;95:106151. [DOI] [PubMed] [Google Scholar]
- 101.de’Angelis N, Khan J, Marchegiani F, et al. Robotic surgery in emergency setting: 2021 WSES position paper. World J Emerg Surg 2022;17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Probst P. A review of the role of robotics in surgery: to DaVinci and beyond!. Mo Med 2023;120:389–396. [PMC free article] [PubMed] [Google Scholar]
- 103.Khandalavala K, Shimon T, Flores L, et al. Emerging surgical robotic technology: a progression toward microbots. Ann Laparosc Endosc Surg 2020;5:3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.


