Abstract
Bacille Calmette–Guérin (BCG) vaccine is designed to provide protection against tuberculosis (TB). However, numerous epidemiological, clinical, and immunological studies have shown that BCG vaccination affects neonatal and infant mortality, which may be related to the reduction of TB-unrelated infections and diseases by BCG vaccine. We aimed to discuss the off-target effects of BCG vaccine on un-TB infections and diseases, as well as the potential mechanism and influencing factors. Literature was retrieved mainly from PubMed using medical subject headings "BCG, variations, and non-specific, heterologous or off-target". Studies have showed that BCG vaccination can prevent various heterologous infections, including respiratory tract infections, leprosy, and malaria, treat viral infections including human papillomavirus and herpes simplex virus infection as immunotherapy, and improve the immune responses as vaccine adjuvant. Besides, BCG vaccine can reduce the recurrence rate of non-muscle-invasive bladder cancer, and may provide protection against autoimmune diseases. These off-target effects of BCG vaccine are thought to be achieved by modulating heterologous lymphocyte responses or inducing trained immunity, which were found to be sex-differentiated and affected by the BCG vaccine strains, sequence or time of vaccination.
Keywords: BCG vaccine, Off-target effects of vaccines, Infections, Non-muscle-invasive bladder cancer, Autoimmune diseases, Trained immunity
Introduction
Bacille Calmette–Guérin (BCG) vaccine, a live attenuated bacterial vaccine derived from Mycobacterium bovis, is one of the earliest vaccines developed in the last century.[1] The BCG vaccine is the only licensed vaccine for the prevention of severe tuberculosis (TB) in children, although the protection diminishes with age.
The BCG vaccine is currently one of the most widely administered vaccines in the world. In 2020, 154 nations have implemented a policy to provide BCG vaccination for the whole population. Among them, 53 countries have recorded a population coverage rate of at least 95% for the whole population.[2] Calmette[3] gathered the mortality data of newborns under 1 year of age in French hospitals from 1926 to 1928, and first reported that BCG vaccination reduced non-TB mortality in children four-fold compared with unvaccinated children. In the following decades, randomized controlled trials (RCTs) and observational studies in different countries provided accumulating evidence that BCG vaccination lessened non-TB neonatal mortality.[4] A World Health Organization (WHO) commissioned systematic review collected the most comprehensive evidence as of March 2013 on the effect of BCG vaccine on all-cause mortality, and reported a 30% reduction in all-cause mortality following neonatal BCG vaccination.[5] Subsequent RCTs established that BCG vaccination reduced neonatal mortality by 38% and 1-year-mortality by 16%, the majority of which were associated with the reduction of TB-unrelated infections and disorders.[6] A RCT of BCG vaccination designed by Prentice et al[7] in 2014 was the first to prospectively assess the effect of BCG vaccine on the morbidity of all-cause infectious diseases rather than all-cause mortality. The trial demonstrated that BCG vaccine could reduce the incidence of heterologous infectious diseases. BCG vaccine not only has a protective effect against TB infection, but also has unspecific effects on TB-unrelated infections and diseases, which is called off-target effect.[8]
Here we summarized the off-target protective effects of BCG vaccine by reviewing the evidence for the impact of BCG vaccination on TB-unrelated infections and diseases, and discussed possible mechanisms that have been proposed to explain off-target effects, involving heterologous lymphocyte responses and trained immunity. Furthermore, we summarized implications for vaccination strategies, and discussed whether BCG vaccine would confer protection against coronavirus disease 2019 (COVID-19).
Off-target Effects of BCG Vaccine
On infections
The decrease in infant mortality attributed to the BCG vaccine has prompted studies on its preventive benefits against respiratory tract infections (RTIs), which is caused by viral or bacterial infections and is one of the leading causes of death in children under 5 years old. A community case-control study by Stensballe et al[9] found that BCG-vaccinated children had a lower risk of RTI caused by respiratory syncytial virus compared with unvaccinated children [Table 1]. Among children from 33 countries, the research with the largest observed population revealed a 17–37% reduction in the risk of suspected acute RTI.[10] And the retrospective epidemiological study in Spain reported that children vaccinated with BCG vaccine had a 41.4% (95% confidence interval [CI]: 40.3–42.5%) drop in hospitalization rates for RTI compared with children not vaccinated with BCG vaccine.[11] Some evidence suggested the BCG vaccine might also be associated with a reduction in RTI among the elderly. One RCT in Indonesia has shown that monthly BCG vaccination for 3 months in older adults substantially reduced the prevalence of RTI compared with placebo.[12] Another RCT in Greece discovered that elderly people received a one-dose BCG vaccine had a 79% (95% CI: 28–94%) lower risk of respiratory infections compared to placebo.[13]
Table 1.
Lists of off-target effects of BCG vaccine on infections and as immunotherapy for viral infections.
| Infections | References | Study types | Sample sizes | Age (years) | Locations | BCG dosage | Control dosage | Treatment duration | Findings |
|---|---|---|---|---|---|---|---|---|---|
| RTI | Stensballe et al[9] | Case-control study | 772 | <5 | Guinea-Bissau | – | BCG-unvaccinated | – | Reduced the risk of infection |
| Hollm-Delgadoet al[10] | Cohort study | 151,322 | <5 | 33 countries | – | BCG-unvaccinated | – | A 17–37% risk reduction in suspected acute RTI | |
| de Castro et al[11] | Retrospective study | 446,915* | <15 | Spain | – | BCG-unvaccinated | – | Reduced hospitalization rates | |
| Wardhana et al[12] | RCT | 34 | 60–75 | Indonesia | 0.1 mL | The solvent of the BCG/0.1 mL | 1 dose/month × 3 | Prevalence of RTI: 8.7% in BCG; 91.3% in control |
|
| Giamarellos-Bourboulis et al[13] | RCT | 198 | ≥65 | Greece | 0.1 mL | Saline/0.1 mL | 1 dose | Reduced the risk of infection | |
| Malaria | Berendsen et al[16] | Cross-sectional study | 34,206 | <5 | Sub-Saharan Africa | – | BCG-unvaccinated | – | Reduced the risk of infection |
| HPV | Metawea et al[17] | Clinical trial | 50 | 30–39 | Egypt | 81.0 mg | Saline/8.0 mL | 1 dose/week × 6 |
Complete clearance: 80% in BCG; 0 in control |
| Podder et al[18] | RCT | 60 | 16–58 | India | 0.1 mL | Tuberculin PPD/0.1 mL | 1 dose/4-week × 3 | Complete clearance: 48.5% in BCG; 18.5% in PPD |
|
| Sharquie et al[19] | Clinical trial | 154 | 6–45 | Iraq | 0.1 mL | Distilled water/0.1 mL | 1–3 doses/month × 3 | Complete clearance: 37.0% in BCG; 13.7% in control |
|
| Salem et al[20] | Clinical trial | 80 | 3–14 | Egypt | 1.0 mL | Saline/– | 1 dose/week × 6 | Complete clearance: 65% in BCG; 0 in control |
|
| HSV | Hippmann et al[22] | Case series | 109 | 7–58 | America | 0.1 mL | Prior-BCG vaccination | 1 dose | Duration of recurrence-free period: 109 (100%) for ≥4–6 months 21 (19%) for ≥3 years 10 (9%) ≥6 years |
BCG: Bacille Calmette-Guérin; HPV: Human papillomavirus; HSV: Herpes simplex virus; PPD: Purified protein derivative; RCT: Randomized controlled trial; RTI: Respiratory tract infection; –: Not available. *The number represented the number of hospitalization episodes.
Clinical evidence suggested that BCG vaccine could also provide protection against other infections, such as leprosy caused by Mycobacterium leprae, and malaria caused by Plasmodium group. The overall protective effect of BCG vaccination against leprosy was 26% (95% CI: 14–37%), according to a meta-analysis of 7 experimental investigations conducted between the 1960s and 2001.[14] Until a more specific vaccine is available, the BCG vaccine is the best line of defense against contracting leprosy, which is why the WHO recommends it for all newborns in high-risk regions.[15] A cross-sectional study in South Africa recruited 34,206 children under 5 years of age to investigate the relationship between BCG vaccination and malaria incidence, which found that the prevalence of malaria was reduced by 6% in the BCG-vaccinated group.[16]
On viruses as immunotherapy
Due to the off-target effects against infections associated with the BCG vaccine, researchers tried to apply the BCG vaccination as non-specific immunotherapy for the treatment of viral infections. The most wildly known example is the aggressive treatment of cutaneous and genital warts caused by human papillomavirus (HPV). A placebo-controlled study with 50 participants revealed that 80% of patients had complete resolution of the lesions after a basic course of treatment (one dose weekly for 6 consecutive weeks), and 12% had complete remission after an intensive course of treatment (three doses weekly for 3 consecutive weeks), while no response was observed in the group receiving topical saline as a control.[17] Clinical trials in various countries have shown that after the use of BCG vaccine, the complete clearance of viral warts associated with HPV was between 37.0% (30/81) and 65.0% (13/20) [Table 1].[18–20] However, the studies have found that the repeated BCG vaccination could increase the incidence of side effects such as pain on the injection side and scarring or abscess formation. In order to address the issue, researchers have found BCG polysaccharide nucleic acid (BCG-PSN), a novel bacterial lipopolysaccharide component extracted from BCG vaccine, as a potential immunotherapy.[21] The patients (n = 120) with warts were randomly assigned to receive either BCG-PSN, BCG vaccination, or saline up to 5 times at 3-week intervals. Comparing the BCG vaccination group with the BCG-PSN group, the BCG-PSN group had a higher percentage of complete remission of warts (77.5% [31/40] vs. 63.9% [23/36]) and a lower rate of adverse effects. Immunotherapy with BCG-PSN was more efficient than the BCG vaccination at removing warts with less serious adverse events.
BCG vaccine was also found to be able to reduce the frequency and duration of relapses of herpes simplex virus (HSV) [Table 1]. With a single dose of BCG, all patients with recurrent herpes simplex remained relapse-free for 4–6 months, 19.3% (21/109) for 3 years, and 9.2% (10/109) for more than 6 years.[22] A systematic review concluded that BCG vaccination resulted in 37% of adults with genital herpes or cold sores not experiencing recurrence and 41% of patients with low frequency or severity recurrences.[23]
On other vaccines as adjuvant
BCG vaccine, in conjunction with other anti-infective vaccinations or previous immunization, may enhance the immune response [Table 2]. BCG vaccine and hepatitis B vaccine, as two vaccines in the national immunization program, must be administered simultaneously to newborns.[24] Compared with hepatitis B vaccine alone, the coadministration of BCG vaccine and hepatitis B vaccine at birth substantially boosted the induction of cytokines (interferon-gamma [IFN-γ], interleukin-5 [IL-5], and interleukin-13 [IL-13]), and increased the antibody response to hepatitis B vaccine.[25] In addition, as shown in Table 2, BCG vaccination at birth has been shown in clinical trials to enhance antibody responses to other vaccines inoculated in accordance with national immunization schedules, including diphtheria, tetanus, pertussis, polio, haemophilus influenzae type b (Hib), and 13-valent pneumococcal conjugate vaccines.[25–27] A randomized, placebo-controlled study by Leentjens et al[28] with 40 healthy participants revealed that BCG vaccination prior to influenza vaccination might lead to a more significant increase and accelerated induction of functional antibody responses against the 2009 pandemic influenza A vaccine strain.
Table 2.
Lists of off-target effects of BCG vaccine on other vaccines.
| References | Study types | Sample sizes | Vaccines | Findings |
|---|---|---|---|---|
| Ota et al[25] | RCT | 104 | At birth, 2 4 months of age: Hepatitis B vaccine; At birth, 1, 2, 3 and 4 months of age: OPV; At 2, 3 and 4 months of age: DPTa |
Enhanced antibody responses to OPV |
| Zimmermann et al[26] | RCT | 471 | At birth or 2, 4, and 5 months of age: BCG; At 6 weeks, 4 and 6 months of age: the combined DTPa–hepatitis B vaccine–IPV–Hib vaccine, PCV13 vaccine, and oral rotavirus vaccine; At 12 months of age: MMR vaccine, the combined meningococcal C and Hib vaccine |
Enhanced antibody responses to diphtheria, tetanus, pertussis, polio, Hib, and 13-valent pneumococcal conjugate vaccines |
| Ritz et al[27] | Prospective non-randomized trial | 108 | At birth: Hepatitis B vaccine; At 2, 4 and 6 months of age: the 7-valent pneumococcal conjugate vaccine, the combined DTPa–hepatitis B vaccine–IPV–Hib vaccine, and oral pentavalent rotavirus vaccine |
Enhanced antibody responses to tetanus vaccine and Hib vaccine |
| Leentjenset al[28] | RCT | 40 | Intramuscular influenza vaccine | Enhanced antibody responses to influenza A |
BCG: Bacille Calmette-Guérin; DPT: Diphtheria-pertussis-tetanus vaccine; DTPa: Diphtheria-tetanus-acellular pertussis vaccine; Hib: Hemophilus influenzae type b; IPV: Inactivated polio vaccine; MMR: Measles-mumps-rubella; OPV: Oral polio vaccine; PCV13: 13-valent conjugate pneumococcal; RCT: Randomized controlled trial.
On non-muscle-invasive bladder cancer (NMIBC)
BCG vaccine for cancer immunotherapy has been shown to be effective against various malignant tumors (including melanoma, prostate cancer, lung cancer, etc.), while the most widely recognized cancer is NMIBC.[1] A meta-analysis reviewing 24 randomized clinical trials concluded that based on the follow-up with a median of 2.5 years and a maximum of 15 years, 9.8% (260/2658) of patients receiving BCG vaccine had tumor progression compared to 13.8% (304/2205) in the control groups (resection or resection with treatment other than BCG vaccine), which showed a 27% lower probability of progression on BCG maintenance therapy (odds ratio [OR] = 0.73, P = 0.001).[29]
Compared with other chemotherapeutic infusion drugs via RCTs, including mitomycin C (MMC) or epirubicin alone, and the combination of epirubicin and alpha-interferon-2a, BCG vaccine had the better ability to reduce the risk of NMIBC's recurrence, distant progression, and mortality.[30–32] The recurrence rate of NMIBC was decreased from 33.9% to 20.6% when MMC was employed in conjunction with BCG immunization, and the duration of disease-free periods was extended.[33] The combinations of interferon plus BCG vaccine, and alpha-interferon-2a plus epirubicin were performed, but these combinations were non-superior to BCG vaccine-alone immunotherapy in preventing relapse and progression.[34,35] However, the combination therapy was only utilized for NMIBC with higher likelihood of recurrence because of its higher incidence of serious adverse events than BCG vaccine alone.[33]
BCG vaccine is currently the most effective intravesical treatment for NMIBC, approved by the U.S. Food and Drug Administration as the first line therapy, not only reducing recurrence, but also preventing progression and reducing death.[36] According to European Association of Urology Guidelines, the recommended treatment regimen is that patients with intermediate-risk NMIBC are administered a 1-year standard-dose BCG vaccine (81 mg) with a weekly induction cycle at weeks 1–6, plus 3 weekly maintenance therapies at 3 months, 6 months, and 12 months.[37] And patients with high-risk NMIBC are administered a 3-year standard-dose BCG vaccine with induction plus 3 weekly maintenance therapies at 3 months, 6 months, 12 months, 18 months, 24 months, 30 months, and 36 months.
On autoimmune diseases
Multiple studies have shown the potential off-target effects of BCG immunization on autoimmune diseases, such as asthma, type 1 diabetes mellitus (T1DM), and multiple sclerosis (MS). BCG vaccination can provoke a T helper 1 (Th1) immune response, while Th1 and T helper 2 (Th2) cells can regulate one another. Anti-inflammatory effects of neonatal BCG vaccination on asthma (Th2-related allergic disease) have been observed.[38] Lung function was significantly improved in asthma patients after BCG inoculation (significantly increased forced expiratory volume, forced expiratory flow, and morning peak expiratory flow), despite no significant change in scores of asthma symptom, but decreased use of asthma medications.[39]
T1DM is an autoimmune disease associated with pancreatic beta cells being attacked and destroyed by the body's immune system. Results from an 8-year follow-up study of participants with chronic T1DM who received 2 doses of the BCG vaccine showed that their glycosylated hemoglobin levels remained close to normal.[40] However, the noticeable impact did not appear until 3 years after administration. The researchers hypothesized that as autoimmune disorders took so long to develop, the time it took to stop or reverse autoimmunity could be comparable.
The BCG vaccination has also been linked to a lower chance of developing MS, an autoimmune illness characterized by demyelination of the central nervous system and driven by an inflammatory immunological response. After the first demyelinating event in the central nervous system, 83 individuals were randomly assigned to receive either the BCG vaccine or placebo in a double-blind, placebo-controlled experiment.[41] The percentage of patients who did not develop MS was considerably lower in the BCG vaccine group than in the placebo group at the conclusion of the experiment (57.6% [19/33] vs. 30.0% [12/40], P = 0.018). Demyelinating episode patients who got the BCG vaccine had a 52% reduced risk of developing MS compared to placebo. BCG vaccination was proved to have early positive benefits in individuals with MS and might have long-term effects, since it decreased the total number of lesions at the first 6 months and disease activity at magnetic resonance imaging throughout the 5 years of follow-up.
Mechanisms Explaining the Off-target Effects of BCG Vaccine
There are two possible mechanisms have been proposed to explain the off-target effects of BCG vaccine on untargeted infections, cancer-related immune response and autoimmune diseases.
Heterologous lymphocyte responses
The first mechanism involves the effects of the BCG vaccine on different diseases via the induction of heterologous lymphocyte responses. Heterologous lymphocyte responses induced by BCG vaccine enhanced immune responses to secondary unrelated pathogen infections.[42] Heterologous lymphocyte responses might involve the activation of cluster of differentiation 4 (CD4)+ and CD8+ memory cells, which modulated Th1 and T helper 17 (Th17) responses with secondary infection to unrelated pathogens. Heterologous production of Th1 and Th17 immune responses against unrelated pathogens was strongly elevated in BCG-vaccinated populations.[43] Heterologous lymphocyte responses might be associated with cytokine production. After being stimulated by allogeneic sources, the BCG-vaccinated population produced considerably more Th1-polarizing cytokine (IFN-γ), as well as more pro-inflammatory response and pro-inflammatory cytokines derived by monocytes (interleukin-1 beta [IL-1β], interleukin-6 [IL-6], and tumor necrosis factor-alpha [TNF-α]), which accelerated the development of the neonatal immune system mediating comprehensive protection against infection and mortality.[44]
The treatment effect of BCG vaccine in NMBIC is thought to be related to the activation of natural killer (NK) cells. Scholars have shown that BCG vaccine can activate the cytotoxic CD56+ NK cell population to recognize cancer cells, which is dependent on the receptor natural killer group 2 member D (NKG2D) or cytokines (interleukin-12 [IL-12], interferon-alpha [IFN-α], interleukin-2 [IL-2], etc.).[45–48] After intravesical BCG instillation, NK cells exhibited marked cytotoxicity to effectively eliminate bladder tumor cells, and the therapeutic benefit of BCG was abolished as NK cells were depleted.[49]
BCG vaccine stimulated the capacity of regulatory T cells (Tregs) to generate anti-inflammatory effects against autoimmune diseases.[50] The off-target effects of BCG vaccine in asthma may be attributed to transforming growth factor-1 beta (TGF-β1), which could inhibit Th1 and Th2 cell responses and promote the differentiation of Treg cells to exert anti-inflammatory effects, and induce the differentiation of Th17 cells to result in the rapid accumulation of various inflammatory cells at the site of inflammation. Asthma is characterized by airway inflammation and airway remodeling, and TGF-β1 with immunomodulatory and fibrogenic activities is one of the key mediators involved in persistent inflammation and tissue remodeling in asthma.[51] Tian et al[52] demonstrated that BCG vaccination might attenuate airway inflammation and airway remodeling by lowering TGF-β1 levels in an experimental mouse model of asthma, therefore, BCG vaccine could be a novel treatment for asthma.
Inducing "trained immunity"
Another plausible mechanism is trained immunity [Figure 1]. Trained immunity, also known as innate memory immunity, protects against unrelated diseases in a T/B cell-independent but monocytes/macrophages manner by inducing adaptive features via long-term reprograming of innate immune cell genetics and metabolism.[53] In severe combined immunodeficiency mice are deficient in both T cells and B cells, lethal inoculum of C. albicans (strain UC820) was injected two weeks after random injection of BCG vaccine or saline, and survival was significantly increased in the BCG vaccine group compared to the saline group, suggesting that T cells and B cells may be not associated with the off-target effects induced by BCG vaccine.[54] Trained immunity was considered to be performed by inducing hematopoietic stem cells and progenitor cells (HSPC) reprograming. BCG vaccine reprogramed HSPC in the bone marrow, resulting in enhanced myelopoiesis, and sustainable induction of epigenetically modified monocytes/macrophages.[55–57] Trained immunity induced by BCG vaccine led to a more open chromatin structure in macrophages and monocytes, which increased the accessibility of transcription factors to DNA to facilitate gene transcription. And BCG vaccination promoted the activation of transcriptionally active histone modifications, such as trimethylation of lysine 4 at histone 3 (H3K4m3) and acetylation of lysine 27 at histone 3 (H3K27ac), and decreased transcriptionally inhibitory histone modifications, such as trimethylation of lysine 9 at histone 3 (H3K9m3). The changes in chromatin structure and histone modifications induced by BCG vaccination ultimately led to the activation of genes encoding pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, which played crucial roles in the immune response, inflammation, and host defense against infections.
Figure 1.
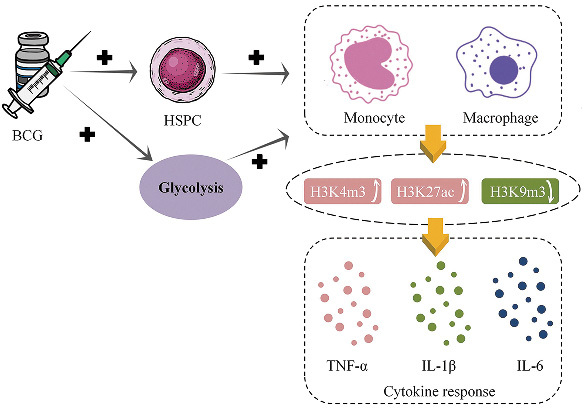
Trained immunity induced by BCG vaccine. BCG: Bacille Calmette–Guérin; H3K27ac: Acetylation of lysine 27 at histone 3; H3K4m3: Trimethylation of lysine 4 at histone 3; H3K9m3: Trimethylation of lysine 9 at histone 3; HSPC: Hematopoietic stem cells and progenitor cells; IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-alpha.
The researchers created a human infection model using live attenuated yellow fever vaccine (YFV) to construct and validate the effect of BCG vaccine on infection with unrelated pathogens in a randomized placebo-controlled human challenge trial.[58] They found H3K27ac increased after vaccination, suggesting that BCG vaccination induced genome-wide epigenetic reprograming of human monocytes in vivo. These epigenetic changes were indeed accompanied by enhanced pro-inflammatory cytokine production (IL-1β, TNF-α, and IL-6) against unrelated pathogens. Compared to those who received the placebo, volunteers who received the BCG vaccine had a lower viral load and lower proportion of yellow fever viremia. The researchers also found a multiplied increase in IL-1β production after BCG vaccination was strongly associated with lower proportion of viremia after YFV administration. In addition, significant differences in epigenetic markers in the monocytes of BCG responders (low yellow fever viremia) and BCG non-responders (high yellow fever viremia) have been shown. Therefore, the increase in monocyte cytokine production induced by BCG vaccine may contribute to improved clinical outcomes after subsequent virus infections.
Trained immunity also has been proposed to mediate the anti-tumor effect of BCG vaccine.[59] Mononuclear cells isolated from patients with bladder cancer were stimulated ex vivo with lipopolysaccharide (LPS) before and after BCG vaccine treatment. And the cytokine responses (IL-1β, TNF-α, and IL-6) were considerably elevated post-treatment compared to pre-treatment, demonstrating that monocytes have been induced by trained immunity.[60] The study also found that BCG-induced trained immunity was favorably influenced by autophagy. The autophagy-related 2B (ATG2B, rs3759601) affected the cytokine response induced by BCG vaccine upon restimulation with unrelated bacterial or fungal stimuli, and significantly increased H3K4m3 to enhance epigenetic reprograming of monocytes. Additionally, the correlation was observed in ATG2B with the progression and recurrence of bladder cancer after BCG intravesical instillation therapy. These results implied that trained immunity, induced by BCG vaccine, via the autophagy pathway might influence the therapeutic impact of BCG in NMIBC patients. Another study used the ratios of cytokine levels (TNF-α, IL-1β, IL-6, IFN-γ, IL-12, and interleukin-10 [IL-10]) released by monocytes before and after BCG vaccine treatment to represent the strength of trained immunity.[61] The IL-12 ratio was significantly higher (five times) in relapse-free patients after BCG vaccine treatments compared with relapse patients. Patients without trained immunity (measured by the IL-12 ratio) had a significantly shorter time to relapse than those with trained immunity (P <0.001). These findings demonstrated the potential link between BCG-induced trained immunity and anti-tumor responses.
Systemic switching from oxidative phosphorylation to aerobic glycolysis may underlie the protective effect of BCG vaccine against autoimmune disorders and nervous system diseases.[62,63] Increases in key early glycolytic enzymes were observed, as were increases in glucose uptake and systemic blood sugar reduction, acceleration of glucose utilization via shunts to the pentose phosphate pathway, decreased utilization of the late glycolytic steps including the Krebs cycle, increased lactate production, and decreased oxidative phosphorylation.[40] And methylation sites in Treg signature genes implicated in the glucose utilization and reestablished tolerance which were driven by systemic metabolic alterations in glycolysis.[40,64] Furthermore, a study found that BCG-induced trained immunity (effects on H3K4m3 and H3K9m3) in monocytes was accompanied by a strong increase in glycolysis.[56] These findings provide evidence to the claim that BCG vaccine may stimulate glycolysis in immunized monocytes trained by BCG vaccine.
Increases in IL-1β were detected in both heterologous lymphocyte responses and trained immunizations, suggesting that this substance was fundamental to antiviral infection.[54,58,65] After being challenged by the virus, mice deficient in the IL-1β receptor or the inflammasome, which consists of components nucleotide-binding and oligomerization domain-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase-1, were more vulnerable to viral infection and had a poor survival rate.[66,67] Further, IL-1β was observed to induce trained immunity in vitro, and this effect was accompanied by epigenetic changes in the levels of histone methylation (H3K4m3 increasing and H3K9me3 decreasing).[58]
Factors Influencing Off-target Effect of BCG Vaccine
BCG vaccine strains
The most prevalent BCG vaccine strains are the Bulgarian, Danish, Russian, and Japanese strains. Compared with BCG vaccine-Bulgarian and BCG vaccine-Russian strains, infants that received BCG vaccine-Danish strain had a higher frequency of CD4+ T cell responses (peaking at day 42 post-immunization) and had persistent, multifunctional CD4+ T cells that were in the memory stage of early differentiation.[68] Moreover, BCG vaccine-Danish strain immunization elicited a higher proportion of multifunctional cytokine response, including IFN-γ, TNF-α, and IL-2, to heterologous vaccine antigens (tetanus and pertussis) compared with BCG vaccine-Bulgarian and BCG vaccine-Russian strains. The study found CD4+ T cell responses to BCG vaccine antigens and other heterologous antigens varied in strength and adaptability depending on the immunity strains of BCG vaccine.
Inoculation time
The timing of vaccination is one of the important factors that may influence the off-target effects of BCG vaccine. An article collected studies on delayed BCG vaccination of low-birth-weight infants before 2014 and found that all-cause mortality after BCG vaccination at birth was reduced compared with newborns who delayed BCG vaccination.[69] And results of a prospective study in Uganda showed that comparing infants immunized at birth to those immunized at 6 weeks of age, the former group had a 29% lower rate of unspecific infections (hazard ratio [HR] = 0.71, P = 0.023).[7] Newborns given BCG vaccine had a greater capacity to proliferate CD4+ and CD8+ T cells to express more IFN-γ alone or in combination with perforin, and more of other cytokines (IL-2 and TNF-α).[70]
Gender disparity
A pool of three clinical trials found a significantly lower mortality rate ratio (MRR [BCG vaccination/no BCG vaccination]) in boys compared with girls in the first week following BCG vaccination (0.36 vs. 0.85). However, girls had significantly lower MRR compared to boys (0.56 vs. 0.91) at weeks 2–4.[71] The finding that BCG immunization offered defense from all-cause mortality as soon as one week after vaccination in boys and 2–4 weeks after vaccination in girls was groundbreaking. It was associated with a stronger proinflammatory cytokine response in girls than in boys at 4 weeks after BCG vaccination. The combination of hepatitis B vaccine and BCG vaccination at birth, however, showed sex-differential impact, as shown in the first RCT on humans to compare the off-target effects of BCG vaccine.[72] In response to the stimulation by unrelated pathogens, males generated more TNF-α, IFN-γ, and less chemokine ligand 2 (CCL2) than females. And BCG vaccination was associated with a decrease in systemic inflammation, with the impact being greater in males than in females.[73] In conclusion, there are discernible disparities between the sexes in the off-target effects of BCG vaccine.
Inoculation sequence
The order of vaccination may affect the off-target effects of the BCG vaccine. In reaction to unrelated stimuli, IL-10 was reduced and interleukin-22 (IL-22) was increased after vaccination with the Vi polysaccharide typhoid vaccine and then the BCG vaccine.[74] In addition, concurrent or subsequent vaccination with BCG vaccine counteracted the immunosuppressive effects caused by tetanus-diphtheria-pertussis inactivated polio vaccine inoculation alone. These effects are short-term potentiation and long-term suppression of monocyte-derived cytokine responses, as well as short- and long-term suppression of T cell responsiveness to unrelated pathogens. Epidemiological studies corroborated this finding, demonstrating a significantly decreased risk of acute lower RTI in BCG vaccine after or simultaneous with diphtheria-pertussis-tetanus (DTP) vaccine, as opposed to DTP after BCG vaccine.[10] Furthermore, a study found that adjusting for background factors and taking the child mortality rate and the combined BCG and first-dose DTP vaccination group as the reference, the BCG pre-vaccination group was associated with a 2-fold higher mortality rate (MRR: 1.94, 95% CI: 1.42–2.63), and children receiving BCG vaccination after first-dose DTP had 1.25-fold higher mortality (MRR: 1.25, 95% CI: 0.91–1.73).[75]
Discussion
According to decades of research on the off-target effects of the BCG vaccine, the BCG immunization could enhance non-specific immune responses against diseases including infections, NMIBC, and autoimmune diseases. Notably, there are studies providing counter-evidence for the off-target effects of the BCG vaccine on infection rates and the development of autoimmune diseases. In two Danish investigations, hospitalization rates for infections were not reduced by newborn BCG immunization.[76,77] Another three studies concluded that BCG vaccination had no effect on the onset or progression of autoimmune disease.[78–80] Therefore, well-designed prospective studies or large-scale clinical RCTs are required to identify the off-target effects of BCG vaccine.
The immunological mechanisms may explain the off-target effects of BCG vaccination, which are trained immunity and heterologous lymphocyte responses induced by BCG vaccine.[43] Trained immunity is mediated by HSPCs to induce epigenetic and metabolic reprograming of innate immune cells like monocytes, resulting in increased production of cytokines, such as IL-1β. The heterologous lymphocyte response elicited by the BCG vaccine modulates responses to TB-unrelated infections and diseases by stimulating CD4+ and CD8+ T cell and NK cell responses. However, the precise biological mechanism of BCG vaccine remains uncertain. It is necessary to further confirm the immune mechanism of the off-target effects of BCG vaccine in order to apply activation to the prevention and treatment of non-targeted infections and diseases.
Implications for vaccination methods arise from the fact that the off-target effects of BCG vaccine may be influenced by BCG vaccine strains, inoculation time, gender, and inoculation sequence. BCG vaccine may act as an immunological adjuvant to enhance immune responses when used in conjunction with other vaccinations, and the sequence in which vaccines are administered can have a significant impact on their efficacy. Studies have found that BCG pre-vaccination or combined vaccination (compared with BCG post-vaccination), or BCG vaccination at birth (compared with delayed BCG vaccination) has lower unspecific infection rate or mortality.[7,10,69,75] Therefore, the sequence and time of vaccinations should be considered when planning immunization schedules or introducing new vaccines into existing schedules. And BCG vaccine should be given priority after delivery in regions with high burden of infectious diseases because of the additional protection that BCG vaccine provides to newborns.[7] Concerns about the impact of BCG vaccine strains on off-target effects also remain. Most of the studies reporting off-target effects of BCG vaccine have used the BCG vaccine-Danish strain phased out in 2015. Off-target effects of BCG vaccine-Japanese strain were shown to be greater than those of BCG vaccine-Russian or BCG vaccine-Danish strains in a study.[81] Therefore, equivalence studies evaluating the off-target effects of different BCG vaccine strains are worthwhile.
Researchers have attempted to exploit the off-target effects of BCG vaccine to design a non-specific immunotherapy or vaccines regimen to treat or prevent COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). RCTs provided evidence for potential protection against COVID-19 provided by BCG vaccination [Supplementary Table 1, http://links.lww.com/CM9/B769].[82–85] However, two other RCTs have found that past BCG vaccination was not associated with increased COVID-19 incidence or symptom severity, nor was it protective against COVID-19, either in childhood or in adulthood [Supplementary Table 1, http://links.lww.com/CM9/B769].[86,87] The immune response of the immune regimen to the two-dose Pfizer-BioNTech vaccine (Pfizer, New York, USA; BioNTech, Mainz, Germany) after 30 days of BCG immunization was stronger compared to the immunization schedule for the only two-dose Pfizer-BioNTech vaccine [Supplementary Table 2, http://links.lww.com/CM9/B769].[88] Exploiting the immunostimulatory properties of BCG vaccine, researchers developed a novel COVID-19 vaccine named BCG:CoVac[89] (University of Sydney and the Centenary Institute, Sydney, Australia) and designed a therapeutic biological product mix named AD26-BCG[90] (Medicine Invention Design Incorporation, Maryland, USA).
In conclusion, our review suggests that the off-target effects of BCG vaccine have beneficial effects on the health of human, and so careful consideration should be given before discontinuing the BCG vaccine. And further studies are needed to confirm the off-target effects of BCG vaccine on TB-unrelated infections and diseases, and the immune mechanisms underlying them, shedding light on future applications of vaccination strategies.
Supplementary Material
Footnotes
How to cite this article: Wu YF, Zhang XY, Zhou L, Lu JY, Zhu FC, Li JX. Research progress in the off-target effects of Bacille Calmette–Guérin vaccine. Chin Med J 2024;137:2065–2074. doi: 10.1097/CM9.0000000000002890
References
- 1.Singh AK, Netea MG, Bishai WR. BCG turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases. J Clin Invest 2021;131: e148291. doi: 10.1172/jci148291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global tuberculosis report 2021. Geneva: Baddeley A Bartens M-C Boon Sd Dias HM Falzon D Floyd K, et al. 2021. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021. [Last accessed on December 1, 2021] [Google Scholar]
- 3.Calmette A. Preventive Vaccination Against Tuberculosis with BCG. Proc R Soc Med 1931;24: 1481–1490. [PMC free article] [PubMed] [Google Scholar]
- 4.Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther 2013;35: 109–114. doi: 10.1016/j.clinthera.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Higgins JP Soares-Weiser K López-López JA Kakourou A Chaplin K Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. Bmj 2016;355: i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biering-Sørensen S Aaby P Lund N Monteiro I Jensen KJ Eriksen HB, et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin Infect Dis 2017;65: 1183–1190. doi: 10.1093/cid/cix525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prentice S Nassanga B Webb EL Akello F Kiwudhu F Akurut H, et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect Dis 2021;21: 993–1003. doi: 10.1016/s1473-3099(20)30653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritschi N, Curtis N, Ritz N. Bacille Calmette Guérin (BCG) and new TB vaccines: Specific, cross-mycobacterial and off-target effects. Paediatr Respir Rev 2020;36: 57–64. doi: 10.1016/j.prrv.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stensballe LG Nante E Jensen IP Kofoed PE Poulsen A Jensen H, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine 2005;23: 1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Hollm-Delgado MG, Stuart EA, Black RE. Acute lower respiratory infection among Bacille Calmette-Guérin (BCG)-vaccinated children. Pediatrics 2014;133: e73–81. doi: 10.1542/peds.2013-2218. [DOI] [PubMed] [Google Scholar]
- 11.de Castro MJ, Pardo-Seco J, Martinón-Torres F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin Infect Dis 2015;60: 1611–1619. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 12.Wardhana, Datau EA, Sultana A, Mandang VV, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones 2011;43: 185–190 [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ Tsilika M Moorlag S Antonakos N Kotsaki A Domínguez-Andrés J, et al. Activate: Randomized clinical trial of BCG vaccination against infection in the elderly. Cell 2020;183: 315–323.e319. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setia MS, Steinmaus C, Ho CS, Rutherford GW. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect Dis 2006;6: 162–170. doi: 10.1016/s1473-3099(06)70412-1. [DOI] [PubMed] [Google Scholar]
- 15.World Health O. BCG vaccine: WHO position paper, February 2018-Recommendations. Vaccine 2018;36: 3408–3410. doi: 10.1016/j.vaccine.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Berendsen ML van Gijzel SW Smits J de Mast Q Aaby P Benn CS, et al. BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in sub-Saharan Africa. BMJ Glob Health 2019;4: e001862. doi: 10.1136/bmjgh-2019-001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metawea B, El-Nashar AR, Kamel I, Kassem W, Shamloul R. Application of viable bacille Calmette-Guérin topically as a potential therapeutic modality in condylomata acuminata: a placebo-controlled study. Urology 2005;65: 247–250. doi: 10.1016/j.urology.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Podder I Bhattacharya S Mishra V Sarkar TK Chandra S Sil A, et al. Immunotherapy in viral warts with intradermal Bacillus Calmette-Guerin vaccine versus intradermal tuberculin purified protein derivative: A double-blind, randomized controlled trial comparing effectiveness and safety in a tertiary care center in Eastern India. Indian J Dermatol Venereol Leprol 2017;83: 411. doi: 10.4103/0378-6323.193623. [DOI] [PubMed] [Google Scholar]
- 19.Sharquie KE, Al-Rawi JR, Al-Nuaimy AA, Radhy SH. Bacille Calmette-Guerin immunotherapy of viral warts. Saudi Med J 2008;29: 589–593. [PubMed] [Google Scholar]
- 20.Salem A, Nofal A, Hosny D. Treatment of common and plane warts in children with topical viable Bacillus Calmette-Guerin. Pediatr Dermatol 2013;30: 60–63. doi: 10.1111/j.1525-1470.2012.01848.x. [DOI] [PubMed] [Google Scholar]
- 21.Ebrahim HM, Asaad AM, El Desoky F, Morsi HM. Bacillus Calmette-Guerin polysaccharide nucleic acid vs Bacillus Calmette-Guerin vaccine in the treatment of warts: A comparative, double-blind, controlled study. Dermatol Ther 2021;34: e14549. doi: 10.1111/dth.14549. [DOI] [PubMed] [Google Scholar]
- 22.Hippmann G, Wekkeli M, Rosenkranz AR, Jarisch R, Götz M. [Nonspecific immune stimulation with BCG in Herpes simplex recidivans. Follow-up 5 to 10 years after BCG vaccination]. Wien Klin Wochenschr 1992;104: 200–204. [PubMed] [Google Scholar]
- 23.Pittet LF, Curtis N. Does bacillus Calmette-Guérin vaccine prevent herpes simplex virus recurrences? A systematic review. Rev Med Virol 2021;31: 1–9. doi: 10.1002/rmv.2151. [DOI] [PubMed] [Google Scholar]
- 24.Table 1: WHO recommendations for routine immunization. Geneva: Organization WH, 2020. Available from: https://www.who.int/publications/m/item/table-1-who-recommendations-for-routine-immunization. [Last accessed on June 20, 2022]. [Google Scholar]
- 25.Ota MO Vekemans J Schlegel-Haueter SE Fielding K Sanneh M Kidd M, et al. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J Immunol 2002;168: 919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann P Donath S Perrett KP Messina NL Ritz N Netea MG, et al. The influence of neonatal Bacille Calmette-Guérin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine 2019;37: 3735–3744. doi: 10.1016/j.vaccine.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Ritz N, Mui M, Balloch A, Curtis N. Non-specific effect of Bacille Calmette-Guérin vaccine on the immune response to routine immunisations. Vaccine 2013;31: 3098–3103. doi: 10.1016/j.vaccine.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 28.Leentjens J Kox M Stokman R Gerretsen J Diavatopoulos DA van Crevel R, et al. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J Infect Dis 2015;212: 1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 29.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002;168: 1964–1970. doi: 10.1097/01.ju.0000034450.80198.1c. [DOI] [PubMed] [Google Scholar]
- 30.Sylvester RJ Brausi MA Kirkels WJ Hoeltl W Calais Da Silva F Powell PH, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin, and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010;57: 766–773. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Järvinen R, Kaasinen E, Sankila A, Rintala E. Long-term efficacy of maintenance bacillus Calmette-Guérin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up. Eur Urol 2009;56: 260–265. doi: 10.1016/j.eururo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Duchek M Johansson R Jahnson S Mestad O Hellström P Hellsten S, et al. Bacillus Calmette-Guérin is superior to a combination of epirubicin and interferon-alpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol 2010;57: 25–31. doi: 10.1016/j.eururo.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Solsona E Madero R Chantada V Fernandez JM Zabala JA Portillo JA, et al. Sequential combination of mitomycin C plus bacillus Calmette-Guérin (BCG) is more effective but more toxic than BCG alone in patients with non-muscle-invasive bladder cancer in intermediate- and high-risk patients: final outcome of CUETO 93009, a randomized prospective trial. Eur Urol 2015;67: 508–516. doi: 10.1016/j.eururo.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Marttila T Järvinen R Liukkonen T Rintala E Boström P Seppänen M, et al. Intravesical Bacillus Calmette-Guérin Versus Combination of Epirubicin and Interferon-α2a in Reducing Recurrence of Non-Muscle-invasive Bladder Carcinoma: FinnBladder-6 Study. Eur Urol 2016;70: 341–347. doi: 10.1016/j.eururo.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd AR, Shepherd E, Brook NR. Intravesical Bacillus Calmette-Guérin with interferon-alpha versus intravesical Bacillus Calmette-Guérin for treating non-muscle-invasive bladder cancer. Cochrane Database Syst Rev 2017;3: Cd012112. doi: 10.1002/14651858.CD012112.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamat AM Bellmunt J Galsky MD Konety BR Lamm DL Langham D, et al. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma. J Immunother Cancer 2017;5: 68. doi: 10.1186/s40425-017-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babjuk M Burger M Capoun O Cohen D Compérat EM Dominguez Escrig JL, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol 2022;81: 75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Li R, Liu EM, Yang XQ, Wang LJ. Combined effects of neonatal Bacillus Calmette-Guerin vaccination and respiratory syncytial infection on experimental asthma in mice (in Chinese). Chin J Pedi 2006;44: 420–424. [PubMed] [Google Scholar]
- 39.Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol 2002;88: 584–591. doi: 10.1016/s1081-1206(10)61890-x. [DOI] [PubMed] [Google Scholar]
- 40.Kühtreiber WM Tran L Kim T Dybala M Nguyen B Plager S, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018;3: 23. doi: 10.1038/s41541-018-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ristori G Romano S Cannoni S Visconti A Tinelli E Mendozzi L, et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology 2014;82: 41–48. doi: 10.1212/01.wnl.0000438216.93319.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodridge HS Ahmed SS Curtis N Kollmann TR Levy O Netea MG, et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol 2016;16: 392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinnijenhuis J Quintin J Preijers F Benn CS Joosten LA Jacobs C, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun 2014;6: 152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen KJ Larsen N Biering-Sørensen S Andersen A Eriksen HB Monteiro I, et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis 2015;211: 956–967. doi: 10.1093/infdis/jiu508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteso G Aguiló N Julián E Ashiru O Ho MM Martín C, et al. Natural Killer Anti-Tumor Activity Can Be Achieved by In Vitro Incubation With Heat-Killed BCG. Front Immunol 2021;12: 622995. doi: 10.3389/fimmu.2021.622995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Cuesta EM Esteso G Ashiru O López-Cobo S Álvarez-Maestro M Linares A, et al. Characterization of a human anti-tumoral NK cell population expanded after BCG treatment of leukocytes. Oncoimmunology 2017;6: e1293212. doi: 10.1080/2162402x.2017.1293212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Cuesta EM López-Cobo S Álvarez-Maestro M Esteso G Romera-Cárdenas G Rey M, et al. NKG2D is a Key Receptor for Recognition of Bladder Cancer Cells by IL-2-Activated NK Cells and BCG Promotes NK Cell Activation. Front Immunol 2015;6: 284. doi: 10.3389/fimmu.2015.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sfakianos JP, Salome B, Daza J, Farkas A, Bhardwaj N, Horowitz A. Bacillus Calmette-Guerin (BCG): Its fight against pathogens and cancer. Urol Oncol 2021;39: 121–129. doi: 10.1016/j.urolonc.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 49.Sonoda T, Sugimura K, Ikemoto S, Kawashima H, Nakatani T. Significance of target cell infection and natural killer cells in the anti-tumor effects of bacillus Calmette-Guerin in murine bladder cancer. Oncol Rep 2007;17: 1469–1474. doi: 10.3892/or.17.6.1469. [PubMed] [Google Scholar]
- 50.Lagranderie M, Guyonvarc'h PM. The interplay between bacillus Calmette-Guérin and Treg cells and its role to prevent or cure inflammatory diseases. Expert Rev Clin Immunol 2014;10: 741–745. doi: 10.1586/1744666x.2014.909286. [DOI] [PubMed] [Google Scholar]
- 51.Yang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy 2012;67: 1193–1202. doi: 10.1111/j.1398-9995.2012.02880.x. [DOI] [PubMed] [Google Scholar]
- 52.Tian X Tian X Huo R Chang Q Zheng G Du Y, et al. Bacillus Calmette-Guerin alleviates airway inflammation and remodeling by preventing TGF-β(1) induced epithelial-mesenchymal transition. Hum Vaccin Immunother 2017;13: 1758–1764. doi: 10.1080/21645515.2017.1313366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Netea MG. Training innate immunity: the changing concept of immunological memory in innate host defence. Eur J Clin Invest 2013;43: 881–884. doi: 10.1111/eci.12132. [DOI] [PubMed] [Google Scholar]
- 54.Kleinnijenhuis J Quintin J Preijers F Joosten LA Ifrim DC Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012;109: 17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.B., de Bree LCJ Groh L Blok BA Chan J van der Velden W, et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020;28: 322–334.e325. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arts RJW Carvalho A La Rocca C Palma C Rodrigues F Silvestre R, et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep 2016;17: 2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Netea MG Domínguez-Andrés J Barreiro LB Chavakis T Divangahi M Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020;20: 375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arts RJW Moorlag S Novakovic B Li Y Wang SY Oosting M, et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018;23: 89–100.e105. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 59.van Puffelen JH Keating ST Oosterwijk E van der Heijden AG Netea MG Joosten LAB, et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat Rev Urol 2020;17: 513–525. doi: 10.1038/s41585-020-0346-4. [DOI] [PubMed] [Google Scholar]
- 60.Buffen K Oosting M Quintin J Ng A Kleinnijenhuis J Kumar V, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog 2014;10: e1004485. doi: 10.1371/journal.ppat.1004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graham CH Paré JF Cotechini T Hopman W Hindmarch CCT Ghaffari A, et al. Innate immune memory is associated with increased disease-free survival in bladder cancer patients treated with bacillus Calmette-Guérin. Can Urol Assoc J 2021;15: E412–E417. doi: 10.5489/cuaj.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faustman DL. Benefits of BCG-induced metabolic switch from oxidative phosphorylation to aerobic glycolysis in autoimmune and nervous system diseases. J Intern Med 2020;288: 641–650. doi: 10.1111/joim.13050. [DOI] [PubMed] [Google Scholar]
- 63.Funes SC, Rios M, Fernández-Fierro A, Di Genaro MS, Kalergis AM. Trained Immunity Contribution to Autoimmune and Inflammatory Disorders. Front Immunol 2022;13: 868343. doi: 10.3389/fimmu.2022.868343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keefe RC Takahashi H Tran L Nelson K Ng N Kühtreiber WM, et al. BCG therapy is associated with long-term, durable induction of Treg signature genes by epigenetic modulation. Sci Rep 2021;11: 14933. doi: 10.1038/s41598-021-94529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaufmann E Sanz J Dunn JL Khan N Mendonça LE Pacis A, et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018;172: 176–190.e119. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 66.Thomas PG Dash P Aldridge JR, Jr. Ellebedy AH Reynolds C Funk AJ, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009;30: 566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen IC Scull MA Moore CB Holl EK McElvania-TeKippe E Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009;30: 556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiravu A Osawe S Happel AU Nundalall T Wendoh J Beer S, et al. Bacille Calmette-Guérin Vaccine Strain Modulates the Ontogeny of Both Mycobacterial-Specific and Heterologous T Cell Immunity to Vaccination in Infants. Front Immunol 2019;10: 2307. doi: 10.3389/fimmu.2019.02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aaby P, Kollmann TR, Benn CS. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nat Immunol 2014;15: 895–899. doi: 10.1038/ni.2961. [DOI] [PubMed] [Google Scholar]
- 70.Lutwama F Kagina BM Wajja A Waiswa F Mansoor N Kirimunda S, et al. Distinct T-cell responses when BCG vaccination is delayed from birth to 6 weeks of age in Ugandan infants. J Infect Dis 2014;209: 887–897. doi: 10.1093/infdis/jit570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biering-Sørensen S, Jensen KJ, Monterio I, Ravn H, Aaby P, Benn CS. Rapid Protective Effects of Early BCG on Neonatal Mortality Among Low Birth Weight Boys: Observations From Randomized Trials. J Infect Dis 2018;217: 759–766. doi: 10.1093/infdis/jix612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pittet LF Cox L Freyne B Germano S Bonnici R Gardiner K, et al. Hepatitis B vaccine co-administration influences the heterologous effects of neonatal BCG vaccination in a sex-differential manner. Vaccine 2022;40: 1334–1341. doi: 10.1016/j.vaccine.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Koeken VA de Bree LCJ Mourits VP Moorlag SJ Walk J Cirovic B, et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J Clin Invest 2020;130: 5591–5602. doi: 10.1172/jci133935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blok BA Arts RJW van Crevel R Aaby P Joosten LAB Benn CS, et al. Differential effects of BCG vaccine on immune responses induced by vi polysaccharide typhoid fever vaccination: an explorative randomized trial. Eur J Clin Microbiol Infect Dis 2020;39: 1177–1184. doi: 10.1007/s10096-020-03813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aaby P, Andersen A, Ravn H, Zaman K. Co-administration of BCG and Diphtheria-tetanus-pertussis (DTP) Vaccinations May Reduce Infant Mortality More Than the WHO-schedule of BCG First and Then DTP. A Re-analysis of Demographic Surveillance Data From Rural Bangladesh. EBioMedicine 2017;22: 173–180. doi: 10.1016/j.ebiom.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stensballe LG Ravn H Birk NM Kjærgaard J Nissen TN Pihl GT, et al. BCG Vaccination at Birth and Rate of Hospitalization for Infection Until 15 Months of Age in Danish Children: A Randomized Clinical Multicenter Trial. J Pediatric Infect Dis Soc 2019;8: 213–220. doi: 10.1093/jpids/piy029. [DOI] [PubMed] [Google Scholar]
- 77.Kjærgaard J. Bacillus Calmette-Guérin vaccination at birth: Effects on early childhood infections, growth, and development. Dan Med J 2016;63: B5304. [PubMed] [Google Scholar]
- 78.Thøstesen LM Stensballe LG Pihl GT Kjærgaard J Birk NM Nissen TN, et al. Neonatal BCG vaccination has no effect on recurrent wheeze in the first year of life: A randomized clinical trial. J Allergy Clin Immunol 2017;140: 1616–1621.e1613. doi: 10.1016/j.jaci.2016.12.990. [DOI] [PubMed] [Google Scholar]
- 79.Corsenac P Parent M Wolfson C Arbour N Duquette P Benedetti A, et al. Bacillus Calmette-Guerin vaccination and multiple sclerosis: A population-based birth cohort study in Quebec, Canada. Eur J Neurol 2022. doi: 10.1111/ene.15290. [DOI] [PubMed] [Google Scholar]
- 80.Corsenac P, Parent M, Benedetti A, Richard H, Stäger S, Rousseau MC. Association between Bacillus Calmette-Guerin vaccination and type 1 diabetes in adolescence: A population-based birth cohort study in Quebec, Canada. Prev Med 2022;154: 106893. doi: 10.1016/j.ypmed.2021.106893. [DOI] [PubMed] [Google Scholar]
- 81.Schaltz-Buchholzer F Bjerregaard-Andersen M Øland CB Golding C Stjernholm EB Monteiro I, et al. Early Vaccination With Bacille Calmette-Guérin-Denmark or BCG-Japan Versus BCG-Russia to Healthy Newborns in Guinea-Bissau: A Randomized Controlled Trial. Clin Infect Dis 2020;71: 1883–1893. doi: 10.1093/cid/ciz1080. [DOI] [PubMed] [Google Scholar]
- 82.Tsilika M Taks E Dolianitis K Kotsaki A Leventogiannis K Damoulari C, et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. Front Immunol 2022;13: 873067. doi: 10.3389/fimmu.2022.873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moorlag S Taks E Ten Doesschate T van der Vaart TW Janssen AB Müller L, et al. Efficacy of BCG Vaccination Against Respiratory Tract Infections in Older Adults During the Coronavirus Disease 2019 Pandemic. Clin Infect Dis 2022;75: e938–e946. doi: 10.1093/cid/ciac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santos AP Werneck GL Dalvi APR Dos Santos CC Tierno P Condelo HS, et al. The effect of BCG vaccination on infection and antibody levels against SARS-CoV-2-The results of ProBCG: a multicenter randomized clinical trial in Brazil. Int J Infect Dis 2023;130: 8–16. doi: 10.1016/j.ijid.2023.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Claus J Ten Doesschate T Gumbs C van Werkhoven CH van der Vaart TW Janssen AB, et al. BCG Vaccination of Health Care Workers Does Not Reduce SARS-CoV-2 Infections nor Infection Severity or Duration: a Randomized Placebo-Controlled Trial. mBio 2023;14: e0035623. doi: 10.1128/mbio.00356-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Upton CM van Wijk RC Mockeliunas L Simonsson USH McHarry K van den Hoogen G, et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine 2022;48: 101414. doi: 10.1016/j.eclinm.2022.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pittet LF Messina NL Orsini F Moore CL Abruzzo V Barry S, et al. Randomized Trial of BCG Vaccine to Protect against Covid-19 in Health Care Workers. N Engl J Med 2023;388: 1582–1596. doi: 10.1056/NEJMoa2212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramos-Martinez E Falfán-Valencia R Pérez-Rubio G Andrade WA Rojas-Serrano J Ambrocio-Ortiz E, et al. Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2. Cells 2021;10: 3179. doi: 10.3390/cells10113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Counoupas C Johansen MD Stella AO Nguyen DH Ferguson AL Aggarwal A, et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. NPJ Vaccines 2021;6: 143. doi: 10.1038/s41541-021-00406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Early Phase Clinical Trial About Therapeutic Biological Product Mix for Treating COVID-19 (AD26-BCG). Geneva: Xu H, 2021. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02403505. [Last accessed on October 6, 2022]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


