Abstract
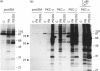
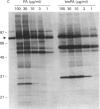
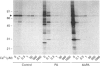
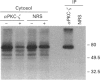
Phosphatidic acid (PA) is produced rapidly in agonist-stimulated cells, but the physiological function of this PA is unknown. We have examined the effects of PA on distinct isoforms of protein kinase C (PKC) using a new cell-free assay system. Addition of PA to cytosol from COS cells overexpressing PKC-alpha, -epsilon or -zeta differentially-activated all three isotypes, as shown by PKC autophosphorylation, and prominent phosphorylation of multiple endogenous substrates. In the absence of Ca2+, the diacylglycerol-insensitive zeta-isotype of PKC was most strongly activated by both PA and bisPA, a newly identified product of activated phospholipase D, with each lipid inducing its own profile of protein phosphorylation. BisPA was also a strong activator of PKC-epsilon, but a weak activator of PKC-alpha. Ca2+, at > or = 0.1 microM, inhibited PA and bisPA activation of PKC-zeta, but did not affect PKC-epsilon activation. In contrast, PKC-alpha was strongly activated by PA only in the presence of Ca2+. BisPA-induced phosphorylations mediated by PKC-zeta could be mimicked in part by other acidic phospholipids and unsaturated fatty acids. PA activation of PKC-zeta was unique in that PA not only stimulated PKC-zeta-mediated phosphorylation of distinctive substrates, but also caused an upward shift in electrophoretic mobility of PKC-zeta, which was not observed with other acidic lipids or with PKC-alpha or -epsilon. We have presented evidence that this mobility shift is not caused by PKC-zeta autophosphorylation, but it coincides with physical binding of PA to PKC-zeta. These results suggest that in cells stimulated under conditions where intracellular Ca2+ is at (or has returned to) basal level, PA may be a physiological activator of PKC-zeta.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bauldry S. A., Elsey K. L., Bass D. A. Activation of NADPH oxidase and phospholipase D in permeabilized human neutrophils. Correlation between oxidase activation and phosphatidic acid production. J Biol Chem. 1992 Dec 15;267(35):25141–25152. [PubMed] [Google Scholar]
- Berra E., Diaz-Meco M. T., Dominguez I., Municio M. M., Sanz L., Lozano J., Chapkin R. S., Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993 Aug 13;74(3):555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Phosphatidate-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6210–6213. doi: 10.1073/pnas.88.14.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. A., Gutowski S., Moomaw C. R., Slaughter C., Sternweis P. C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993 Dec 17;75(6):1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984 Aug 15;795(1):37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Thomas G. M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N. F., Truong O., Hsuan J. J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994 Jan 28;263(5146):523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Stafford A. R., Lester D. S. Lipid vesicles which can bind to protein kinase C and activate the enzyme in the presence of EGTA. Eur J Biochem. 1992 Sep 1;208(2):327–332. doi: 10.1111/j.1432-1033.1992.tb17190.x. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fukami K., Furuhashi K., Inagaki M., Endo T., Hatano S., Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992 Sep 10;359(6391):150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- Fukami K., Takenawa T. Phosphatidic acid that accumulates in platelet-derived growth factor-stimulated Balb/c 3T3 cells is a potential mitogenic signal. J Biol Chem. 1992 Jun 5;267(16):10988–10993. [PubMed] [Google Scholar]
- Gebbink M. F., van Etten I., Hateboer G., Suijkerbuijk R., Beijersbergen R. L., Geurts van Kessel A., Moolenaar W. H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991 Sep 23;290(1-2):123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Leibersperger H., Kittstein W., Marks F. Protein kinase C zeta and eta in murine epidermis. TPA induces down-regulation of PKC eta but not PKC zeta. FEBS Lett. 1992 Jul 28;307(2):151–155. doi: 10.1016/0014-5793(92)80756-7. [DOI] [PubMed] [Google Scholar]
- Ha K. S., Exton J. H. Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts. J Cell Biol. 1993 Dec;123(6 Pt 2):1789–1796. doi: 10.1083/jcb.123.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Protein kinase C activation in mixed micelles. Mechanistic implications of phospholipid, diacylglycerol, and calcium interdependencies. J Biol Chem. 1986 Jun 5;261(16):7184–7190. [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa W., Bandyopadhyay G. K., Wallace D., Nandi S. Phospholipids containing polyunsaturated fatty acyl groups are mitogenic for normal mouse mammary epithelial cells in serum-free primary cell culture. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4122–4126. doi: 10.1073/pnas.86.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes F. J., Prestle J., Eis S., Oberhagemann P., Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994 Feb 25;269(8):6140–6148. [PubMed] [Google Scholar]
- Kahn R. A., Yucel J. K., Malhotra V. ARF signaling: a potential role for phospholipase D in membrane traffic. Cell. 1993 Dec 17;75(6):1045–1048. doi: 10.1016/0092-8674(93)90314-g. [DOI] [PubMed] [Google Scholar]
- Khan W. A., Blobe G., Halpern A., Taylor W., Wetsel W. C., Burns D., Loomis C., Hannun Y. A. Selective regulation of protein kinase C isoenzymes by oleic acid in human platelets. J Biol Chem. 1993 Mar 5;268(7):5063–5068. [PubMed] [Google Scholar]
- Kochs G., Hummel R., Fiebich B., Sarre T. F., Marmé D., Hug H. Activation of purified human protein kinase C alpha and beta I isoenzymes in vitro by Ca2+, phosphatidylinositol and phosphatidylinositol 4,5-bisphosphate. Biochem J. 1993 Apr 15;291(Pt 2):627–633. doi: 10.1042/bj2910627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. Phospholipid functional groups involved in protein kinase C activation, phorbol ester binding, and binding to mixed micelles. J Biol Chem. 1989 Sep 5;264(25):14797–14805. [PubMed] [Google Scholar]
- Liscovitch M., Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J Biol Chem. 1989 Jul 15;264(20):11762–11767. [PubMed] [Google Scholar]
- Liyanage M., Frith D., Livneh E., Stabel S. Protein kinase C group B members PKC-delta, -epsilon, -zeta and PKC-L(eta). Comparison of properties of recombinant proteins in vitro and in vivo. Biochem J. 1992 May 1;283(Pt 3):781–787. doi: 10.1042/bj2830781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Rossi M. W., Fujiki T., Phillips W. A., Disa S., Queen C. F., Johnston R. B., Jr, Rosen O. M., Corkey B. E., Korchak H. M. Protein kinase C isotypes and signaling in neutrophils. Differential substrate specificities of a translocatable calcium- and phospholipid-dependent beta-protein kinase C and a phospholipid-dependent protein kinase which is inhibited by long chain fatty acyl coenzyme A. J Biol Chem. 1991 May 15;266(14):9285–9294. [PubMed] [Google Scholar]
- Marais R. M., Parker P. J. Purification and characterisation of bovine brain protein kinase C isotypes alpha, beta and gamma. Eur J Biochem. 1989 Jun 1;182(1):129–137. doi: 10.1111/j.1432-1033.1989.tb14809.x. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Dunlop M. Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem J. 1990 Sep 1;270(2):427–435. doi: 10.1042/bj2700427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. E., Marais R. M., Parker P. J. The phosphorylation of protein kinase C as a potential measure of activation. Biochem J. 1989 Jul 1;261(1):131–136. doi: 10.1042/bj2610131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Moritz A., De Graan P. N., Gispen W. H., Wirtz K. W. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992 Apr 15;267(11):7207–7210. [PubMed] [Google Scholar]
- Nakanishi H., Brewer K. A., Exton J. H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993 Jan 5;268(1):13–16. [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- Newton A. C., Koshland D. E., Jr Phosphatidylserine affects specificity of protein kinase C substrate phosphorylation and autophosphorylation. Biochemistry. 1990 Jul 17;29(28):6656–6661. doi: 10.1021/bi00480a015. [DOI] [PubMed] [Google Scholar]
- Newton A. C., Koshland D. E., Jr Protein kinase C autophosphorylates by an intrapeptide reaction. J Biol Chem. 1987 Jul 25;262(21):10185–10188. [PubMed] [Google Scholar]
- Nishikawa K., Yamamoto S., Otsuka C., Kato R. Characterization of endogenous substrates for novel-type protein kinase C as well as conventional-type protein kinase C in primary cultured mouse epidermal cells. Cell Signal. 1992 Nov;4(6):757–776. doi: 10.1016/0898-6568(92)90057-f. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J. W., Newton A. C. Interaction of protein kinase C with phosphatidylserine. 1. Cooperativity in lipid binding. Biochemistry. 1992 May 19;31(19):4661–4667. doi: 10.1021/bi00134a018. [DOI] [PubMed] [Google Scholar]
- Pacini L., Limatola C., Frati L., Luly P., Spinedi A. Muscarinic stimulation of SK-N-BE(2) human neuroblastoma cells elicits phosphoinositide and phosphatidylcholine hydrolysis: relationship to diacylglycerol and phosphatidic acid accumulation. Biochem J. 1993 Jan 1;289(Pt 1):269–275. doi: 10.1042/bj2890269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears C., Schaap D., Parker P. J. The regulatory domain of protein kinase C-epsilon restricts the catalytic-domain-specificity. Biochem J. 1991 May 15;276(Pt 1):257–260. doi: 10.1042/bj2760257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido T. C., Mizuno K., Konno Y., Osada S., Ohno S., Suzuki K. Purification and characterization of protein kinase C epsilon from rabbit brain. Biochemistry. 1992 Jan 21;31(2):482–490. doi: 10.1021/bi00117a026. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J., Bristol A., Kriz R., Knopf J. Unique substrate specificity and regulatory properties of PKC-epsilon: a rationale for diversity. FEBS Lett. 1989 Jan 30;243(2):351–357. doi: 10.1016/0014-5793(89)80160-7. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J. Expression, purification, and characterization of protein kinase C-epsilon. J Biol Chem. 1990 May 5;265(13):7301–7307. [PubMed] [Google Scholar]
- Schaap D., de Widt J., van der Wal J., Vandekerckhove J., van Damme J., Gussow D., Ploegh H. L., van Blitterswijk W. J., van der Bend R. L. Purification, cDNA-cloning and expression of human diacylglycerol kinase. FEBS Lett. 1990 Nov 26;275(1-2):151–158. doi: 10.1016/0014-5793(90)81461-v. [DOI] [PubMed] [Google Scholar]
- Schaap D., van der Wal J., van Blitterswijk W. J., van der Bend R. L., Ploegh H. L. Diacylglycerol kinase is phosphorylated in vivo upon stimulation of the epidermal growth factor receptor and serine/threonine kinases, including protein kinase C-epsilon. Biochem J. 1993 Feb 1;289(Pt 3):875–881. doi: 10.1042/bj2890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Copeland T. D., Mark G. E., Rapp U. R., Oroszlan S. Detection of the myristylated gag-raf transforming protein with raf-specific antipeptide sera. Virology. 1985 Oct 15;146(1):78–89. doi: 10.1016/0042-6822(85)90054-6. [DOI] [PubMed] [Google Scholar]
- Selbie L. A., Schmitz-Peiffer C., Sheng Y., Biden T. J. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993 Nov 15;268(32):24296–24302. [PubMed] [Google Scholar]
- Shinomura T., Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. S., Chauhan A., Brockerhoff H., Chauhan V. P. Activation of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. Biochem Biophys Res Commun. 1993 Aug 31;195(1):104–112. doi: 10.1006/bbrc.1993.2016. [DOI] [PubMed] [Google Scholar]
- Stabel S., Parker P. J. Protein kinase C. Pharmacol Ther. 1991;51(1):71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- Tigyi G., Dyer D., Matute C., Miledi R. A serum factor that activates the phosphatidylinositol phosphate signaling system in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1521–1525. doi: 10.1073/pnas.87.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumura A., Tsutsumi T., Tsukatani H. Transbilayer movement and metabolic fate of ether-linked phosphatidic acid (1-O-Octadecyl-2-acetyl-sn-glycerol 3-phosphate) in guinea pig peritoneal polymorphonuclear leukocytes. J Biol Chem. 1992 Apr 15;267(11):7275–7283. [PubMed] [Google Scholar]
- Walker T. R., Watson S. P. Synergy between Ca2+ and protein kinase C is the major factor in determining the level of secretion from human platelets. Biochem J. 1993 Jan 1;289(Pt 1):277–282. doi: 10.1042/bj2890277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways D. K., Cook P. P., Webster C., Parker P. J. Effect of phorbol esters on protein kinase C-zeta. J Biol Chem. 1992 Mar 5;267(7):4799–4805. [PubMed] [Google Scholar]
- Zhao Z., Shen S. H., Fischer E. H. Stimulation by phospholipids of a protein-tyrosine-phosphatase containing two src homology 2 domains. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4251–4255. doi: 10.1073/pnas.90.9.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Hilkmann H. Rapid attenuation of receptor-induced diacylglycerol and phosphatidic acid by phospholipase D-mediated transphosphatidylation: formation of bisphosphatidic acid. EMBO J. 1993 Jul;12(7):2655–2662. doi: 10.1002/j.1460-2075.1993.tb05926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., Hilkmann H., van Blitterswijk W. J. Diacylglycerol kinase in receptor-stimulated cells converts its substrate in a topologically restricted manner. J Biol Chem. 1994 Feb 11;269(6):4098–4102. [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992 Jul 1;285(Pt 1):235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]