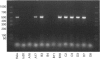
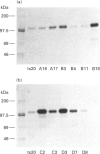
Abstract
The ubiquitin conjugation system is a multi-step pathway in which ubiquitin is activated and conjugated to acceptor proteins, one function of which is to target acceptor proteins for rapid degradation within the cell. The conjugation system is involved in many aspects of cellular functions, including the cell cycle. Several cell-cycle arrest mutant cell lines have been characterized and appear to harbour a mutant ubiquitin-activating enzyme, E1, as their primary defect. One such cell line is ts20, which is derived from Chinese hamster ovary E36 cells. This cell line has been used to characterize some of the potential functions of the ubiquitin conjugation system in vivo, such as its involvement in the maturation of autophagic vacuoles. The present study describes the complete rescue of the complex ts20 phenotype following the expression of the cDNA for human E1. Stable transfectants expressing the human E1 cDNA in the CMVneo expression vector were measured for ubiquitin-conjugation activity, protein degradation and growth in culture at the nonpermissive temperature. This rescue confirms that the phenotype observed in the ts20 cells is due to a defect in the E1 enzyme. Thus, the ts20 cell line will serve as a useful tool to delineate the functions of the ubiquitin system in vivo.
Full text
PDF

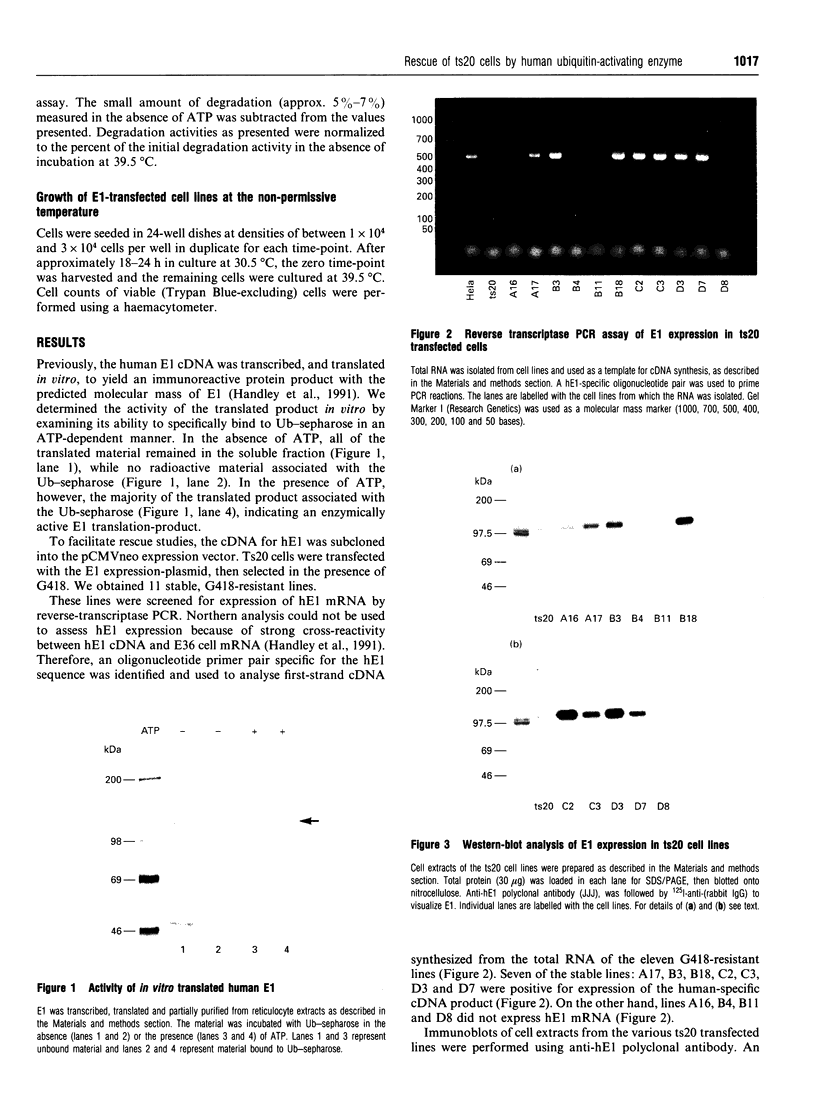
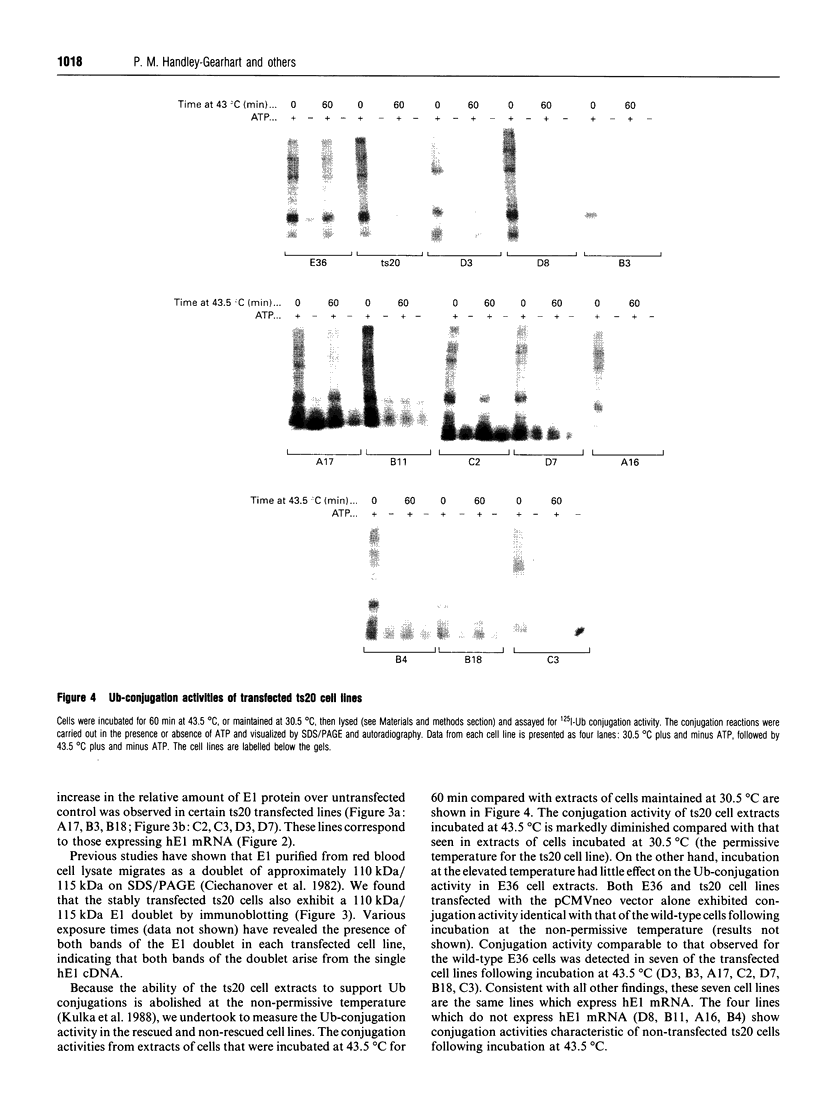

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Kaneda S., Itoh Y., Yasuda H., Murakami Y., Sugasawa K., Hanaoka F., Seno T. Complementation by a cloned human ubiquitin-activating enzyme E1 of the S-phase-arrested mouse FM3A cell mutant with thermolabile E1. Cell Struct Funct. 1992 Apr;17(2):113–122. doi: 10.1247/csf.17.113. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., DiGiuseppe J. A., Bercovich B., Orian A., Richter J. D., Schwartz A. L., Brodeur G. M. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Elias S., Heller H., Hershko A. "Covalent affinity" purification of ubiquitin-activating enzyme. J Biol Chem. 1982 Mar 10;257(5):2537–2542. [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Crosby S. D., Puetz J. J., Simburger K. S., Fahrner T. J., Milbrandt J. The early response gene NGFI-C encodes a zinc finger transcriptional activator and is a member of the GCGGGGGCG (GSG) element-binding protein family. Mol Cell Biol. 1991 Aug;11(8):3835–3841. doi: 10.1128/mcb.11.8.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D., Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gropper R., Brandt R. A., Elias S., Bearer C. F., Mayer A., Schwartz A. L., Ciechanover A. The ubiquitin-activating enzyme, E1, is required for stress-induced lysosomal degradation of cellular proteins. J Biol Chem. 1991 Feb 25;266(6):3602–3610. [PubMed] [Google Scholar]
- Haas A. L., Warms J. V., Hershko A., Rose I. A. Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J Biol Chem. 1982 Mar 10;257(5):2543–2548. [PubMed] [Google Scholar]
- Handley P. M., Mueckler M., Siegel N. R., Ciechanover A., Schwartz A. L. Molecular cloning, sequence, and tissue distribution of the human ubiquitin-activating enzyme E1. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):258–262. doi: 10.1073/pnas.88.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield P. M., Callis J., Vierstra R. D. Cloning of ubiquitin activating enzyme from wheat and expression of a functional protein in Escherichia coli. J Biol Chem. 1990 Sep 15;265(26):15813–15817. [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ganoth D., Pehrson J., Palazzo R. E., Cohen L. H. Methylated ubiquitin inhibits cyclin degradation in clam embryo extracts. J Biol Chem. 1991 Sep 5;266(25):16376–16379. [PubMed] [Google Scholar]
- Hershko A., Heller H., Elias S., Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983 Jul 10;258(13):8206–8214. [PubMed] [Google Scholar]
- Kong S. K., Chock P. B. Protein ubiquitination is regulated by phosphorylation. An in vitro study. J Biol Chem. 1992 Jul 15;267(20):14189–14192. [PubMed] [Google Scholar]
- Kudo M., Sugasawa K., Hori T., Enomoto T., Hanaoka F., Ui M. Human ubiquitin-activating enzyme (E1): compensation for heat-labile mouse E1 and its gene localization on the X chromosome. Exp Cell Res. 1991 Jan;192(1):110–117. doi: 10.1016/0014-4827(91)90164-p. [DOI] [PubMed] [Google Scholar]
- Kulka R. G., Raboy B., Schuster R., Parag H. A., Diamond G., Ciechanover A., Marcus M. A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J Biol Chem. 1988 Oct 25;263(30):15726–15731. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenk S. E., Dunn W. A., Jr, Trausch J. S., Ciechanover A., Schwartz A. L. Ubiquitin-activating enzyme, E1, is associated with maturation of autophagic vacuoles. J Cell Biol. 1992 Jul;118(2):301–308. doi: 10.1083/jcb.118.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Gropper R., Schwartz A. L., Ciechanover A. Purification, characterization, and rapid inactivation of thermolabile ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. J Biol Chem. 1989 Feb 5;264(4):2060–2068. [PubMed] [Google Scholar]
- McGrath J. P., Jentsch S., Varshavsky A. UBA 1: an essential yeast gene encoding ubiquitin-activating enzyme. EMBO J. 1991 Jan;10(1):227–236. doi: 10.1002/j.1460-2075.1991.tb07940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek M. T., Grant E. P., Gramm C., Goldberg A. L., Rock K. L. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993 Jun 10;363(6429):552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Goto H., Kaneda S., Yamao F., Seno T., Handley P., Schwartz A. L., Nishimoto T. tsBN75 and tsBN423, temperature-sensitive x-linked mutants of the BHK21 cell line, can be complemented by the ubiquitin-activating enzyme E1 cDNA. Biochem Biophys Res Commun. 1992 Apr 30;184(2):1015–1021. doi: 10.1016/0006-291x(92)90692-e. [DOI] [PubMed] [Google Scholar]
- Paolini R., Kinet J. P. Cell surface control of the multiubiquitination and deubiquitination of high-affinity immunoglobulin E receptors. EMBO J. 1993 Feb;12(2):779–786. doi: 10.1002/j.1460-2075.1993.tb05712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Pickart C. M., Rose I. A. Functional heterogeneity of ubiquitin carrier proteins. J Biol Chem. 1985 Feb 10;260(3):1573–1581. [PubMed] [Google Scholar]
- Russell B., Dix D. J. Mechanisms for intracellular distribution of mRNA: in situ hybridization studies in muscle. Am J Physiol. 1992 Jan;262(1 Pt 1):C1–C8. doi: 10.1152/ajpcell.1992.262.1.C1. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Kohn K. W., Bonner W. M. Metabolism of ubiquitinated histones. J Biol Chem. 1981 Jun 10;256(11):5916–5920. [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E., Sheinin R. Molecular cloning, primary structure and expression of the human X linked A1S9 gene cDNA which complements the ts A1S9 mouse L cell defect in DNA replication. EMBO J. 1990 Sep;9(9):2923–2929. doi: 10.1002/j.1460-2075.1990.tb07483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]