Abstract
Background
Monolysocardiolipin acyltransferase (MLCL AT) catalyzes the acylation of monolysocardiolipin to cardiolipin in mammalian tissues. We previously reported that cardiac cardiolipin levels, MLCL AT and cardiolipin synthase activities were all elevated in rats made hyperthyroid by thyroxine treatment. In this study, we examined if cardiac mitochondrial MLCL AT activity was dependent upon the biosynthesis and level of cardiolipin in the heart. Rat heart mitochondrial MLCL AT activity was determined under conditions in which the levels of cardiac cardiolipin and cardiolipin synthase activity were either reduced or unaltered using four different disease models in the rat. In addition, these parameters were examined in a murine model of cardiac cell differentiation.
Results
In rats made hypothyroid by treatment with 6-n-propyl-2-thiouracil in the drinking water for 34 days, cardiac cardiolipin content was decreased 29% (p < 0.025) and this was associated with a 32% decrease (p < 0.025) in cardiolipin synthase and a 35% reduction (p < 0.025) in MLCL AT activities. Streptozotocin-induced diabetes or hyperinsulinemia in rats did not affect cardiac cardiolipin content nor MLCL AT and cardiolipin synthase activities. Finally, cardiolipin content, MLCL AT and cardiolipin synthase activities were unaltered during murine P19 teratocarcinoma cell differentiation into cardiac myocytes. In all models, phospholipase A2 activities were unaltered compared with controls.
Conclusion
We propose a general model in which the expression of MLCL AT activity is regulated in concert with the biosynthesis and level of cardiolipin in the heart.
Background
Phospholipids are important structural and functional components of the biological membrane [1]. Structurally, as major components of the biological membrane, they define compartmentalization of organelles and the protective barrier, the cell membrane, which surrounds cells. An important class of phospholipids are the polyglycerophospholipids. Cardiolipin (CL), the first polyglycerophospholipid discovered, was isolated from beef heart by Pangborn in 1942 [2]. In the heart, CL represents approximately 12–16% of the entire cardiac phospholipid mass and is found exclusively in mitochondria [3].
In mammalian tissues CL is required for the reconstituted activity of a number of key mitochondrial enzymes involved in cellular energy metabolism including for example cytochrome c oxidase, carnitine palmitoyltransferase, creatine phosphokinase, pyruvate translocator, mono-, di- and tricarboxylate carriers, glycerol-3-phosphate dehydrogenase, phosphate transporter, ATP/ADP translocase and ATP synthase [4]. Under experimental conditions in which CL was removed or digested away from these proteins with phospholipases, denaturation and complete loss in activity of many of these proteins was observed. CL interaction with these proteins was specific since substitution with other phospholipids did not fully reconstitute activity. The fatty acyl composition of CL also appeared to be important for this functional reconstitution. For example, the activity of delipidated rat liver cytochrome c oxidase was reconstituted by the addition of CL [5]. The specific activity of reconstituted cytochrome c oxidase varied significantly with different fatty acyl compositions of CL. A strong positive correlation has been established between fatty acid unsaturation of CL and antioxidant production in cells [6]. In staurosporine-treated granulosa cells undergoing apoptosis CL levels were reduced [7]. Peroxidation of CL induced release of cytochrome c from mitochondria into the cytosol and this was associated with the induction of apoptosis [8-10]. Suppression of CL peroxidation inhibited release of cytochrome c from mitochondria [11]. Thus, the activities of the enzymes that synthesize and remodel CL play a pivotal role in maintaining the content and molecular composition of CL and hence may regulate a plethora of cellular processes from energy metabolism to apoptosis.
In mammalian tissues CL is synthesized by condensation of phosphatidylglycerol with cytidine-5'-diphosphate-1,2-diacyl-sn-glycerol catalyzed by CL synthase [for review see [12]]. Thyroxine treatment of rats stimulated the expression of rat liver and heart mitochondrial CL synthase activities [13,14]. The increase in CL synthase activity accounted for the elevated levels of CL observed in these organs. We recently identified and characterized the activity of monolysocardiolipin acyltransferase (MLCL AT), the enzyme responsible for monolysocardiolipin (MLCL) acylation to CL in mammalian tissues [15]. In another study, we showed that thyroxine treatment of rats elevated cardiac MLCL AT activity and postulated that MLCL AT may be a rate-limiting enzyme for the molecular remodeling of CL in the heart [16]. The above studies prompted us to examine if cardiac MLCL AT activity was linked to CL biosynthesis and content in the heart. Our results, using four different disease models in the rat in which the level of cardiac CL is either reduced, elevated or unaltered, demonstrate that this is the case. We also observe this relationship in a model of murine cardiac cell differentiation.
Results
Cardiac CL content, CL synthase and MLCL AT activities are reduced in hypothyroid rats
In previous studies we observed that cardiac MLCL AT activity was elevated when the cardiac CL content and CL synthase activity were elevated in hyperthyroid rats [14,16]. We examined if cardiac CL content, CL synthase and MLCL AT activities were reduced in hypothyroid rats. Rats were made hypothyroid by the addition of 0.05% PTU to their drinking water for 34 days. This protocol was shown to produce decreased serum thyroid hormone levels and result in cardiac atrophy in the rat [23]. As seen in Table 1, in rats that received PTU there was a 48% decrease (p < 0.025) in heart weight compared to controls. In addition, the heart to body weight ratio decreased, indicative of cardiac atrophy. Growth failure was demonstrated by the decreased body weights of the hypothyroid animals compared to controls. As a further control, the activity of an inner mitochondrial membrane marker, succinate dehydrogenase, was determined. Cardiac mitochondrial succinate dehydrogenase activity was reduced 23% (p < 0.025) from 30 ± 3 μmol/min·mg to 23 ± 3 μmol/min·mg protein in PTU-treated rats. These are documented characteristics of hypothyroidism [24].
Table 1.
Body weight, heart weight, heart CL content, CL synthase and MLCL AT activities in normal and hypothyroid rats.
| Control | Hypothyroid | |
| Heart weight (g) | 1.23 ± 0.03 | 0.64 ± 0.04* |
| Body weight (g) | ||
| Before PTU treatment | 206 ± 4 | 205 ± 4 |
| After PTU treatment | 441 ± 19 | 275 ± 20* |
| Heart weight:body weight (×100) | 0.28 ± 0.02 | 0.23 ± 0.03* |
| nmol/mg heart | ||
| CL content | 6.1 ± 0.1 | 4.3 ± 0.4* |
| pmol/min·mg protein | ||
| CL synthase activity | 3.1 ± 0.3 | 2.1 ± 0.2* |
| MLCL AT activity | 40 ± 6 | 26 ± 3* |
Rats were made hypothyroid by administration of 0.05% PTU in the drinking water for 34 days. The hearts were harvested, weighed and CL content, CL synthase and MLCL AT activities determined. Results represent the mean ± standard deviation of ten animals *p < 0.025.
Heart mitochondrial fractions were prepared from rats made hypothyroid by the addition of 0.05% PTU to their drinking water for 34 days and CL content, CL synthase and MLCL AT activities determined. We initially determined the activity of cardiac mitochondrial PA:CTP cytidylyltransferase, a rate-limiting enzyme of CL biosynthesis [21]. PA:CTP cytidylyltransferase activity was 15.1 ± 1.2 pmol/min/mg protein and unaltered (14.7 ± 1.1 pmol/min/mg protein) in cardiac mitochondrial fractions prepared from hypothyroid rats. Hence, PA:CTP cytidylyltransferase served as a control for a mitochondrial enzyme not affected by hypothyroidism. When compared to controls, heart mitochondria prepared from hypothyroid rats exhibited a 29% decrease (p < 0.025) in CL content, a 32% decrease (p < 0.025) in CL synthase activity and a 35% decrease (p < 0.025) in MLCL AT activity (Table 1). PLA2 activity was 4.2 ± 0.7 nmol/min·mg protein and unaltered (4.0 ± 0.5 nmol/min·mg protein) in cardiac mitochondria prepared from hypothyroid rats. Thus, cardiac mitochondrial CL content, CL synthase and MLCL AT activities were all reduced in hypothyroid rats.
Cardiac CL content, CL synthase and MLCL AT activities are unaltered in streptozotocin-induced diabetic rats and in hyperinsulinemic rats
Previously we showed that cardiac phosphatidylglycerol levels were reduced in streptozotocin-induced diabetic rats but CL synthase activity and CL content were unaltered [20]. We examined if streptozotocin-induced diabetes in rats altered MLCL AT activity or if hyperinsulinemia in rats altered CL synthase and MLCL AT activities in cardiac mitochondria. Rats were made diabetic by injection of steptozotocin or hyperinsulinemic by intraperitoneal addition of insulin. Subsequently, the hearts were removed and mitochondrial fractions prepared. Cardiac CL synthase activities were 3.0 ± 0.5 pmol/min·mg protein in hyperinsulinemic rats and did not differ from control (3.1 ± 0.6 pmol/min·mg protein) non-insulin injected animals. Cardiac CL content was 5.9 ± 0.5 nmol/mg heart and unaltered compared to controls (6.1 ± 0.1 nmol/mg heart). Cardiac MLCL AT activities were 38 ± 6 pmol/min·mg protein in diabetic rats and 43 ± 9 pmol/min·mg protein in hyperinsulinemic rats and did not differ from controls (40 ± 6 pmol/min·mg protein saline injected and 41 ± 9 pmol/min·mg protein non-insulin injected animals, respectively). Thus, in streptozotocin-induced diabetes and hyperinsulinemia, conditions in which the CL content and CL synthase activities were unaltered, MLCL AT activity was unaltered.
CL content, CL synthase and MLCL AT activities are unaltered during cardiac cell differentiation
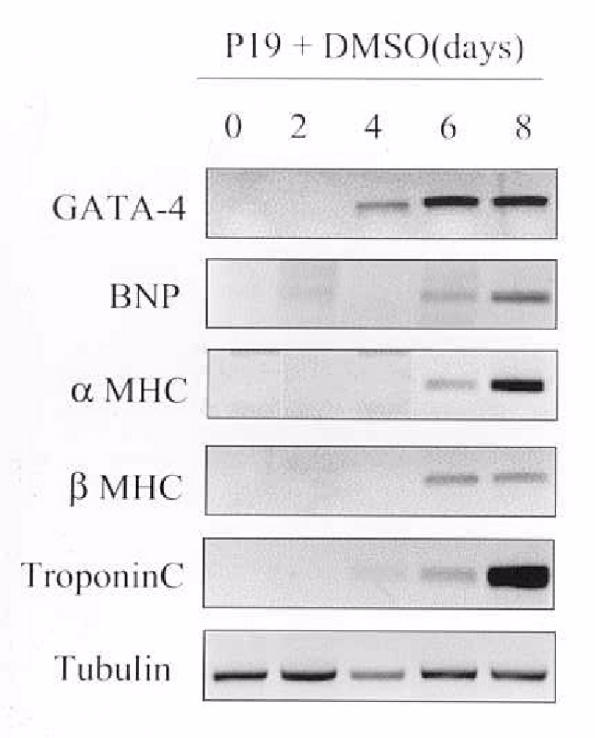
As a distinct model, we examined if CL synthase activity was altered in murine P19 teratocarcinoma cells induced to undergo differentiation into cardiac myocytes. We chose this model since differentiation of murine P19 cells into cardiac myocytes results in an increase in phosphatidylethanolamine biosynthesis, phosphatidylethanolamine mass and lysophosphatidylethanolamine acyltransferase activities [17,25]. The cells were harvested at various times, 0–8 days post DMSO addition, and MRNA analysis of markers of cardiac cell differentiation performed on cell lysates. GATA-4 is a member of the GATA family of zinc finger transcription factors and is an early marker of cardiac cell differentiation. As seen in Figure 1, GATA-4 was expressed at 4 days post DMSO addition relative to the constitutive expression of tubulin. As expected GATA-4 expression preceded the expression of other cardiac genes including B-natriuretic peptide (BNP), alpha myosin heavy chain (αMHC), beta myosin heavy chain (βMHC) and Troponin C relative to the constitutive expression of tubulin. Thus, the P19 cells used in this study differentiated into cardiac myocytes. As previously shown CL content, MLCL AT and PLA2 activities were unaltered during P19 cell differentiation into cardiac myocytes [17,25]. CL synthase activity was 2.7 ± 0.5 pmol/min·mg protein in undifferentiated and unaltered (2.8 ± 0.3 pmol/min·mg protein) in differentiated P19 cells. Together, the above five models using hyper- and hypothyroid, diabetic and hyperinsulinemic rats and murine P19 cell differentiation into cardiac myocytes all indicate that expression of mammalian cardiac mitochondrial MLCL AT activity appears to be regulated in concert with the biosynthesis and content of CL in the heart.
Figure 1.
Expression of genes during differentiation of P19 cells into cardiac myocytes. P19 cells were incubated with 1% DMSO for up to 8 days. At various times, 0–8 days post DMSO addition, cells were harvested and mRNA levels of GATA-4, BNP, α MHC, β MHC, troponin C and tubulin were determined by quantitative RT-PCR analysis.
Discussion
Previous and current studies in the mammalian heart and liver support the hypothesis that CL content is regulated in concert with the level of CL synthase activity
In the CL biosynthetic pathway, PG is converted to CL by condensation with CDP-DG catalyzed by CL synthase [3]. In vitro studies have indicated that alteration in cellular CL levels appears to have functional consequences. For example, reduction in the content of CL was shown to reduce oxygen consumption in mitochondria prepared from rat liver [26]. Thus, maintenance of the appropriate content of CL in mammalian mitochondria is essential for proper mitochondrial function. Thyroxine treatment of rats was shown to stimulate the activity of rat liver mitochondrial CL synthase 2.5-fold [13]. This elevation in rat liver mitochondrial CL synthase activity was suggested to account for the elevated levels of CL observed in livers prepared from hyperthyroid rats. In addition, CL synthase was shown to be elevated in heart mitochondria prepared from hyperthyroid rats and this was correlated with an increase in cardiac CL content [14].
Prior to the current study, CL synthase activity had not been determined in any model of hypothyroidism. Hypothyroidism in the rat resulted in a 25% reduction in cardiac CL synthase activity. This reduction in CL synthase activity likely accounted for the reduced levels of cardiac CL observed in hearts prepared from hypothyroid animals. Previous studies in the rat have indicated that hypothyroidism also results in reduced CL levels in the liver [for review see [4]]. Thus, it is reasonable to assume that CL synthase activity would also be reduced in the liver of hypothyroid animals. In the current study, CL synthase activity was unaltered in diabetic and hyperinsulinemic rats and in a model of murine cardiac cell differentiation. In these models, the content of CL was unaltered. These data suggest that the level of CL produced in the mammalian heart is regulated in concert with the level of CL synthase activity.
Previous and current studies in the heart support the hypothesis that cardiac MLCL AT activity may be regulated in concert with CL content and CL synthase activity
The data presented in this paper are entirely consistent with the conclusion that the expression of MLCL AT activity in the heart is regulated in concert with the biosynthesis and content of cardiac CL. Previously, we demonstrated that thyroxine-treatment of rats resulted in an increase in cardiac CL content, CL synthase and MLCL AT activities [14,16]. In the present study rats made hypothyroid with PTU in the drinking water had reduced cardiac CL content, CL synthase and MLCL AT activities. In contrast, in streptozotocin-induced diabetes and hyperinsulinemia, pathological conditions in which cardiac mitochondial CL content and CL synthase were unaltered [20], MLCL AT activities were unaltered. In addition, CL content, CL synthase and MLCL AT activities were unaltered during cardiac cell differentiation. It is reasonable to propose that when the rate of synthesis and level of CL is either reduced or elevated expression of the activities of the enzymes that remodel CL should be correspondingly reduced or elevated. The activity of cardiac mitochondrial PLA2 was high (100-fold) relative to cardiac mitochondrial MLCL AT activity and was unaltered in all models examined [20,25]. Since MLCL AT activity was either increased or decreased under conditions in which elevated or reduced CL remodelling was required, i.e. elevated or reduced CL synthesis, it is possible that MLCL AT may be rate-limiting for MLCL acylation to CL in the mammalian heart. However, it should be considered that other factors such as the intra-mitochondrial level of MLCL may be limiting for the acylation of MLCL to CL.
Conclusions
A summary of our findings is presented in Table 2. In hyperthyroidism, when cardiac CL synthase activity and CL content are elevated an increase in MLCL AT activity is observed. In hypothyroidism, when cardiac CL synthase activity and CL content are reduced a decrease in MLCL AT activity is observed. Finally, when cardiac CL synthase activity and CL content are unaltered in streptozotocin-induced diabetes, hyperinsulinemia and murine P19 cell differentiation into cardiac myocytes, MLCL AT activity is unaltered. Thus, expression of MLCL AT activity is regulated in concert with the biosynthesis and content of cardiac CL.
Table 2.
Summary of mammalian cardiac mitochondrial MLCL AT activities, CL synthase activities and CL content in various cardiac models.
| CL content | CL Synthase Activity | MLCL AT Activity | References | |
| Hyperthyroidism | increased | increased | increased | [14,16] |
| Hypothyroidism | decreased | decreased | decreased | [this paper] |
| STZ-induced diabetes | unaltered | unaltered | unaltered | [[20], this paper] |
| Hyperinsulinemia | unaltered | unaltered | unaltered | [this paper] |
| Murine P19 cell differentiation into cardiac myocytes | unaltered | unaltered | unaltered | [[17,25], this paper] |
Materials and Methods
Male Sprague Dawley rats (125–175 g) were used throughout the study and were housed in a temperature and light controlled room. They were maintained on Purina rat chow and tap water ad libitium. Treatment of animals conformed to the Guidelines of the Canadian Council on Animal Care. Rats were made hypothyroid by administration of (0.5% w/v) 6-n-propyl-2-thiouracil (PTU) in their drinking water for 34 days. Rats were made diabetic by injection of 60 mg/Kg steptozotocin. Hyperglycemia was confirmed 24 h later by urine and blood glucose analysis. Rats were made hyperinsulinemic by intraperitoneal addition of 3 units/day of insulin for 28 days. Murine P19 teratocarcinoma cells were kindly provided by Dr. Mona Nemer, Institute of Cardiovascular Research, University of Montreal, Montreal, Quebec, Canada. [1-14C]Linoleoyl-Coenzyme A was obtained from American Radiochemical Co., St. Louis MO. All other radiochemicals were obtained from Dupont, Winnipeg, Canada. MLCL was obtained from Avanti Polar Lipids, Alabaster, AL. Thin-layer chromatography (Silica gel 60, 0.25 mm thickness) plates were obtained from BDH, Toronto, Canada. Cell culture and reagents were products of Canadian Life Technologies (GIBCO) Burlington, Ontario, Canada. Ecolite scintillant was obtained from ICN Biochemicals, Costa Mesa, CA.. Lipids standards were obtained from Serdary Research Laboratories, Englewood Cliffs, NJ., USA. All other biochemicals were of analytical grade and obtained from either Fisher Scientific, Edmonton, Canada, Sigma Chemical Co., St. Louis, MO. or CanLab Division of Baxter Co. Winnipeg, Canada.
The protocol for differentiation and culturing of murine P19 teratocarcinoma cells into cardiac myocytes was performed as described [17]. P19 cells (5 × 105 cells/ml) were placed into 60 mm bacterial dishes, 1% dimethylsulfoxide (DMSO) was added and incubation continued for 48 h. The cells began to aggregate at this point. Cells were then transferred to a 100 mm bacterial dish and 1% DMSO was added for another 48 h. The cells in these 100 mm bacterial dishes differentiated into the cardiac cell lineage within eight days. At various days (0–8) post DMSO addition cells were harvested and mRNA expression of GATA-4, BNP, αMHC, βMHC, troponin C and tubulin were determined using quantitative RT-PCR analysis as described [18].
A 10% homogenate from rat hearts or P19 cells was prepared in buffer (0.25 M sucrose, 0.145 M NaCl, 10 mM Tris-HCl, pH 7.4) and centrifuged for 10 min at 600 × g (Beckman J2-H with JA-20 rotor). The resulting pellet was washed once, resuspended in 5 ml buffer by 15 strokes of a hand-held Dounce (tight fitting) tissue grinder and designated the crude nuclear fraction. The supernatant from the first centrifugation was centrifuged at 10,000 × g for 10 min. The resulting pellet was resuspended in 1.5 ml buffer as described above and used as the source of mitochondrial fraction for enzyme assays. Protein in this fraction was determined by the method of Bradford [19]. Phospholipase A2 (PLA2) was determined as described using phosphatidyl [14C]glycerol as substrate [20]. CL synthase and phosphatidic acid (PA):CTP cytidylyltransferase activities were determined as described [21]. MLCL AT activities were determined as described [15]. Mitochondrial fractions (50 μg) were incubated for 30 min at 25°C in 50 mM Tris-HCL, pH 8.0, 33 μM [1-14C]linoleoyl-Coenzyme A (68,700 dpm/nmol), 0.3 mM MLCL in a final volume of 0.35 ml. The reaction was initiated by the addition of [1-14C]linoleoyl-Coenzyme A substrate and terminated by addition of 3 ml of chloroform:methanol (2:1, by vol). 0.8 ml of KCL was added to facilitate phase separation. The aqueous phase was removed and the organic phase dried under nitrogen and resuspended in 25 μl of chloroform:methanol (2:1, by vol). A 20 μl aliquot was placed on a thin layer plate and CL was separated from other phospholipids in a solvent system containing chloroform:hexane:methanol:acetic acid (50:30:10:5, by vol). The silica gel corresponding to CL was removed and placed in plastic scintillation vials with 5 ml of aqueous counting scintillant. Radioactivity incorporated into CL was determined approximately 24 h later using a liquid scintillation counter. CL content was determined as described [21]. Mitochondrial succinate dehydrogenase activity was determined as described [22]. Students t-test was used for the determination of statistical significance. The level of significance was defined as p < 0.025.
List of abbreviations
CL, Cardiolipin; MLCL AT, monolysocardiolipin acyltransferase; MLCL, monolysocardiolipin; PTU, 6-n-propyl-2-thiouracil; PLA2, phospholipase A2; PA, phosphatidic acid; DMSO, dimethylsulfoxide; CTP, cytidine-5'-triphosphate; ATP, adenosine-5'-triphosphate; ADP, adenosine-5'-diphosphate; BNP, B-natriuretic peptide; αMHC, alpha myosin heavy chain; βMHC, beta myosin heavy chain; mRNA, messenger ribonucleic acid; STZ, Stre ptozotocin
Authors contribution
Mr. William A. Taylor intitiated the experimental and edited the manuscript.
Dr. Fred Y. Xu performed experimental studies related to the P19 cells and edited the manuscript.
Mr. Brian J. Ma performed experimental studies related to the hyperinsulinemic rat model and edited the manuscript.
Dr. Thomas C. Mutter performed experimental studies related to the hypothyroid rat model.
Mr. Vernon W. Dolinsky initiated experimental studies related to the hypothyroid rat model and edited the manuscript.
Prof. Grant M. Hatch conceived of the study, participated in its design and coordination, wrote and edited the manuscript.
All authors have read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Dr. Mona Nemer for Figure 1. This work was supported by an operating grant from the Canadian Institutes of Health Research to G.M.H. G.M.H. is a CIHR Scientist.
Contributor Information
William A Taylor, Email: taylorw@ms.umanitoba.ca.
Fred Y Xu, Email: fyxu@ms.umanitoba.ca.
Brian J Ma, Email: bjma@ualberta.ca.
Thomas C Mutter, Email: mutter@ms.umanitoba.ca.
Vernon W Dolinsky, Email: dolinsky@ualberta.ca.
Grant M Hatch, Email: hatchgm@ms.umanitoba.ca.
References
- White DA. The phospholipid composition of mammalian tissues. In: GB Ansell, JN Hawthorne, RMC Dawson, editor. In Form and Function of Phospholipids. Elsevier, Amsterdam; 1973. pp. 441–482. [Google Scholar]
- Pangborn M. Isolation and purification of a serologically active phospholipid from beef heart. J Biol Chem. 1942;143:247–256. [Google Scholar]
- Hostetler KY. Polyglycerophospholipids: phosphatidylglycerol, diphosphatidylglycerol and bis(monoacylglycerol)phosphate. In: JN Hawthorne, GB Ansell, editor. In Phospholipids. Elsevier, Amsterdam.; 1982. pp. 215–261. [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Yamaoka-Koseki S, Urade R, Kito M. Cardiolipins from rats fed different diets affect bovine heart cytochrome C oxidase activity. J Nutr. 1991;121:956–958. doi: 10.1093/jn/121.7.956. [DOI] [PubMed] [Google Scholar]
- Watkins SM, Carter LC, German JB. Docosahexanoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J Lipid Res. 1998;39:1583–1588. [PubMed] [Google Scholar]
- Khan SM, Dauffenbach LM, Yeh J. Mitochondria and caspases in induced apoptosis in human luteinized granulosa cells. Biochem Biophys Res Comm. 2000;269:542–545. doi: 10.1006/bbrc.2000.2321. [DOI] [PubMed] [Google Scholar]
- Shidoji Y, Hayashi K, Komura S, Ohishi N, Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem Biophys Res Comm. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- Ushmorov A, Ratter F, Lehmann V, Droge W, Schirrmacher V, Umansky V. Nitric-oxide-induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome c release. Blood. 1999;93:2342–2352. [PubMed] [Google Scholar]
- Poot M, Pierce RH. Detection of changes in mitochondrial function during apoptosis by simultaneous staining with multiple fluorescent dyes and correlated multiparameter flow cytometry. Cytometry. 1999;35:311–317. doi: 10.1002/(SICI)1097-0320(19990401)35:4<311::AID-CYTO3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Mitochondrial phospholipid hydropeoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GM. Biosynthesis, remodelling and trafficking in the heart and mammalian cells. Int J Mol Med. 1998;1:33–41. doi: 10.3892/ijmm.1.1.33. [DOI] [PubMed] [Google Scholar]
- Hostetler KY. Effect of thyroxine on the activity of cardiolipin synthase in rat liver. Biochim Biophys Acta. 1991;1086:139–140. doi: 10.1016/0005-2760(91)90165-E. [DOI] [PubMed] [Google Scholar]
- Cao SG, Cheng P, Angel A, Hatch GM. Thyroxine stimulates phosphatidylglycerolphosphate synthase activity in rat heart mitochondria. Biochim Biophys Acta. 1995;1256:241–244. doi: 10.1016/0005-2760(95)00035-B. [DOI] [PubMed] [Google Scholar]
- Ma BJ, Taylor WA, Dolinsky VW, Hatch GM. Acylation of monolysocardiolipin in rat heart. J Lipid Res. 1999;40:1837–1845. [PubMed] [Google Scholar]
- Mutter T, Dolinsky VW, Ma BJ, Taylor WA, Hatch GM. Thyroxine stimulates the acylation of monolysocardiolipin in rat heart. Biochem J. 2000;346:403–406. doi: 10.1042/0264-6021:3460403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu FY, Kardami E, Nemer M, Choy PC, Hatch GM. Elevation in phosphatidylethanolamine is an early but not essential event for cardiac cell differentiation. Expt Cell Res. 2000;256:358–364. doi: 10.1006/excr.2000.4849. [DOI] [PubMed] [Google Scholar]
- Grepin C, Robitaille L, Antakly T, Nemer M. Inhibition of transcription factor GATA-4 expression blocks in vitro cardiac muscle cell differentiation. Mol Cell Biol. 1995;15:4095–4102. doi: 10.1128/mcb.15.8.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hatch GM, Cao SG, Angel A. Decrease in cardiac phosphatidylglycerol in streptozotocin-induced diabetic rats does not affect cardiolipin biosynthesis: Evidence for distinct pools of phosphatidylglycerol in the heart. Biochem J. 1995;306:759–764. doi: 10.1042/bj3060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GM, McClarty G. Regulation of cardiolipin biosynthesis in H9c2 cardiac myoblasts by cytidine-5'-triphosphate. J Biol Chem. 1996;271:25810–25816. doi: 10.1074/jbc.271.42.25810. [DOI] [PubMed] [Google Scholar]
- Hovius R, Lambrachts H, Nicolay K, de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta. 1990;1021:217–226. doi: 10.1016/0005-2736(90)90036-N. [DOI] [PubMed] [Google Scholar]
- Heron MI, Rakusan K. Geometry of coronary capillaries in hyperthyroid and hypothyroid rat heart. Amer J Physiol. 1994;267:H1024–H1031. doi: 10.1152/ajpheart.1994.267.3.H1024. [DOI] [PubMed] [Google Scholar]
- Klein I, Ojamaa K. The Thyroid. In: Utiger RD, Braverman LE, editor. A fundamental and clinical text. 7. Lippencott-Raven, New York; 1996. pp. 799–801. [Google Scholar]
- Fotheringham J, Xu FY, Nemer M, Kardami E, Choy PC, Hatch GM. Lysophosphatidylethanolamine acyltransferase activity is elevated during cardiac cell differentiation. Biochim Biophys Acta. 2000;1485:1–10. doi: 10.1016/S1388-1981(00)00025-1. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Urade R, Kito M. Cardiolipin molecular species in heart mitochondria are sensitive to essential fatty acid-deficient dietary lipids. J Nutr. 1990;120:415–421. doi: 10.1093/jn/120.5.415. [DOI] [PubMed] [Google Scholar]