Abstract
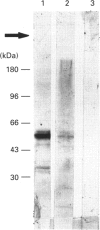
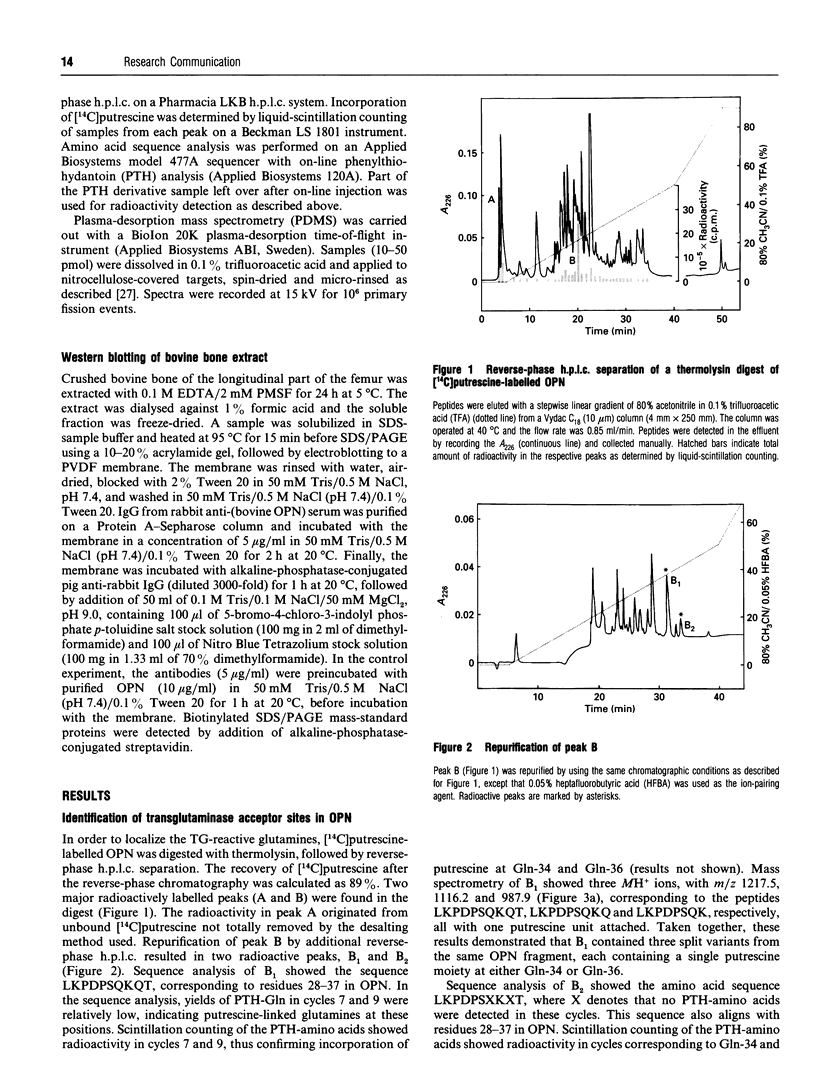
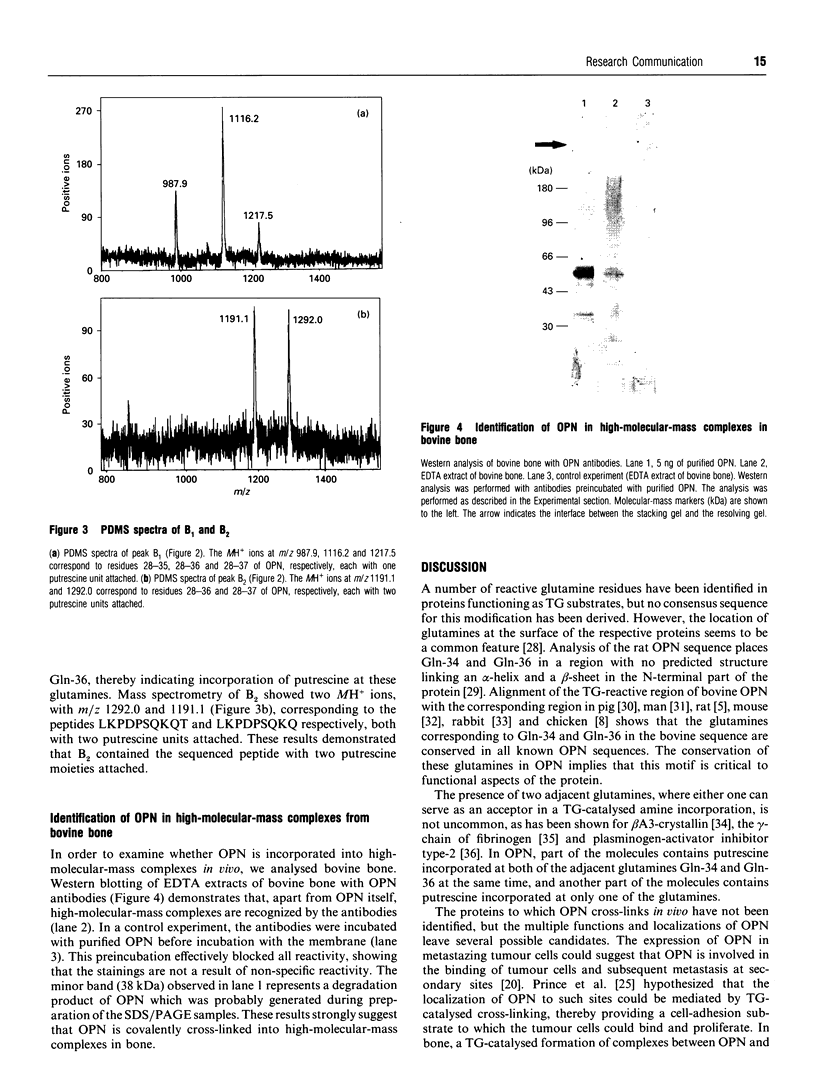
Here we report the identification of two transglutaminase-reactive glutamines (Gln-34 and Gln-36) in bovine osteopontin (OPN). Sequence alignment revealed that these glutamines are conserved in all known OPN sequences, indicating a functional importance of this region of the protein. Furthermore, immunological analysis of bovine bone demonstrated that OPN is present in high-molecular-mass complexes in vivo. These findings support the functional aspects of a transglutaminase-catalysed cross-linking of OPN in facilitating cellular attachment and tissue calcification.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeschlimann D., Paulsson M., Mann K. Identification of Gln726 in nidogen as the amine acceptor in transglutaminase-catalyzed cross-linking of laminin-nidogen complexes. J Biol Chem. 1992 Jun 5;267(16):11316–11321. [PubMed] [Google Scholar]
- Aeschlimann D., Wetterwald A., Fleisch H., Paulsson M. Expression of tissue transglutaminase in skeletal tissues correlates with events of terminal differentiation of chondrocytes. J Cell Biol. 1993 Mar;120(6):1461–1470. doi: 10.1083/jcb.120.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninati S., Senger D. R., Cordella-Miele E., Mukherjee A. B., Chackalaparampil I., Shanmugam V., Singh K., Mukherjee B. B. Osteopontin: its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J Biochem. 1994 Apr;115(4):675–682. doi: 10.1093/oxfordjournals.jbchem.a124395. [DOI] [PubMed] [Google Scholar]
- Berbers G. A., Feenstra R. W., van den Bos R., Hoekman W. A., Bloemendal H., de Jong W. W. Lens transglutaminase selects specific beta-crystallin sequences as substrate. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7017–7020. doi: 10.1073/pnas.81.22.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnola P., Bet P., Quarto R., Gennari M., Cancedda R. cDNA cloning and gene expression of chicken osteopontin. Expression of osteopontin mRNA in chondrocytes is enhanced by trypsin treatment of cells. J Biol Chem. 1991 May 25;266(15):9944–9949. [PubMed] [Google Scholar]
- Chen Y., Bal B. S., Gorski J. P. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992 Dec 5;267(34):24871–24878. [PubMed] [Google Scholar]
- Craig A. M., Bowden G. T., Chambers A. F., Spearman M. A., Greenberg A. H., Wright J. A., McLeod M., Denhardt D. T. Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts. Int J Cancer. 1990 Jul 15;46(1):133–137. doi: 10.1002/ijc.2910460124. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Denhardt D. T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993 Dec;7(15):1475–1482. [PubMed] [Google Scholar]
- Fet V., Dickinson M. E., Hogan B. L. Localization of the mouse gene for secreted phosphoprotein 1 (Spp-1) (2ar, osteopontin, bone sialoprotein 1, 44-kDa bone phosphoprotein, tumor-secreted phosphoprotein) to chromosome 5, closely linked to Ric (Rickettsia resistance). Genomics. 1989 Aug;5(2):375–377. doi: 10.1016/0888-7543(89)90074-8. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Shirasawa T., Esaki Y., Yoshiki S., Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993 Dec;92(6):2814–2820. doi: 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. H., Schüler E., Woodrow G., Richardson M., Goss N., Højrup P., Petersen T. E., Rasmussen L. K. A unique interhelical insertion in plasminogen activator inhibitor-2 contains three glutamines, Gln83, Gln84, Gln86, essential for transglutaminase-mediated cross-linking. J Biol Chem. 1994 May 27;269(21):15394–15398. [PubMed] [Google Scholar]
- Kiefer M. C., Bauer D. M., Barr P. J. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res. 1989 Apr 25;17(8):3306–3306. doi: 10.1093/nar/17.8.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohri K., Nomura S., Kitamura Y., Nagata T., Yoshioka K., Iguchi M., Yamate T., Umekawa T., Suzuki Y., Sinohara H. Structure and expression of the mRNA encoding urinary stone protein (osteopontin). J Biol Chem. 1993 Jul 15;268(20):15180–15184. [PubMed] [Google Scholar]
- Kohri K., Suzuki Y., Yoshida K., Yamamoto K., Amasaki N., Yamate T., Umekawa T., Iguchi M., Sinohara H., Kurita T. Molecular cloning and sequencing of cDNA encoding urinary stone protein, which is identical to osteopontin. Biochem Biophys Res Commun. 1992 Apr 30;184(2):859–864. doi: 10.1016/0006-291x(92)90669-c. [DOI] [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Gay S., Austin R. L., Bhown M., Finkelman R. D., Butler W. T. A comparative immunocytochemical study on the subcellular distributions of 44 kDa bone phosphoprotein and bone gamma-carboxyglutamic acid (Gla)-containing protein in osteoblasts. J Bone Miner Res. 1987 Aug;2(4):337–346. doi: 10.1002/jbmr.5650020411. [DOI] [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Oosawa T., Gay S., Bronckers A. L., Butler W. T. Immunohistochemical demonstration of a 44-KD phosphoprotein in developing rat bones. J Histochem Cytochem. 1987 Jul;35(7):707–715. doi: 10.1177/35.7.3295029. [DOI] [PubMed] [Google Scholar]
- Nielsen P. F., Roepstorff P. Sample preparation dependent fragmentation in 252-Cf plasma desorption mass spectrometry of the polycyclic antibiotic, nisin. Biomed Environ Mass Spectrom. 1988 Aug;17(2):137–141. doi: 10.1002/bms.1200170212. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Dickie D., Krumdieck C. L. Osteopontin, a substrate for transglutaminase and factor XIII activity. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1205–1210. doi: 10.1016/0006-291x(91)90669-x. [DOI] [PubMed] [Google Scholar]
- Purves L., Purves M., Brandt W. Cleavage of fibrin-derived D-dimer into monomers by endopeptidase from puff adder venom (Bitis arietans) acting at cross-linked sites of the gamma-chain. Sequence of carboxy-terminal cyanogen bromide gamma-chain fragments. Biochemistry. 1987 Jul 28;26(15):4640–4646. doi: 10.1021/bi00389a008. [DOI] [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter N. M., Farach-Carson M. C., Butler W. T. Evidence for the formation of a complex between osteopontin and osteocalcin. J Bone Miner Res. 1992 Aug;7(8):877–885. doi: 10.1002/jbmr.5650070804. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A., Tenen D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989 Jun 13;996(1-2):43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Shanahan C. M., Cary N. R., Metcalfe J. C., Weissberg P. L. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994 Jun;93(6):2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraga H., Min W., VanDusen W. J., Clayman M. D., Miner D., Terrell C. H., Sherbotie J. R., Foreman J. W., Przysiecki C., Neilson E. G. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. H., Denhardt D. T. Molecular cloning of a tumor promoter-inducible mRNA found in JB6 mouse epidermal cells: induction is stable at high, but not at low, cell densities. J Cell Biochem. 1987 May;34(1):13–22. doi: 10.1002/jcb.240340103. [DOI] [PubMed] [Google Scholar]
- Sørensen E. S., Petersen T. E. Purification and characterization of three proteins isolated from the proteose peptone fraction of bovine milk. J Dairy Res. 1993 May;60(2):189–197. doi: 10.1017/s0022029900027503. [DOI] [PubMed] [Google Scholar]
- Tezuka K., Sato T., Kamioka H., Nijweide P. J., Tanaka K., Matsuo T., Ohta M., Kurihara N., Hakeda Y., Kumegawa M. Identification of osteopontin in isolated rabbit osteoclasts. Biochem Biophys Res Commun. 1992 Jul 31;186(2):911–917. doi: 10.1016/0006-291x(92)90832-6. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Domenicucci C., Goldberg H. A., Wrana J. L., Sodek J. Characterization of fetal porcine bone sialoproteins, secreted phosphoprotein I (SPPI, osteopontin), bone sialoprotein, and a 23-kDa glycoprotein. Demonstration that the 23-kDa glycoprotein is derived from the carboxyl terminus of SPPI. J Biol Chem. 1990 May 5;265(13):7583–7589. [PubMed] [Google Scholar]
- Zhang Q., Wrana J. L., Sodek J. Characterization of the promoter region of the porcine opn (osteopontin, secreted phosphoprotein 1) gene. Identification of positive and negative regulatory elements and a 'silent' second promoter. Eur J Biochem. 1992 Jul 15;207(2):649–659. doi: 10.1111/j.1432-1033.1992.tb17092.x. [DOI] [PubMed] [Google Scholar]