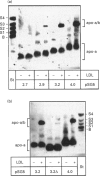
Abstract
Lipoprotein-a [Lp(a)], one of the most atherogenic lipoproteins, is composed of a low-density lipoprotein (LDL) core in addition to an apo-a of variable size which is linked to apoB by a disulphide bridge. Lp(a) synthesized in vitro by incubation of recombinant apo-a (r-apo-a) with LDL is physico-chemically indistinguishable from native Lp(a). The synthesis of Lp(a) in vitro proceeds in two steps. In the first step, one of the unique kringle-IVs (K-IVs) in apo-a binds to a Lys residue of apoB; in the second step, Cys-4057 of K-IV type-9 (T-9) forms a disulphide bridge with Cys-3734 of LDL. Here we have produced r-apo-a with different combinations of unique K-IVs and shown that K-IV T-6 is required for the first step of Lp(a) assembly. For the second step not only is K-IV T-9 essential, but also the distance between T-6 and T-9 requires a length of two K-IVs. These findings give additional insight into the mode of Lp(a) assembly and are of relevance in the search for apo-a mutants influencing Lp(a) levels and for the development of Lp(a)-lowering medications.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azrolan N., Gavish D., Breslow J. L. Plasma lipoprotein(a) concentration is controlled by apolipoprotein(a) (apo(a)) protein size and the abundance of hepatic apo(a) mRNA in a cynomolgus monkey model. J Biol Chem. 1991 Jul 25;266(21):13866–13872. [PubMed] [Google Scholar]
- Brunner C., Kraft H. G., Utermann G., Müller H. J. Cys4057 of apolipoprotein(a) is essential for lipoprotein(a) assembly. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11643–11647. doi: 10.1073/pnas.90.24.11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow M. J., Stoltzfus L. J., Lawn R. M., Rubin E. M. Expression of human apolipoprotein B and assembly of lipoprotein(a) in transgenic mice. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2130–2134. doi: 10.1073/pnas.91.6.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Chiesa G., Hobbs H. H. Sequence polymorphisms in the apolipoprotein (a) gene. Evidence for dissociation between apolipoprotein(a) size and plasma lipoprotein(a) levels. J Clin Invest. 1993 Apr;91(4):1630–1636. doi: 10.1172/JCI116370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaw A., Boerwinkle E., Cohen J. C., Hobbs H. H. Comparative analysis of the apo(a) gene, apo(a) glycoprotein, and plasma concentrations of Lp(a) in three ethnic groups. Evidence for no common "null" allele at the apo(a) locus. J Clin Invest. 1994 Jun;93(6):2526–2534. doi: 10.1172/JCI117263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara J., Jr, Spurlino J., Jan A. Y., Yang C. Y., Tulinsky A., Prasad B. V., Gaubatz J. W., Morrisett J. D. Proposed mechanisms for binding of apo[a] kringle type 9 to apo B-100 in human lipoprotein[a]. Biophys J. 1993 Mar;64(3):686–700. doi: 10.1016/S0006-3495(93)81428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G., Hermann A., Aktuna D., Petek W. Dissociation-enhanced lanthanide fluorescence immunoassay of lipoprotein(a) in serum. Clin Chem. 1992 Jun;38(6):853–859. [PubMed] [Google Scholar]
- Koschinsky M. L., Côté G. P., Gabel B., van der Hoek Y. Y. Identification of the cysteine residue in apolipoprotein(a) that mediates extracellular coupling with apolipoprotein B-100. J Biol Chem. 1993 Sep 15;268(26):19819–19825. [PubMed] [Google Scholar]
- Kostner G. M., Grillhofer H. K. Lipoprotein(a) mediates high affinity low density lipoprotein association to receptor negative fibroblasts. J Biol Chem. 1991 Nov 5;266(31):21287–21292. [PubMed] [Google Scholar]
- Krempler F., Kostner G. M., Roscher A., Haslauer F., Bolzano K., Sandhofer F. Studies on the role of specific cell surface receptors in the removal of lipoprotein (a) in man. J Clin Invest. 1983 May;71(5):1431–1441. doi: 10.1172/JCI110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., Lawn R. M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987 Nov 12;330(6144):132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- Perombelon Y. F., Soutar A. K., Knight B. L. Variation in lipoprotein(a) concentration associated with different apolipoprotein(a) alleles. J Clin Invest. 1994 Apr;93(4):1481–1492. doi: 10.1172/JCI117126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader D. J., Cain W., Zech L. A., Usher D., Brewer H. B., Jr Variation in lipoprotein(a) concentrations among individuals with the same apolipoprotein (a) isoform is determined by the rate of lipoprotein(a) production. J Clin Invest. 1993 Feb;91(2):443–447. doi: 10.1172/JCI116221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A. M., Fless G. M. Lipoprotein (a). Heterogeneity and biological relevance. J Clin Invest. 1990 Jun;85(6):1709–1715. doi: 10.1172/JCI114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Menzel H. J., Kraft H. G., Duba H. C., Kemmler H. G., Seitz C. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J Clin Invest. 1987 Aug;80(2):458–465. doi: 10.1172/JCI113093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. L., Rainwater D. L., Lanford R. E. Intracellular maturation of apolipoprotein[a] and assembly of lipoprotein[a] in primary baboon hepatocytes. J Lipid Res. 1993 Mar;34(3):509–517. [PubMed] [Google Scholar]
- Zatloukal K., Wagner E., Cotten M., Phillips S., Plank C., Steinlein P., Curiel D. T., Birnstiel M. L. Transferrinfection: a highly efficient way to express gene constructs in eukaryotic cells. Ann N Y Acad Sci. 1992 Oct 28;660:136–153. doi: 10.1111/j.1749-6632.1992.tb21066.x. [DOI] [PubMed] [Google Scholar]