Abstract
We reported the main results of the Japanese Registry of Neuroendovascular Therapy (JR-NET) 4, a nationwide surveillance of therapy (NET) in Japan from January 2015 to December 2019. JR-NET 4 registered consecutive patients who underwent NETs by Japan Society of Neuroendovascular Therapy (JSNET)-certified specialists. The primary endpoint was functional independence (mRS score of 0-2) at 30 days post-NET, with secondary endpoints focusing on technical success and major adverse events within 30 days.
A total of 63,230 patients and 60,354 NET procedures from 166 participating centers were analyzed. During the study period, NET cases have consistently increased, with an increase in the proportion of elderly patients. A significant trend shift was observed in the distribution of NET procedures, with endovascular treatment for acute ischemic stroke that showed a dramatic increase in 5 years. This trend aligns with key randomized clinical trials from 2015 that presented the efficacy of this treatment. Clinical outcomes at 30 days posttreatment revealed that endovascular treatment for acute ischemic stroke and other NETs maintained safety and effectiveness despite varying prevalence of functional independence between target diseases. The study also observed a steady increase in emergency treatment cases, reflecting the increase in acute ischemic stroke, a time-sensitive medical condition.
This comprehensive surveillance highlights the trend of NET practices in Japan, driven by clinical evidence and advancements in treatment devices. Although these findings were specific to Japan, they offer valuable insights into the broader trends in NETs and acute stroke care.
Keywords: nationwide surveillance, endovascular treatment, registry study, clinical outcome, safety endpoint
Introduction
Since the previous reports from the Japanese Registry of Neuroendovascular Therapy (JR-NET) 3, which comprehensively summarized the annual trends of neuroendovascular treatment (NET) from 2010 to 2014 in Japan,1) some significant advancements have been made in both clinical evidence and treatment devices for NET. Notably, endovascular treatment (EVT) for acute ischemic stroke (AIS) because of large vessel occlusion emerged as an important treatment based on its efficacy proved in pivotal randomized clinical trials (RCTs) published in 2015.2-5) In 2018, the efficacy of EVT has also been demonstrated for patients with longer time windows from onset in the following RCTs based on patient selection via perfusion imaging.6,7) Additionally, after JR-NET 3, new embolic materials for treating shunt-related diseases and a new device, a flow diverter for cerebral aneurysms, were introduced in Japan. To keep up with these advancements, the Japanese Society of Neuroendovascular Therapy (JSNET), which includes 2,209 specialists on September 1, 2023, has continued to conduct nationwide surveillance, JR-NET 4.
This main report of JR-NET 4 aimed to present the latest trends in NETs in Japan from January 2015 to December 2019, following the JR-NET 1 and 2 and JR-NET 3.
Materials and Methods
I. Study design and participants
JR-NET 4 was a nationwide surveillance that registered patients who received NETs at 166 participating centers in Japan from January 2015 to December 2019. The study protocol summarized briefly here is available online with the full text (http://www.jrnet.umin.jp). All members of the writing committee assumed responsibility for ensuring the accuracy and completeness of the data, as well as the study's adherence to the protocol. Before the investigators proceeded with the study, the institutional review board of the principal investigator at Kobe City Medical Center General Hospital on December 18, 2019, and the local institutional review boards at each participating center approved the study protocol.
Consecutive patients who received NET from JSNET-certificated specialists as practitioners, supervisors, or assistants during the study period were eligible for JR-NET 4. Even for NETs carried out in the participating centers, cases that did not involve JSNET specialists were excluded from the registry. Any patients considered ineligible judged by physicians were also excluded.
II. Data collection
Using a registration system developed by the Translational Research Informatics Center (http://www.tri-kobe.org/), patient information and clinical data were anonymized and retrospectively registered on the web.
We collected data on patient characteristics (age, sex, modified Rankin scale [mRS] score before the procedure8)), details of NETs (either elective or emergency procedure, target diseases, anesthesia methods, operative methods, the type of practitioner [senior trainer and specialist certified by JSNET, or nonspecialist], and the number of scrub-in physicians), and clinical outcomes (mRS at 30 days after NETs, achievement of technical success, and adverse events within 30 days).
A score of 0 on mRS indicates no disability. Contrarily, scores of 1 or 2 indicate slight disability (some help required with ADL but independent), scores of 3-5 indicate moderate to severe, with varying levels of assistance required, and a score of 6 indicates death.8)
III. Primary and secondary endpoints
The primary endpoint of this study was functional independence after NETs, defined as an mRS score of 0-2 at 30 days. The secondary endpoints included the achievement of technical success during the NETs and the occurrence of major adverse events (MAEs) within 30 days following NETs.
Adverse events (AEs) were categorized as either minor or major based on changes in mRS scores, with an increase of one point constituting a minor event and an increase of two or more points indicating a major event. This classification is consistent with that used in JR-NET 1 and 2 and JR-NET 3.1,9)
IV. Statistical analysis
Categorical variables were presented as numbers and percentages and compared using a chi-square test. Continuous variables were presented as mean and standard deviation or median and interquartile range. Statistical analyses were performed using JMP 15.0.2 software (SAS Institute, Cary, North Carolina, USA). The statistical significance of intergroup differences was assessed using the t-test for quantitative scales, Pearson's χ2 test; p < 0.05 was considered significant.
Results
Patients' characteristics
In this study (Table 1), involving 749 cumulative total number of board-certified physicians at 166 centers in JR-NET 4 (shown in Appendix), we analyzed 63,230 patients (mean age, 67.0 ± 14.7 years; female, 50.3%; mRS 1 [1-2]). The total number of NET cases has been increasing steadily every year from 10,257 cases in 2015 to 15,530 in 2019 (Table 1). As for the age distribution of patients, the proportion of elderly patients aged ≥80 years increased from 16.1% in 2015 to 21.9% in 2019 (p < 0.001); conversely, the proportion of younger patients aged <40 years decreased from 5.0% in 2015 to 4.1% in 2019 (p = 0.002) (Fig. 1A).
Table 1.
Annual trends of JR-NET 4 data
| 2015 (n = 10,257) | 2016 (n = 11,019) | 2017 (n = 12,551) | 2018 (n = 13,873) | 2019 (n = 15,530) | Total (n = 63,230) | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 66.0 (14.6) | 66.3 (14.6) | 66.9 (14.7) | 67.4 (14.8) | 67.9 (14.8) | 67.0 (14.7) |
| Female, n (%) | 5,049 (49.3) | 5,610 (50.9) | 6,382 (50.9) | 6,998 (50.5) | 7,717 (49.8) | 31,756 (50.3) |
| mRS, median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) |
| Procedures | ||||||
| Aneurysm treatment, n (%) | 4,231 (41.3) | 4,599 (41.7) | 5,179 (41.3) | 5,554 (40.1) | 5,865 (37.8) | 25,428 (40.2) |
| Dome embolization, n (%) | 3,934 (38.4) | 4,334 (39.3) | 4,914 (39.2) | 5,266 (38.0) | 5,589 (36.0) | 24,037 (38.0) |
| Parental artery occlusion, n (%) | 297 (2.9) | 265 (2.4) | 265 (2.1) | 288 (2.1) | 276 (1.8) | 1,391 (2.2) |
| Angioplasty/stenting, n (%) | 2,365 (23.0) | 2,359 (21.5) | 2,611 (20.9) | 2,752 (19.9) | 3,126 (20.1) | 13,213 (20.9) |
| Carotid artery stenting, n (%) | 1,846 (18.0) | 1,862 (16.9) | 2,016 (16.1) | 2,117 (15.3) | 2,356 (15.2) | 10,197 (16.1) |
| Intracranial artery, n (%) | 259 (2.5) | 216 (2.0) | 286 (2.3) | 299 (2.2) | 391 (2.5) | 1,451 (2.3) |
| Extracranial artery, n (%) | 260 (2.5) | 281 (2.6) | 309 (2.5) | 336 (2.4) | 379 (2.4) | 1,565 (2.5) |
| Brain & spine AVM embolization, n (%) | 365 (3.6) | 302 (2.7) | 317 (2.5) | 361 (2.6) | 422 (2.7) | 1,767 (2.8) |
| DAVF embolization | 545 (5.3) | 618 (5.6) | 654 (5.2) | 684 (4.9) | 747 (4.8) | 3,248 (5.1) |
| Tumor embolization | 453 (4.4) | 517 (4.7) | 554 (4.4) | 601 (4.3) | 620 (4.0) | 2,745 (4.3) |
| Reperfusion therapy for acute ischemic stroke, n (%) | 1,757 (17.1) | 2,126 (19.3) | 2,689 (21.4) | 3,296 (23.8) | 4,085 (26.3) | 13,953 (22.1) |
| Practitioner | ||||||
| Senior trainers, JSNET-certified, median (IQR) | 1 (0-1) | 1 (0-1) | 1 (0-1) | 1 (0-1) | 1 (0-1) | 1 (0-1) |
| Specialists, JSNET-certified, median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) |
| Non-specialists, median (IQR) | 1 (1-2) | 2 (1-2) | 2 (1-2) | 2 (1-2) | 2 (1-2) | 2 (1-2) |
JR-NET, Japanese Registry of Neuroendovascular Therapy; AVM, arteriovenous malformation; DAVF, dural arteriovenous fistula; JSNET, Japanese Society of Neuroendovascular Therapy
Fig. 1.
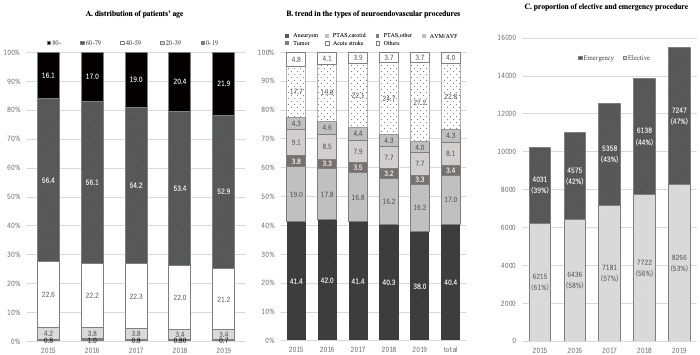
Annual changes during JR-NET 4.
(A) Distribution of patients’ age during JR-NET 4. (B) The types of neuroendovascular procedures. (C) Proportion of elective and emergency procedures of neuroendovascular procedures. JR-NET: Japanese Registry of Neuroendovascular Therapy
Procedures
During the study period, a total of 60,354 NET procedures were registered in JR-NET 4, of which 24,037 cases (40.2%) were treatments for cerebral aneurysms; 13,213 cases (20.9%) were angioplasty or stenting for the carotid artery, vertebral artery, or subclavian artery (VA/SCA); 1,767 cases (2.8%) were for arteriovenous malformations (AVMs) of brain and spine; 3,248 cases (5.1%) were for dural arteriovenous fistulas (DAVFs); 2,745 cases (4.3%) were tumor embolization; and 13,953 cases (22.1%) were EVT for AIS. Among these procedures, the proportion of EVT for AIS dramatically increased from 17.1% in 2015 to 26.3% in 2019 (p < 0.001) (Fig. 1B). The proportion of emergency treatment cases also increased year by year, that is, from 39% in 2015 to 47% in 2019 (Fig. 1C), and this trend has continued since the study period of JR-NET 3. The proportion of NET procedures with JSNET-certified senior trainers, specialists, and non-specialists in charge remained relatively constant during the study period (Table 1).
mRS scores before and after the procedure
More than 90% of patients had mRS scores of 0-2 before the procedure, which indicated no symptoms or slight disability (Fig. 2). At 30 days after NETs, the patients who maintained mRS scores of 0-2 decreased from 71.4% in 2015 to 66.3% in 2019 (Fig. 2). Figure 3 shows the clinical outcomes of each type of treatment based on mRS scores at 30 days. The data indicate favorable outcomes (mRS 0-2) for 53.5% of patients with ruptured aneurysms and 95.1% with unruptured aneurysms. Over 85% of patients treated via angioplasty and stenting for carotid artery, VA/SCA, DAVF embolization, and tumor embolization achieved favorable outcomes at 30 days. Conversely, 66.9%, 71.3%, and only 33.1% of those treated for intracranial artery disease, AVM, and AIS had an mRS score of 0-2 at 30 days.
Fig. 2.
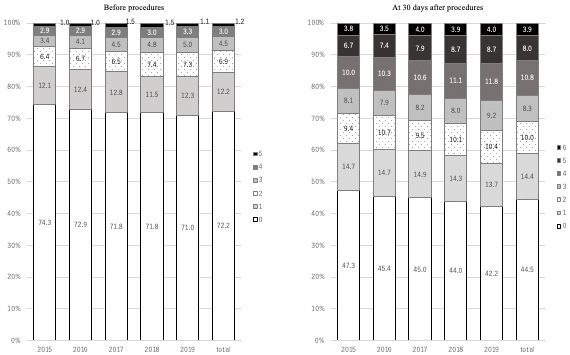
Proportions of modified Rankin scale (mRS) scores before and after procedures.
Fig. 3.
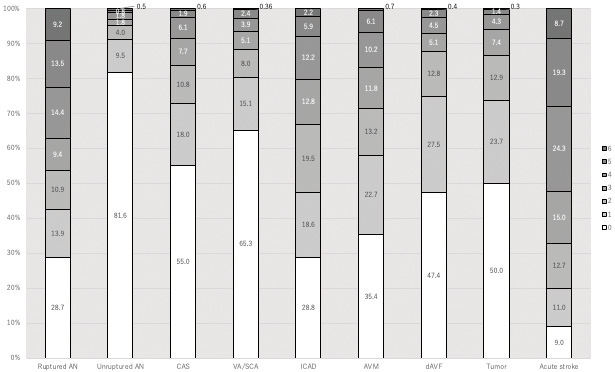
Proportions of modified Rankin scale (mRS) scores at 30 days after each procedure.
Adverse events after endovascular treatment
Figure 4 presents the frequency of procedural AEs after each type of NET. Death and MAEs that worsened mRS score by 2 or more occurred in 3.9% and 1.4% of patients treated for ruptured and unruptured aneurysms, respectively. MAEs and death mostly occurred in EVT for AIS and ruptured aneurysm, approximately 3%-4%. In another procedure, the occurrence of death and MAEs was almost approximately 1%-2%.
Fig. 4.
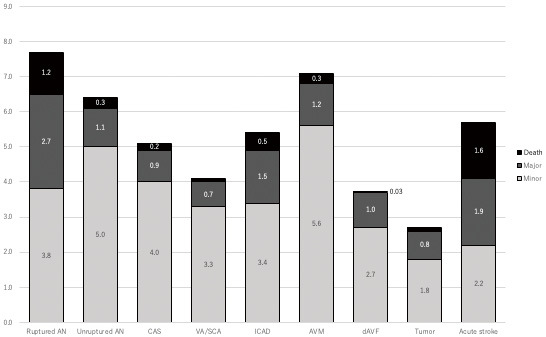
Adverse events associated with each procedure.
Discussion
This nationwide surveillance from 2015 to 2019, following JR-NET 1 and 2 (registered from January 2005 to December 2006 and January 2007 to December 2009) and JR-NET 3 (from January 2010 to December 2014), revealed the recent status of NETs in Japan. The number of registered cases has steadily increased year by year, with the proportion of EVT for AIS and emergency treatment cases rising significantly. Clinical outcomes 30 days after treatments were relatively poorer in patients who underwent EVT for AIS, but the efficacy and safety of overall NETs were almost maintained.
During the study period, the notable trend shift in NETs was the increase in EVT for AIS. In 2015, key RCTs highlighted the efficacy of EVT for acute large vessel occlusion compared with medical treatment,2-5) which leads to its endorsement as a Grade A recommendation in guidelines by the Japan Stroke Society and the American Heart Association within the same year.10) Additionally, the DAWN and DEFUSE 3 trials, published in 2018, demonstrated the efficacy of EVT in patients with longer time windows, up to 16 or 24 h after onset or last known well, using patient selection based on perfusion imaging.6,7) Even after JR-NET 4, the efficacy of EVT has also been exhibited in patients with large ischemic cores and with posterior circulation LVO.11-13) Therefore, EVT for AIS would continue to increase. The results of this study reflect not only the increased contribution of NETs in recent acute stroke care but also the changing social needs for NETs in Japan as a consequence. EVT for AIS often requires emergency treatment because of its high time dependency,14) which likely led to some changes in the working practices of neurovascular interventionists. Appropriate placement of specialists, stroke center certification, or the enhancement of a prehospital stroke patient transport system would be needed to provide this important treatment sustainably and uniformly to citizens.
In terms of the advancements of devices, during the study period, some new devices for NETs were approved in Japan, such as Onyx Liquid Embolic System (Medtronic, Minneapolis, Minnesota, USA) for dural arteriovenous fistula treatment, flow diverters for aneurysm treatment (Pipeline Flex with Shield Technology [Medtronic, Minneapolis, Minnesota, USA] approved in Japan in 2015, and FRED [Microvention, Tustin, California, USA] in 2019), and stent retriever for AIS (REVIVE SE [Codman Neuro/DePuy Synthes, Raynham, Massachusetts, USA]). An important change from the JR-NET 3 era is the improvement in the “device lag.” In Japan, until around 2010, the delay in introducing medical devices was a social issue compared to Western countries, so-called device lag. However, efforts by the Ministry of Health, Labour and Welfare to shorten the approval process for medical devices and activate clinical trials using new devices initiated in Japan have resolved this issue. The significant contribution of JSNET in this regard should be noted. However, there is still a “drug gap” between Japan and Western countries in drugs used in NETs, such as GP IIb/IIIa inhibitor, Tenecteplase, or Ticagrelor, which should be considered a next issue to address in the future.
This study is not without some limitations. First, considering that this study was retrospective surveillance, detailed data regarding individual patients or long-term outcomes were not acquired. Second, this study covered approximately 40% of total NET cases in Japan, and the survey's completeness was insufficient. However, there was no significant change in the number and composition of participating facilities in JR-NET 4 compared to JR-NET 3. Within this context, we were able to identify a shift in trends. Therefore, it is believed that this shift accurately reflects the general tendencies across Japan to a certain extent. Finally, this study was conducted in Japan, and its results may not necessarily apply to other countries or regions.
Conclusions
This study showed that NETs conducted in Japan have maintained certain safety and effectiveness and that the presence of EVT for AIS was rapidly increasing. Details regarding each NET or target disease will be described in the subanalyzes of JR-NET 4. Continuing the surveillance to document the recent status of NETs will also be an important theme of this JR-NET, as it would lead to the discovery of new medical needs in the field of neurosurgery in Japan.
Sources of Funding
This study was endorsed by JSNET on November 20, 2019, and partly supported by a research grant from the JSNET in 2019 and 2020, and by the Kobayashi Foundation in 2019.
Conflicts of Interest Disclosure
All authors who are members and nonmembers of the Japan Neurosurgical Society (JNS) have registered self-reported COI disclosure statements through the JNS website. Nobuyuki Sakai reports a research grant from Biomedical Solutions, Medtronic, Terumo, and TG Medical; lecturer's fees from Asahi-Intec, Biomedical Solutions, Daiichi-Sankyo, Kaneka, Medtronic, and Terumo; membership on the advisory boards for Johnson & Johnson, Medtronic and Terumo outside the submitted work. Other coauthors have no conflict of interest for this manuscript.
Supplementary Material
Acknowledgments
The JR-NET 4 Study Group: Principal investigator; Nobuyuki Sakai, Kobe City Medical Center General Hospital, Kobe, Japan; Investigators: Koji Iihara and Hirotoshi Imamura, National Cerebral and Cardiovascular Center, Suita, Osaka, Japan, Akira Ishii, Kyoto University, Kyoto, Japan, Yuji Matsumaru, Tsukuba University, Tsukuba, Ibaraki, Japan, Chiaki Sakai, Kobe City Medical Center General Hospital, Kobe, Japan, Tetsu Satow, Kindai University, Osaka-Sayama, Japan, Shinichi Yoshimura, Hyogo Medical University, Nishinomiya, Hyogo, Japan, and Certified Specialist of Japanese Society of Neuroendovascular Therapy.
References
- 1).Sakai N, Uchida K, Iihara K, et al. : Japanese surveillance of neuroendovascular therapy in JR-NET - part II. Japanese registry of NeuroEndovascular treatment 3. Main report. Neurol Med Chir (Tokyo) 59: 106-115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Berkhemer OA, Fransen PS, Beumer D, et al. : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11-20, 2015 [DOI] [PubMed] [Google Scholar]
- 3).Saver JL, Goyal M, Bonafe A, et al. : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372: 2285-2295, 2015 [DOI] [PubMed] [Google Scholar]
- 4).Goyal M, Demchuk AM, Menon BK, et al. : Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372: 1019-1030, 2015 [DOI] [PubMed] [Google Scholar]
- 5).Campbell BC, Mitchell PJ, Kleinig TJ, et al. : Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372: 1009-1018, 2015 [DOI] [PubMed] [Google Scholar]
- 6).Albers GW, Marks MP, Kemp S, et al. : Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378: 708-718, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Nogueira RG, Jadhav AP, Haussen DC, et al. : Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378: 11-21, 2018 [DOI] [PubMed] [Google Scholar]
- 8).van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J: Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19: 604-607, 1988 [DOI] [PubMed] [Google Scholar]
- 9).Sakai N, Yoshimura S, Taki W, et al. : Recent trends in neuroendovascular therapy in Japan: analysis of a nationwide survey--Japanese registry of neuroendovascular therapy (JR-NET) 1 and 2. Neurol Med Chir (Tokyo) 54 Suppl 2: 1-8, 2014 [DOI] [PubMed] [Google Scholar]
- 10).Powers WJ, Derdeyn CP, Biller J, et al. : 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46: 3020-3035, 2015 [DOI] [PubMed] [Google Scholar]
- 11).Yoshimura S, Sakai N, Yamagami H, et al. : Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med 386: 1303-1313, 2022 [DOI] [PubMed] [Google Scholar]
- 12).Jovin TG, Li C, Wu L, et al. : Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med 387: 1373-1384, 2022 [DOI] [PubMed] [Google Scholar]
- 13).Tao C, Nogueira RG, Zhu Y, et al. : Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med 387: 1361-1372, 2022 [DOI] [PubMed] [Google Scholar]
- 14).Goyal M, Menon BK, van Zwam WH, et al. : Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387: 1723-1731, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


