Abstract
Background: A renal biopsy is essential for the identification and management of renal disorders. Although considered an invasive operation, it is necessary for a definitive diagnosis and treatment of many renal diseases. The primary goal of this study was to assess the clinicopathological aspect of renal diseases undergoing biopsy in children receiving tertiary care.
Patients and Methods: Children (≤18 years) hospitalized with nephrotic syndrome were the subjects of this cross-sectional study, and comprehensive assessments confirmed the need for a kidney biopsy. Included were 277 children who met the inclusion and exclusion criteria. Data on patient outcomes, biopsy indications, complications, histopathologic results, and demographic information were documented.
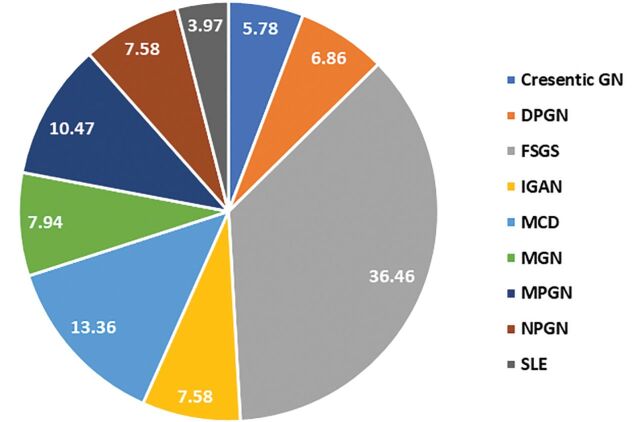
Results: Of the 277 patients who underwent renal biopsy, 63.2% were male, and 36.8% were female. Average age of the patients was 15 ± 2.9 years, with age distribution ranging from 3 to 18 years. The most frequent indication for renal biopsy was atypical age of <1 and >10-years-old (91.7%), steroid-resistant (5.1%), asymptomatic hematuria (21.3%), abnormal glomerular filtration rate (16.2%), and proteinuria (14.8%). The most common histopathological findings were focal segmental glomerulosclerosis (FSGS) (36.5%), followed by minimal change disease (MCD) (13.4%), membranoproliferative glomerulonephritis (MPGN) (10.5%), membranous glomerulonephritis (MGN) (7.94%), IgA nephropathy (IGAN) (7.58%), non-proliferative glomerulonephritis (NPGN) (7.58%), diffuse proliferative glomerulonephritis (DPGN) (6.9%), crescentic GN (5.8%), and systemic lupus erythematosus (SLE) (3.97%). The high frequency of positive samples was seen in SLE, followed by DPGN, MPGN, IGAN, and MGN. In contrast, MCD, crescentic GN, and NPGN showed negativity in all differential item functioning (DIF) parameters.
Conclusion: Renal biopsy is a safe and effective procedure in the diagnosis and treatment of in children with nephrotic syndrome. FSGS had the highest frequency in examined biopsies.
Keywords: Children, Histopathological diagnosis, Nephrotic syndrome, Renal biopsy
One of the most common pediatric kidney diseases is childhood nephrotic syndrome, which affects 1–17 children per 100,000 and 16 children per 100,000 under the age of 16.1 Hyperlipidemia, low albumin, widespread edema, and severe proteinuria are the hallmarks of nephrotic syndrome, a kidney disease.2 In children, renal disorders are highly prevalent and a substantial cause of morbidity. A frequent kidney condition in children, idiopathic nephrotic syndrome varies in its patient features throughout different regions of the world.3–5 Depending on the area and ethnicity, 1.15-16.9 children per 100,000 have idiopathic nephrotic syndrome.1 The general frequency of nephrotic syndrome varies by geographic location, with a 6-fold increase in the Indian population as compared to children in Europe.6
Numerous physiological systems, including the gastrointestinal, central nervous system, endocrine, and cardiovascular, are severely impacted by chronic kidney disease. The pathologist faces a great deal of difficulty in diagnosing and classifying renal illness because of the complexity and variety of these diseases.7,8 At first, the illness shows no symptoms, but as kidney function steadily declines, symptoms including exhaustion, high blood pressure, and stunted development are seen. Kidney illnesses of all kinds can affect children anywhere. These illnesses or their symptoms include a broad spectrum of presentations, such as kidney involvement in metabolic disorders, acute glomerulonephritis, vesicoureteral reflux, hematuria, acute kidney damage, and nephrotic syndrome.9–11
Adults with nephrotic syndrome have a more diverse etiology than children. To confirm the histologic diagnosis, kidney biopsies are carried out on adults at an early stage of the illness. Invasive kidney biopsies provide a risk of bleeding, especially in younger patients. Regarding the rationale for kidney biopsies for childhood-onset nephrotic syndrome, medical professionals have recently held divergent views. Kidney biopsies should be considered when a child is older than 10 years-of-age or under the age of one and exhibits extrarenal symptoms including purpura, hypertension, renal failure, and persistent hematuria.12 For establishing a histological diagnosis, prognosis, and available treatments, renal biopsy is the gold standard.13 Renal biopsy is a vital technique for prognostication, therapy, and etiology in addition to patient diagnosis. It has been observed that in half of the cases, kidney biopsy results in a different initial diagnosis, and in one-third of cases, a different treatment plan. According to studies, the most popular percutaneous approach is safer and more successful when guided by ultrasonography.14,15 A number of non-invasive techniques for identifying early renal problems have been put forth recently; these techniques mostly include the use of omics technologies (genomics, proteomics, and metabolomics) to assess plasma and urine biomarkers. The usefulness of these indicators in patient diagnosis and therapy, however, is still being investigated. Several research investigations have demonstrated that biopsy enhances clinical procedures for individuals with kidney problems.9,16
Patients and Methods
The purpose of this study was to assess the clinicopathological aspect of renal disorders in children receiving tertiary treatment by biopsy This was a hospital based, observational, cross-sectional study in a tertiary care institute. Demographic information including gender and age, clinical symptoms before kidney biopsy, clinical diagnosis, indications for renal biopsy, and histopathological findings were documented in predesigned forms.
KDIGO 2021 guidelines17 were followed in developing the diagnostic criteria for pediatric nephrotic syndrome used in the present study. Nephrotic syndrome, extensive hematuria, severe proteinuria, decreased kidney function, and a low level of C3 that persists for more than 2 months were all regarded indicators that a kidney biopsy was necessary in children with acute nephritic syndrome. Moreover, patients with atypical age at presentation (age <12 months or >10 years),18,19 as well as patients with persistent hematuria with proteinuria underwent kidney biopsy. Infants under 1-year-old and over 18-years-old, parents who did not provide consent, patients with bleeding diathesis and abnormal coagulation parameters, uncontrolled hypertension, acute pyelonephritis, single kidney, and severe anemia were among the exclusion criteria that would exclude them from the study.
Children who met the criteria for renal biopsy had their kidneys biopsied after their parents provided informed permission. A surgeon performed an open kidney biopsy on patients who were younger than a year old. A pediatric nephrologist used real-time sonography guidance to do needle biopsies on other children (those older than 1-year). Every patient had two samples obtained from them. While some of the samples underwent immunofluorescence analysis, all of them underwent periodic acid-Schiff (PAS) and hematoxylin and eosin (H&E) staining. A pathologist assessed these samples following staining. Following kidney biopsy, a pediatric nephrologist monitored each child. Histologic diagnoses were classified into the following groups: Crescentic glomerulonephritis, focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), membranoproliferative glomerulonephritis (MPGN), diffuse proliferative glomerulonephritis (DPGN), IgA nephropathy (IGAN), membranous glomerulonephritis (MGN), non-proliferative glomerulonephritis (NPGN), and systemic lupus erythematosus (SLE).
Statistical Analysis
For statistical analysis data were compiled into a Microsoft excel spreadsheet and then analyzed using STATA version 17. Continuous data were expressed as means or medians with standard derivations or ranges, as appropriate. Also, categorical variables were shown as numbers and percentages and compared using chi-square test. A P value <0.05 was considered statistically significant.
Results
Demographic Data and Clinical Presentation
Of the 277 patients who underwent renal biopsy, 175 (63.2%) were male, and 102 (36.8%) were female. The average age of the patients was 15 ± 2.9 years, with age distribution ranging from 3 years to 18 years. During the procedure, 47 patients had edema, 23 patients had Stage-I hypertension, and 17 patients had rash. The laboratory examinations revealed proteinuria in 41 patients, hematuria in 59 patients, and anemia in 15 patients. The serum creatinine levels ranged from 24 to144 (11.8 ± 1.8) μmol/L, urine protein from 1.8 to 3.8 (40.1 ± 9.5) g/L, and albumin from 14 to 62 (40.1 ± 9.5) g/L. The mean glomerular filtration rate was 81.5 ± 9.5. The mean triglycerides and cholesterol of the study subjects were 128.4 ± 51.3 mg/dL and 172.5 ± 80.7 mg/dL, respectively (Table 1). In addition, we found an increase in serum C3 and C4 in 13% and 12% of cases, respectively.
Table 1.
Patient demographics
| Characteristics | Variable | Number | Percentage |
|---|---|---|---|
| Gender | Male | 175 | 63.2 |
| Female | 102 | 36.8 | |
| Age at biopsy, mean in years (SD) | 15 ± 2.9 (Range 3-18) | ||
| Age categories | 1-5 years | 5 | 1.8 |
| 6-10 years | 18 | 6.5 | |
| 11-15 years | 109 | 39.4 | |
| 16-18 years | 145 | 52.4 | |
| Blood pressure | Normal | 114 | 41.2 |
| Pre-Hypertension | 140 | 50.5 | |
| Stage-I HTN | 23 | 8.3 | |
| Hemoglobin (g/dL), mean ± SD | 11.8 ± 1.8 (Range 5.6-16.8) | ||
| Serum creatinine (μmol/L), mean ± SD | 67.3 ± 15 (Range 24-144) | ||
| Urine protein (g/L), mean ± SD) | 40.1 ± 9.5 (Range 1.8-3.84) | ||
| Serum albumin (g/L), mean ± SD | 40.1 ± 9.5 (Range 14-62) | ||
| Glomerular filtration rate (mL/min/1.73 m2), mean ± SD | 81.5 ± 9.5 (Range 0-94) | ||
| Triglycerides (mg/dL), mean ± SD | 128.4 ± 51.3 (Range 30-337) | ||
| Cholesterol (mg/dL), mean ± SD | 172.5 ± 80.7 (Range 98-628) | ||
| Edema | Present | 47 | 17 |
| Absent | 230 | 83 | |
| Hematuria | Present | 59 | 21.3 |
| Absent | 218 | 78.7 | |
| Hemoptysis | Present | 76 | 27.4 |
| Absent | 201 | 72.6 | |
| Rashes | Present | 17 | 6.1 |
| Absent | 260 | 93.9 | |
| Complement C3 | Increased | 36 | 13 |
| Normal | 241 | 87 | |
| Complement C4 | Increased | 25 | 9 |
| Normal | 252 | 91 | |
| Antinuclear antibody (ANA) | Positive | 12 | 4.3 |
| Negative | 265 | 95.7 | |
| Anti-dsDNA | Positive | 11 | 4 |
| Negative | 266 | 96 | |
| Anti-neutrophil cytoplasmic antibody (ANCA) | Positive | 17 | 6.1 |
| Negative | 260 | 93.9 | |
| Anti-Glomerular Basement Membrane antibodies (Anti-GBM) | Positive | 0 | 0 |
| Negative | 277 | 100 | |
| Direct immunofluorescence (DIF)- Full-house pattern | Present | 19 | 6.9 |
| Absent | 258 | 93.1 |
Of 277 patients with nephrotic syndrome, the most frequent indication for renal biopsy was age ˂1-year-old and ˃10-years-old (91.7%), 14 cases (5.1%) were steroid-resistant. Asymptomatic hematuria was the indication in 21.3%, abnormal glomerular filtration rate in 16.2%, and proteinuria in 14.8% of cases. The frequency of indications for renal biopsy in patients with nephrotic syndrome is presented in Table 2.
Table 2:
Indications for kidney biopsy in children with nephrotic syndrome
| Indications for kidney biopsy | Values |
|---|---|
| Age >10 years | 254/277 (91.7%) |
| Hematuria | 59/277 (21.3%) |
| Proteinuria | 41/277 (14.8%) |
| Abnormal glomerular filtration rate | 45/277 (16.2%) |
| Hypertension or hematuria or elevated serum creatinine level | 82/277 (29.6%) |
| Nephritis (hypertension and hematuria and elevated serum creatinine level) | 1/277 (0.4%) |
| Steroid resistance | 14/277 (5.1%) |
Values in table are presented as the number of patients with the percentage in parenthesis
Histopathological Diagnosis
Of the 277 patients, the most common histopathological findings in kidney biopsied patients were FSGS (36.5%), followed by MCD (13.4%), MPGN (10.5%), MGN (7.94%), IGAN (7.58%), NPGN (7.58%), DPGN (6.9%), crescentic glomerulonephritis (5.8%), and SLE (3.97%). Refer to Figure 1 for histopathological findings of renal biopsy samples of the study population.
Figure 1.
Histopathological findings of renal biopsy samples of the study population. GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MPGN, membranoproliferative glomerulonephritis; DPGN, diffuse proliferative glomerulonephritis; IGAN, IgA nephropathy; MGN, membranous glomerulonephritis; NPGN, non-proliferative glomerulonephritis; SLE, systemic lupus erythematosus
Figure 2 depicts the distribution of children by age according to the pathological diagnoses. The most common idiopathic glomerular diseases were: FSGS (n=54) in the 16-18 age group and FSGS (n=36) in 11-15 age group. The most common diagnoses in the 1-5 years-of-age group were FSGS and NPGN. In crescentic glomerulonephritis in all age groups, majority of them were ANCA positive.
Figure 2.
Distribution of children by age according to the pathological diagnoses. GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MPGN, membranoproliferative glomerulonephritis; DPGN, diffuse proliferative glomerulonephritis; IGAN, IgA nephropathy; MGN, membranous glomerulonephritis; NPGN, non-proliferative glomerulonephritis; SLE, systemic lupus erythematosus
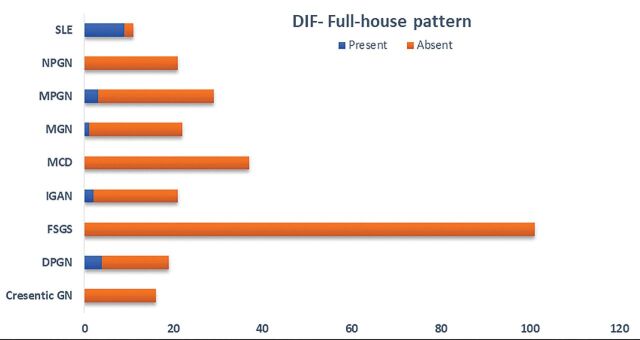
Figure 3 shows the presence of full-house pattern in main pathological findings during direct immunofluorescence. Overall, 19 (6.9%) cases were positive in immunofluorescence imaging. The high frequency of positive samples was seen in SLE, followed by DPGN, MPGN, IGAN, and MGN. Patients with lupus nephritis had a full-house pattern by immunofluorescence; i.e., concurrent positive staining for IgA, IgG, IgM, C3, and C1q. Maximum positivity was in IgG followed by C3 and C1q, respectively. Antinuclear antibody (ANA) was positive in 12 cases, and Anti-dsDNA was seen in 11 cases. In contrast, MCD, crescentic glomerulonephritis, and NPGN showed negativity in all DIF parameters.
Figure 3.
Direct immunofluorescence (DIF)- Full-house pattern in main pathological findings. GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MPGN, membranoproliferative glomerulonephritis; DPGN, diffuse proliferative glomerulonephritis; IGAN, IgA nephropathy; MGN, membranous glomerulonephritis; NPGN, non-proliferative glomerulonephritis; SLE, systemic lupus erythematosus
Discussion
Due to genetics, environmental factors, and illness awareness, childhood renal disorders are diverse and differ between geographical areas. Renal biopsy samples may frequently be histologically examined to determine the diagnosis, prognosis, and treatment plan for renal illness. Since its inception in 1934, percutaneous renal biopsy has been a commonly utilized procedure for the assessment of several nephropathies. It is among the most crucial instruments for making diagnoses, keeping track of, and directing therapy. However, there are a number of potential consequences, including infection, injury to surrounding organs, arteriovenous fistulas, perirenal hematoma, severe hematuria, and kidney loss.9,13,20,21 Renal biopsy is still very important in the therapy of pediatric renal illness, even with the advancements in laboratory and radiologic procedures.22 A kidney biopsy is a critical diagnostic technique that has a strong predictive value for a variety of adult and pediatric renal illnesses. Children’s lack of participation and their differing kidney sizes make kidney biopsies challenging. Nonetheless, doctors may now perform biopsies more easily due to the development of surgical procedures and the use of ultrasound-guided biopsy. Thus, it can be concluded that kidney biopsy is now a reliable and accurate diagnostic technique due to technological advancements.9,13
According to the findings of this study, age between ˂ 1 and ˃10 years was the most common reason for renal biopsy (91.7%), and 14 instances (5.1%) involved steroid resistance. In 21.3% of instances, asymptomatic hematuria, in 16.2% of cases, aberrant glomerular filtration rate, and in 14.8% of cases, proteinuria was the cause. In a study conducted in Iran, steroid resistance (28.84%) was reported to be the most frequent reason for renal biopsy, followed by age between ˂1 and ˃10 years (23.07%).23 About 65% of kidney biopsies in the Indian research by Nammalwar et al24 were due to steroid-resistant nephrotic syndrome. According to this study, FSGS (36.5%) was the most commonly seen histological finding in kidney biopsied individuals. This was followed by MCD (13.4%), MPGN (10.5%), MGN (7.94%), IGAN (7.58%), NPGN (7.58%), DPGN (6.9%), crescentic glomerulonephritis (5.8%), and SLE (3.97%). Recent years have seen a rise in the occurrence of FSGS in children with nephrotic syndrome.25,26 Our study’s outcome is in line with these studies. According to Alhasan et al,14 lupus nephritis was the most prevalent cause identified in 20.7% of the patients, whereas secondary glomerulonephritis accounted for 42.3% of the cases. In a study, the histological diagnoses of the patients were: 1.4% congenital NS, 4.2% lgM nephropathy, 2.8% MesGN, 2.8% hereditary nephritis, 25% MCD, 44.4% FSGS, and 2.8% other causes.2
Primary glomerulonephritis is the most commonly identified diagnosis in investigations assessing histopathological diagnoses in both pediatric and adult patients. Apart from this FSGS, MPGN, and IgAN are also commonly observed diagnosis.27 The disparities in biopsy indications caused by age, geographic region, and ethnicity are assumed to be the cause of these discrepancies in biopsy diagnosis. In another study with 60 cases (16.5%) having the most prevalent histological diagnosis, FSGS came in second with 7.7% of cases, followed by MesPGN with 7.7% and IgAN with 7.7% of cases.15
Classic glomerulonephritis signs and symptoms such as proteinuria, hemoglobinuria, and hypertension are usually evident during a clinical assessment. For the majority of patients, complement dysregulation may be identified by measuring complement C3 and C4 and doing an autoantibody screen. The absence of substantial immunoglobulin deposits can be used to differentiate immune-complex glomerulonephritis from C3 glomerulopathy by further renal biopsy.29 We showed elevated blood levels of C3 and C4 were a significant predictor of prognosis in IgAN patients. Predicting the renal prognosis of these individuals can be enhanced by combining the analysis of serum C3 and C4 levels into a single model.
Light microscopy only provides the morphological pattern; thus, to obtain more accurate results, we must connect the results of light microscopy with clinical aspects using electron microscopy and immunofluorescence. In every kidney biopsy, direct immunofluorescence microscopy was performed. Positive samples showed a high frequency of C3, IgG, IgA, IgM, and C1q, in that order. By binding fluorescein-labelled antibodies to kidney-specific antigens or immunoglobulins in biopsy specimens, the DIF can identify the presence of antigens or immunoglobulins and, thus, aid in the diagnosis of immune-related disorders in kidney pathology. Without DIF, conditions such as C1q nephropathy, C3 glomerulopathy, and IgA nephropathy cannot be detected. 6.9% of the patients in our research had positive immunofluorescence imaging results. SLE had the highest frequency of positive samples, with DPGN, MPGN, IGAN, and MGN following closely after. Immunofluorescence analysis revealed a full-house pattern in lupus nephritis patients or concomitant positive staining for IgA, IgG, IgM, C3, and C1q. IgG had the highest level of positivity, followed by C3 and C1q, in that order. Twelve cases had positive antinuclear antibodies (ANA), and eleven cases had positive anti-dsDNA tests. On the other hand, all DIF metrics displayed negative for MCD, crescentic glomerulonephritis, and NPGN. Most patients with crescentic glomerulonephritis across all age categories were positive for ANCA. Similar observations have been reported by Amatya et al, all the cases of SLE were positive for ANA and dsDNA.30
According to earlier research, problems after a kidney biopsy can range from 4% to 36%.31,32 By choosing the right needle size and limiting the number of biopsy attempts, the degree of problems can be decreased in relation to the patient’s size and age. Another reason for the variation in the frequency of problems is biopsies conducted by nephrologists or radiologists.33 A meta-analysis revealed children may safely undergo biopsy procedures, because fewer than 1% of patients required blood transfusions, and only 11% of patients experienced hematuria.34 As a result, kidney biopsies are regarded as a safe diagnostic technique with few adverse effects.35 The results of our study, which showed no cases of mortality or nephrectomy following renal biopsy, are similar to the findings of another study, which found no major consequences following renal biopsy.36
Conclusion
The gold standard for diagnosing, treating, and predicting the course of treatment for many renal pathologies—particularly glomerular and systemic disorders that call for prompt and effective medical attention—is renal biopsy. With early and adequate therapy, it allows treatments to slow down this process, particularly in individuals who may advance to end-stage renal failure. Histopathological results showed that FSGS was the most common finding in the samples under examination.
References
- 1.Mahamat Abderraman G, Djidita Hagré Y, Mahamat HA, et al. Idiopathic Nephrotic Syndrome in Children in Chad: Epidemiology and Clinical Outcomes. J Clin Med. 2023;12(24):7626. doi: 10.3390/jcm12247626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alatas C, Tabel Y, Elmas AT, Selcuk SZ.. Evaluation of Children with Nephrotic Syndrome: A Single- Center Experience. Annals of Nephrology. 2020;5(1):78-83. doi: 10.36959/832/404. [DOI] [Google Scholar]
- 3.Roy SM, Das MK, Basu S.. Clinicopathological spectrum of renal biopsies in children – A single center experience from Eastern India. Journal of Medical and Scientific Research. 2023;11(3):176-180. doi: 10.17727/JMSR.2023/11-33. [DOI] [Google Scholar]
- 4.Al Battashi M, Al Nadhairi Z, Al Riyami A, et al. Pediatric Kidney Biopsies in Oman: A Retrospective Study. Oman Med J. 2023;38(3):e503-e503. doi: 10.5001/omj.2023.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad OA, Elkalla NM, Moursi F.. Percutaneous Renal Biopsy in Egyptian Children: A Five-year Single-Center Experience. Saudi J Kidney Dis Transpl. 2022;33(1):106-110. doi: 10.4103/1319-2442.367803 [DOI] [PubMed] [Google Scholar]
- 6.Yadav S, Kandalkar B.. Epidemiology of Pediatric Renal Diseases and its Histopathological Spectrum – A Single-Center Experience from India. Saudi J Kidney Dis Transpl. 2021;32(6):1744-1753. doi: 10.4103/1319-2442.352437. [DOI] [PubMed] [Google Scholar]
- 7.Senan EM, Al-Adhaileh MH, Alsaade FW, et al. Diagnosis of Chronic Kidney Disease Using Effective Classification Algorithms and Recursive Feature Elimination Techniques. J Healthc Eng. 2021;2021:1004767. doi: 10.1155/2021/1004767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng C, Liu F.. Artificial intelligence in renal pathology: Current status and future. Bosn J Basic Med Sci. 2022;23(2):225-234. doi: 10.17305/bjbms.2022.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambella A, Barreca A, Biancone L, et al. Spectrum of Kidney Injury Following COVID-19 Disease: Renal Biopsy Findings in a Single Italian Pathology Service. Biomolecules. 2022;12(2):298. doi: 10.3390/biom12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivarelli M, Gibson K, Sinha A, Boyer O.. Childhood nephrotic syndrome. Lancet. 2023;402(10404):809-824. doi: 10.1016/S0140-6736(23)01051-6. [DOI] [PubMed] [Google Scholar]
- 11.Mattoo TK, Sanjad S.. Current Understanding of Nephrotic Syndrome in Children. Pediatr Clin North Am. 2022;69(6):1079-1098. doi: 10.1016/j.pcl.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Kano Y, Takagi Y, Imataka G, Yoshihara S.. Treatment Strategy for Pediatric Patients with Nephrotic Syndrome with Microscopic Hematuria at the Onset: A Retrospective Study of the Need for Kidney Biopsy. Dokkyo Medical Journal. 2023;2(2):114-122. 10.51040/dkmj.2022-055. [DOI] [Google Scholar]
- 13.Schnuelle P. Renal Biopsy for Diagnosis in Kidney Disease: Indication, Technique, and Safety. J Clin Med. 2023;12(19):6424. doi: 10.3390/jcm12196424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhasan K, Aloudah NM, Bakhit AA, et al. Renal histopathology spectrum in children with kidney diseases in Saudi Arabia, 1998-2017. Saudi Med J. 2020;41(4):369-375. doi: 10.15537/smj.2020.4.24999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cakici EK, Yazilitas F, Under C, et al. Indications and Outcomes of Renal Biopsies in Children: A Single-Center 12-Year Experience. Turkish Journal of Nephrology. 2019;28(4):250-256. doi: 10.5152/turkjnephrol.2019.3664. [DOI] [Google Scholar]
- 16.Bernard L, Wang AR, Menez S, et al. ; Kidney Precision Medicine Project . Kidney Biopsy Utility: Patient and Clinician Perspectives from the Kidney Precision Medicine Project. Kidney Med. 2023;5(10):100707. doi: 10.1016/j.xkme.2023.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group . KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4) (4S):S1-S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Ehren R, Benz MR, Brinkkötter PT, et al. ; German Society for Pediatric Nephrology . Pediatric idiopathic steroid-sensitive nephrotic syndrome: diagnosis and therapy—short version of the updated German best practice guideline (S2e) — AWMF register no. 166-001, 6/2020. Pediatr Nephrol. 2021;36(10):2971-2985. doi: 10.1007/s00467-021-05135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekassy Z, Lindström M, Rosenblad T, Aradóttir S, Sartz L, Tullus K.. Is kidney biopsy necessary in children with idiopathic nephrotic syndrome? Acta Paediatr. 2023;112(12):2611-2618. doi: 10.1111/apa.16959. [DOI] [PubMed] [Google Scholar]
- 20.Pilania RK, Venkatesh GV, Nada R, et al. Renal Biopsy in Children—Effect on Treatment Decisions: A Single-Center Experience. Indian J Pediatr. 2021;88(10):1036-1039. doi: 10.1007/s12098-021-03721-9. [DOI] [PubMed] [Google Scholar]
- 21.Bakdash K, Schramm KM, Annam A, Brown M, Kondo K, Lindquist JD.. Complications of Percutaneous Renal Biopsy. Semin Intervent Radiol. 2019;36(2):097-103. doi: 10.1055/s-0039-1688422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Printza N, Bosdou J, Pantzaki A, et al. Percutaneous ultrasound-guided renal biopsy in children: a single centre experience. Hippokratia. 2011;15(3):258-261. [PMC free article] [PubMed] [Google Scholar]
- 23.Momtaz HE, Dehghan A, Fazeli A.. Pathologic findings of renal biopsies in children; an 11-year experience from a single center in West of Iran. Journal of Nephropathology. 2022;11(1):1-6. doi: 10.34172/jnp.2022.04. [DOI] [Google Scholar]
- 24.Nammalwar BR, Vijayakumar M, Prahlad N.. Experience of renal biopsy in children with nephrotic syndrome. Pediatr Nephrol. 2006;21(2):286-288. doi: 10.1007/s00467-005-2084-5. [DOI] [PubMed] [Google Scholar]
- 25.Shabaka A, Tato Ribera A, Fernández-Juárez G.. Focal Segmental Glomerulosclerosis: State-of-the-Art and Clinical Perspective. Nephron J. 2020;144(9):413-427. doi: 10.1159/000508099. [DOI] [PubMed] [Google Scholar]
- 26.Shoji J, Mii A, Terasaki M, Shimizu A.. Update on Recurrent Focal Segmental Glomerulosclerosis in Kidney Transplantation. Nephron J. 2020;144(Suppl. 1):65-70. doi: 10.1159/000510748. [DOI] [PubMed] [Google Scholar]
- 27.Su S, Yu J, Wang Y, Wang Y, Li J, Xu Z.. Clinicopathologic correlations of renal biopsy findings from northeast China: A 10-year retrospective study. Medicine (Baltimore). 2019;98(23):e15880. doi: 10.1097/MD.0000000000015880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdul-Aziz R, Deng R, Liu L, Tarsi S, Waz WR, Wu X.. Complete Renal Recovery in Pediatric Patient with C3 Glomerulonephritis: A Case Report. Case Rep Nephrol Dial. 2021;11(3):261-269. doi: 10.1159/000518714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amatya M, Pant AD.. Clinical and histopathological study of renal biopsy in Nepalese children: A single center experience. PLoS One. 2022;17(10):e0276172. doi: 10.1371/journal.pone.0276172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding JJ, Lin SH, Huang JL, et al. Risk factors for complications of percutaneous ultrasound-guided renal biopsy in children. Pediatr Nephrol. 2020;35(2):271-278. doi: 10.1007/s00467-019-04367-8. [DOI] [PubMed] [Google Scholar]
- 31.Hayatghaibi SE, Ashton DJ, Orth RC.. Pediatric percutaneous renal biopsies: comparison of complications between real-time ultrasound guidance and pre-procedure ultrasound-aided skin-marking techniques. Pediatr Radiol. 2019;49(5):626-631. doi: 10.1007/s00247-018-4321-7. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal S, Siddiqui WJ, Shahid N, et al. A Comparison between Kidney Allograft Biopsies Performed by Nephrologists and Surgeons Versus Interventional Radiologists. Cureus. 2019;11(12):e6315. doi: 10.7759/cureus.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varnell CD Jr, Stone HK, Welge JA.. Bleeding Complications after Pediatric Kidney Biopsy. Clin J Am Soc Nephrol. 2019;14(1):57-65. doi: 10.2215/CJN.05890518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettit C, Kanagaratnam R, Coughlan F, Graf N, Hahn D, Durkan A.. Kidney biopsy adequacy and complications in children - does technique matter?. Eur J Pediatr. 2022;181(7):2677-2684. doi: 10.1007/s00431-022-04464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalra S, Mukund B, Kumar M, Kanitkar M.. Comparative analysis of blind vs real-time ultrasound-guided pediatric renal biopsies: A cross-sectional study. Med J Armed Forces India. 2023;79(4):409-413. doi: 10.1016/j.mjafi.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]