Abstract
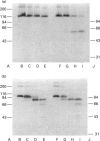
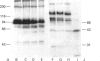
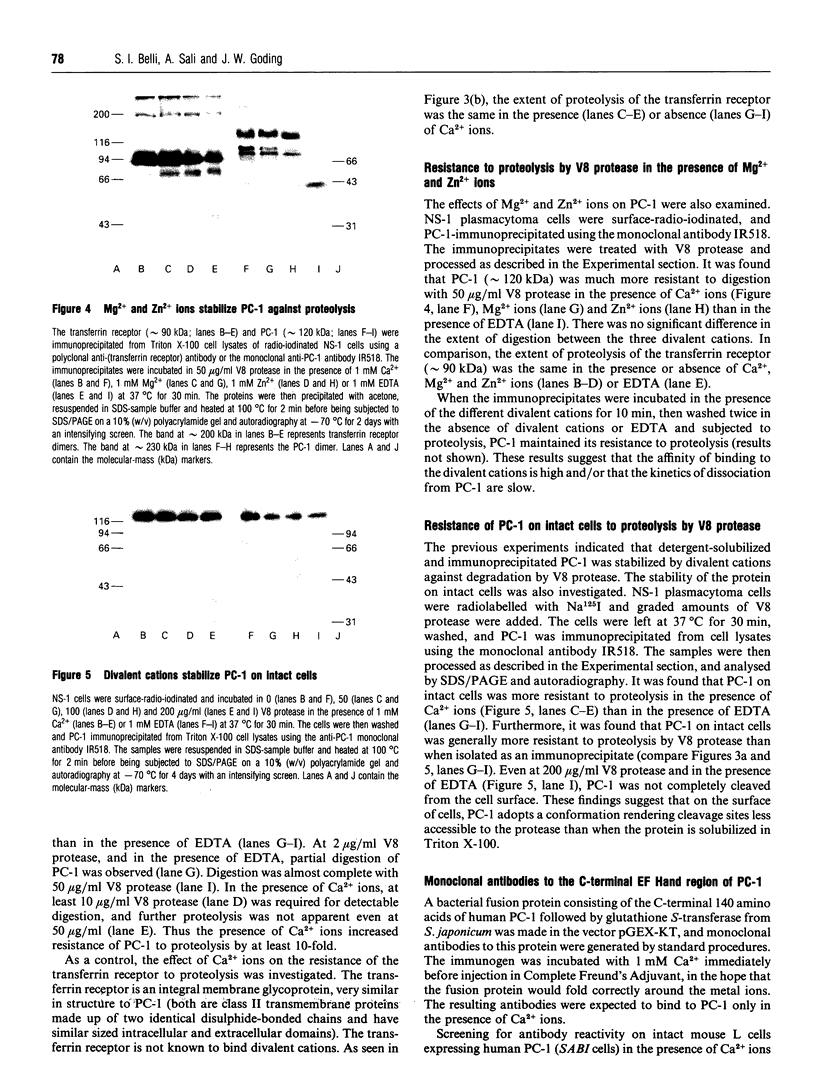
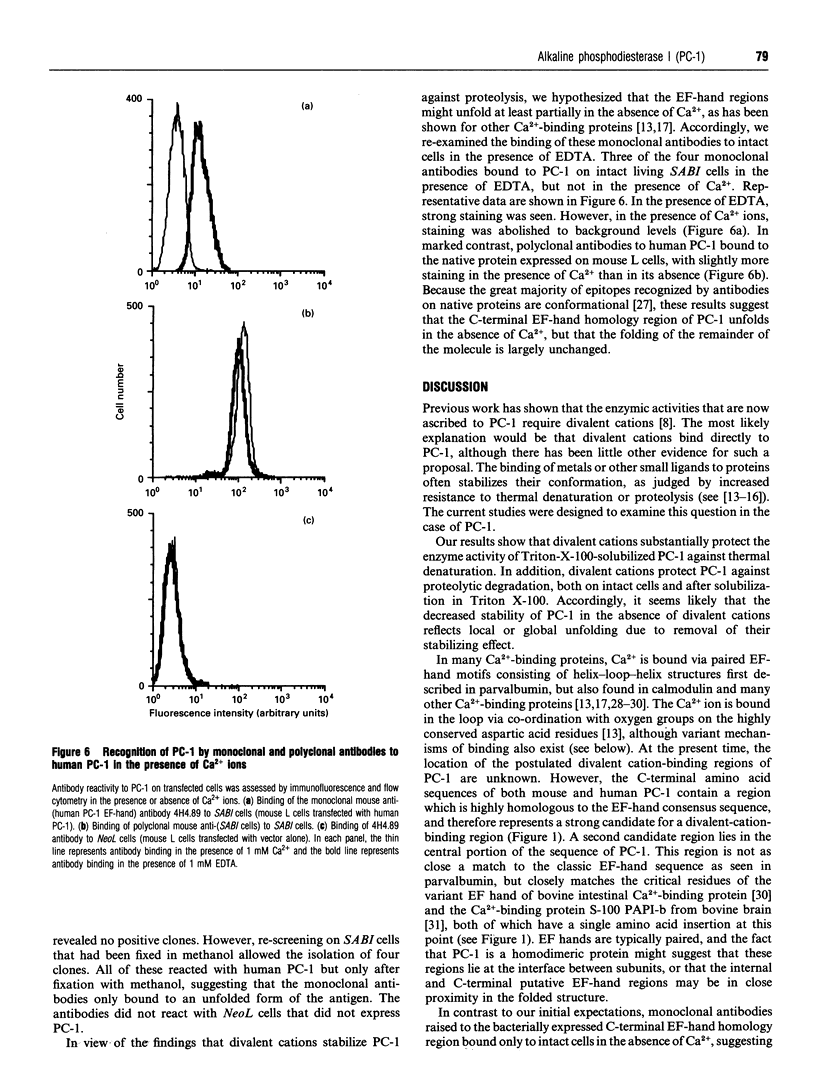
The plasma cell-membrane glycoprotein PC-1 is an ectoenzyme with alkaline phosphodiesterase I/5'-nucleotide phosphodiesterase (EC 3.1.4.1) and nucleotide pyrophosphatase (EC 3.6.1.9) activities. It contains sequence motifs which closely match the consensus EF-hand (helix-loop-helix) Ca(2+)-binding regions of parvalbumin, troponin-C and calmodulin, and its enzymic activity is increased in the presence of divalent cations and decreased in the presence of chelating agents. We have undertaken experiments to determine whether divalent cations affect the conformation of the PC-1 protein, as assessed by their effect on thermal stability, resistance to proteolysis and binding of polyclonal antibodies to the whole native protein and monoclonal antibodies to a putative Ca(2+)-binding region. Divalent cations were found to protect solubilized PC-1 against thermal denaturation and proteolysis. They also stabilized PC-1 on intact cells; this form was much more resistant to proteolysis than Triton X-100 solubilized PC-1. Ca2+, Mg2+ and Zn2+ ions were equally effective. Monoclonal antibodies to the bacterially expressed C-terminal EF-hand homology region only bound to mammalian PC-1 in the absence of Ca2+. In contrast, the great majority of polyclonal antibodies to native PC-1 bound regardless of whether Ca2+ was present or not, but with increased binding when Ca2+ was present. These results provide evidence that divalent cations bind to PC-1 and stabilize its conformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belli S. I., van Driel I. R., Goding J. W. Identification and characterization of a soluble form of the plasma cell membrane glycoprotein PC-1 (5'-nucleotide phosphodiesterase). Eur J Biochem. 1993 Oct 1;217(1):421–428. doi: 10.1111/j.1432-1033.1993.tb18261.x. [DOI] [PubMed] [Google Scholar]
- Buckley M. F., Loveland K. A., McKinstry W. J., Garson O. M., Goding J. W. Plasma cell membrane glycoprotein PC-1. cDNA cloning of the human molecule, amino acid sequence, and chromosomal location. J Biol Chem. 1990 Oct 15;265(29):17506–17511. [PubMed] [Google Scholar]
- Drapeau G. R., Boily Y., Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972 Oct 25;247(20):6720–6726. [PubMed] [Google Scholar]
- Evans W. H., Hood D. O., Gurd J. W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem J. 1973 Dec;135(4):819–826. doi: 10.1042/bj1350819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi I., Kato H., Horie K., Yano T., Hori Y., Kobayashi H., Inoue T., Suzuki H., Fukui S., Tsukahara M. Molecular cloning of cDNAs for human fibroblast nucleotide pyrophosphatase. Arch Biochem Biophys. 1992 May 15;295(1):180–187. doi: 10.1016/0003-9861(92)90504-p. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Shen F. W. Structure of the murine plasma cell alloantigen PC-1: comparison with the receptor for transferrin. J Immunol. 1982 Dec;129(6):2636–2640. [PubMed] [Google Scholar]
- Hakes D. J., Dixon J. E. New vectors for high level expression of recombinant proteins in bacteria. Anal Biochem. 1992 May 1;202(2):293–298. doi: 10.1016/0003-2697(92)90108-j. [DOI] [PubMed] [Google Scholar]
- Harahap A. R., Goding J. W. Distribution of the murine plasma cell antigen PC-1 in non-lymphoid tissues. J Immunol. 1988 Oct 1;141(7):2317–2320. [PubMed] [Google Scholar]
- Isobe T., Okuyama T. The amino-acid sequence of S-100 protein (PAP I-b protein) and its relation to the calcium-binding proteins. Eur J Biochem. 1978 Sep 1;89(2):379–388. doi: 10.1111/j.1432-1033.1978.tb12539.x. [DOI] [PubMed] [Google Scholar]
- Kelly S. J., Butler L. G. Enzymic hydrolysis of phosphonate esters. Biochem Biophys Res Commun. 1975 Sep 2;66(1):316–321. doi: 10.1016/s0006-291x(75)80330-5. [DOI] [PubMed] [Google Scholar]
- Kelly S. J., Dardinger D. E., Butler L. G. Hydrolysis of phosphonate esters catalyzed by 5'-nucleotide phosphodiesterase. Biochemistry. 1975 Nov 4;14(22):4983–4988. doi: 10.1021/bi00693a030. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Kuroki R., Kawakita S., Nakamura H., Yutani K. Entropic stabilization of a mutant human lysozyme induced by calcium binding. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6803–6807. doi: 10.1073/pnas.89.15.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki R., Taniyama Y., Seko C., Nakamura H., Kikuchi M., Ikehara M. Design and creation of a Ca2+ binding site in human lysozyme to enhance structural stability. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6903–6907. doi: 10.1073/pnas.86.18.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landt M., Butler L. G. 5'-Nucleotide phosphodiesterase: isolation of covalently bound 5'-adenosine monophosphate, an intermediate in the catalytic mechanism. Biochemistry. 1978 Oct 3;17(20):4130–4135. doi: 10.1021/bi00613a004. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Moss S. E., Crumpton M. J. The lipocortins and the EF hand proteins: Ca2(+)-binding sites and evolution. Trends Biochem Sci. 1990 Jan;15(1):11–12. doi: 10.1016/0968-0004(90)90118-u. [DOI] [PubMed] [Google Scholar]
- Pantoliano M. W., Whitlow M., Wood J. F., Rollence M. L., Finzel B. C., Gilliland G. L., Poulos T. L., Bryan P. N. The engineering of binding affinity at metal ion binding sites for the stabilization of proteins: subtilisin as a test case. Biochemistry. 1988 Nov 1;27(22):8311–8317. doi: 10.1021/bi00422a004. [DOI] [PubMed] [Google Scholar]
- Persechini A., Moncrief N. D., Kretsinger R. H. The EF-hand family of calcium-modulated proteins. Trends Neurosci. 1989 Nov;12(11):462–467. doi: 10.1016/0166-2236(89)90097-0. [DOI] [PubMed] [Google Scholar]
- Rebbe N. F., Tong B. D., Finley E. M., Hickman S. Identification of nucleotide pyrophosphatase/alkaline phosphodiesterase I activity associated with the mouse plasma cell differentiation antigen PC-1. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5192–5196. doi: 10.1073/pnas.88.12.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Skinner M. A. Murine plasma cell antigen PC-1 has a region homologous to the active site of bovine intestinal 5'-nucleotide phosphodiesterase I (EC 3.1.4.1). Nucleic Acids Res. 1991 Nov 11;19(21):6049–6049. doi: 10.1093/nar/19.21.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearne P. A., van Driel I. R., Grego B., Simpson R. J., Goding J. W. The murine plasma cell antigen PC-1: purification and partial amino acid sequence. J Immunol. 1985 Jan;134(1):443–448. [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Szebenyi D. M., Obendorf S. K., Moffat K. Structure of vitamin D-dependent calcium-binding protein from bovine intestine. Nature. 1981 Nov 26;294(5839):327–332. doi: 10.1038/294327a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckwell D. S., Brass A., Humphries M. J. Homology modelling of integrin EF-hands. Evidence for widespread use of a conserved cation-binding site. Biochem J. 1992 Jul 1;285(Pt 1):325–331. doi: 10.1042/bj2850325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufty R. M., Kretsinger R. H. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science. 1975 Jan 17;187(4172):167–169. doi: 10.1126/science.1111094. [DOI] [PubMed] [Google Scholar]
- van Driel I. R., Goding J. W. Plasma cell membrane glycoprotein PC-1. Primary structure deduced from cDNA clones. J Biol Chem. 1987 Apr 5;262(10):4882–4887. [PubMed] [Google Scholar]