Abstract
Aims: LCZ696 (sacubitril/valsartan) exerts cardioprotective effects. Recent studies have suggested that it improves the endothelial function; however, the underlying mechanisms have not been thoroughly investigated. We investigated whether LCZ696 ameliorates diabetes-induced endothelial dysfunction.
Methods: Diabetes was induced using streptozotocin in 8-week-old male C57BL/6 mice. Diabetic mice were randomly assigned to receive LCZ696 (100 mg/kg/day), valsartan (50 mg/kg/day), or a vehicle for three weeks. The endothelium-dependent and endothelium-independent vascular responses of the aortic segments were determined based on the response to acetylcholine and sodium nitroprusside, respectively. Human umbilical vein endothelial cells (HUVEC) and aortic segments obtained from C57BL/6 mice were used to performin vitro andex vivo experiments, respectively.
Results: LCZ696 and valsartan reduced the blood pressure in diabetic mice (P<0.05). The administration of LCZ696 (P<0.001) and valsartan (P<0.01) ameliorated endothelium-dependent vascular relaxation, but not endothelium-independent vascular relaxation, under diabetic conditions. LCZ696, but not valsartan, increased eNOSSer1177 (P=0.06) and Akt (P<0.05) phosphorylation in the aorta. In HUVEC, methylglyoxal (MGO), a major precursor of advanced glycation end products, decreased eNOSSer1177 phosphorylation (P<0.05) and increased eNOSThr495 phosphorylation (P<0.001). However, atrial natriuretic peptide (ANP) reversed these effects. ANP also ameliorated the MGO-induced impairment of endothelium-dependent vascular relaxation in the aortic segments (P<0.05), although L-NAME completely blocked this effect (P<0.001).
Conclusion: LCZ696 ameliorated diabetes-induced endothelial dysfunction by increasing the bioavailability of ANP. Our findings suggest that LCZ696 has a vascular protective effect in a diabetic model and highlight that it may be more effective than valsartan.
Keywords: Diabetes, Sacubitril/valsartan, Natriuretic peptide, Endothelial dysfunction
Abbreviation list: ACE, angiotensin-converting enzyme; Ach, acetylcholine; AGEs, advanced glycation end-products; ANOVA, analysis of variance; ANP, atrial natriuretic peptide; ARB, angiotensin receptor blocker; BP, blood pressure; cGMP, cyclic guanosine monophosphate; CVD, cardiovascular disease; eNOS, endothelial nitric oxide synthase; HUVEC, human umbilical vein endothelial cells; L-NAME, N-nitro-L-arginine methyl ester; MGO, methylglyoxal; NO, nitric oxide; NP, natriuretic peptide; RAAS, renin-angiotensin-aldosterone system; SNP, sodium nitroprusside; STZ, streptozotocin.
Introduction
Endothelial dysfunction is fundamental to the development of cardiovascular disease 1 - 3) . Shear stress, hyperglycemia, and increased oxidative stress on the endothelium are known to trigger the inappropriate activation of the renin-angiotensin-aldosterone system (RAAS), accompanied by suppression of the natriuretic peptide system, thus leading to an impaired endothelial function. Nitric oxide (NO), which is predominantly synthesized in the endothelium, is not only a well-known vasodilator, but it is also recognized as a crucial antiplatelet, antithrombotic, and anti-inflammatory vasoprotective agent 4 - 6) .
Over the past few decades, RAAS blocking agents, including ACE inhibitors, ARBs, and combination treatment with ACE and neprilysin inhibitors (such as omapatrilat) have been extensively used for the treatment of hypertension and cardiovascular disease 7) . While omapatrilat has shown superior protective effects compared to ACE inhibitors, its use has been limited because of the occurrence of severe angioedema caused by suppressed bradykinin degeneration 8 , 9) .
LCZ696, also known as sacubtril/valsartan, is a first-in-class dual-inhibiting drug comprising valsartan, an angiotensin II receptor blocker, and sacubitril, a neprilysin inhibitor responsible for the degradation of natriuretic peptides (NPs), in a 1:1 molar ratio. LCZ696 increases not only atrial natriuretic peptide (ANP) and plasma and urinary cGMP but also the angiotensin II level, although the functions of angiotensin II are blocked by valsartan 10) . In the Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study, LCZ696 has shown superiority to the ACE inhibitor enalapril, and the results were consistent in diabetic subgroups 11 , 12) . In addition, the systolic and diastolic blood pressure (BP) reduction was significantly greater in the LCZ696 200 and 400 mg daily treated groups than in the valsartan 160 and 320 mg daily treated groups, as demonstrated in a double-blind randomized (PARAMOUNT) trial 13) .
Pre-clinical studies have demonstrated LCZ696 to have a protective effect on hypertension 14) , cardiac fibrosis 15) , hypertrophy 16) , inflammation 17) , and vascular dysfunction 14 , 18) . The administration of LCZ696 improved endothelium-dependent vascular relaxation in response to Ach in spontaneously hypertensive rats fed a high-salt diet, while valsartan treatment failed to ameliorate it. Interestingly, LCZ696 did not affect endothelium-independent relaxation 14) . Furthermore, LCZ696 demonstrated superiority to valsartan in promoting vascular relaxation by increasing NO bioavailability in heart failure-induced rats after 8 weeks of treatment 18) . These studies suggest that LCZ696 increased NP bioavailability, which might result in a better endothelial function and cardioprotective effects through NO bioavailability at least partially 19 - 21) .
However, there is a notable gap in the existing literature regarding the specific influence of LCZ696 on the endothelium-dependent function, specifically in the context of endothelial NO synthesis and its underlying mechanisms during hyperglycemia. Therefore, the present study aimed to test the hypothesis that treatment with LCZ696 ameliorates diabetes-induced endothelial dysfunction by promoting eNOS activity.
2.Methods
2.1. Animals and Drug Administration
C57BL/6J wild-type mice were purchased from Japan SLC, Inc (Japan). LCZ696 and valsartan were supplied by Novartis Pharma AG (Basel, Switzerland). Eight-week-old male mice were intraperitoneally injected with a single dose of either streptozotocin (STZ, 180 mg/kg, Santa Cruz) or vehicle to induce diabetes. Three days after the injection, diabetic mice were randomly divided into LCZ696 (100 mg/kg/day), valsartan (50 mg/kg/day), and vehicle groups, and the drugs were added to the drinking water for three weeks. These doses were chosen based on previous studies, in which the same doses of LCZ and valsartan were used without observing any severe adverse effects in mice 22 , 23) . Aortic segments obtained from 8-week-old male C57BL/6J mice were used for an ex vivo vascular reactivity assay. Mice were maintained under a controlled temperature (23±1℃) with a 12-h artificial light and dark cycle. All experimental procedures conformed to the guidelines for animal experimentation at Tokushima University. The Animal Care and Use Committee of Tokushima University reviewed and approved the study protocol under #T2020-127.
2.2. Measurement of the Blood Pressure and Metabolic Parameters
The BP was measured using a tail-cuff system in conscious mice (Softron). At the time of sacrifice, blood was collected from the heart into EDTA-containing tubes, and plasma was separated by centrifugation (9000 rpm for 15 min) at 4℃ and stored until further analyses at −80℃. Plasma total cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were measured at Sanritsu Zelkova Examination Center (Japan).
2.3. Vascular Reactivity Assay
Vascular reactivity was analyzed as previously documented 24) . After three weeks of LCZ696 and valsartan administration, the descending thoracic aorta was isolated and cut into 2-mm rings with special care taken to preserve the endothelium and mounted in organ baths filled with modified Krebs-Henseleit buffer (118.4 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 11.1 mM glucose) that was aerated (95% O2 and 5% CO2) and warmed (37℃). The changes in isometric tension were recorded using a polygraph (LabChart). Aortic rings were primed with 31.4 mM KCl and then pre-contracted with phenylephrine, producing a submaximal (60% of maximum) contraction. After a plateau was attained, the rings were exposed to increasing concentrations of acetylcholine (Ach; 10−9 to 10−4 M) and sodium nitroprusside (SNP; 10−9 to 10−4 M) to obtain cumulative concentration–response curves. The endothelium-dependent and endothelium-independent vascular reactivity was analyzed in response to Ach and SNP, respectively. In our ex vivo experiment, aortic segments were incubated with 1mM methylglyoxal (MGO) with or without 100 nM ANP and 100 µM N-nitro-l-arginine methyl ester (l-NAME) for 1 h before examining vascular reactivity.
2.4. Cell Culture Experiments
Human umbilical vein endothelial cells (HUVEC) were purchased from Life Technologies and cultured in EGM-2 medium (Lonza). HUVEC (passages 4–6) were stimulated with 500 µM MGO for 30 min in the presence or absence of 10 nM ANP in EBM-2 (Lonza) containing 2% FBS (Cytiva).
2.5. Western Blot Analysis
Radioimmunoprecipitation assay (RIPA) buffer containing a protease inhibitor cocktail and phosphatase inhibitors was used to prepare the protein lysates of HUVEC or aortic tissue. Proteins were separated by SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Hybond-P; GE Healthcare). After blocking with 5% bovine serum albumin or 5% skimmed milk for 1 h at room temperature, the membranes were incubated overnight at 4℃ with primary antibodies against phosphorylated-eNOSSer1177, phosphorylated-eNOSThr495, eNOS (BD Biosciences), phosphorylated-AktSer473, Akt (Cell Signaling Technology), or β-actin (Sigma). After five washes with TBS-T buffer, each membrane was incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The signal was detected using a luminescent image analyzer (LAS-1000, Fuji Film) with ECL-plus reagent (GE Healthcare).
2.6. Statistical Analysis
All numerical values are expressed as the mean±SEM. The unpaired Student’s t-test was used to compare the parameters between the two groups. Comparisons between multiple groups were performed using one-way analysis of variance (ANOVA) followed by Holm-Sidak’s multiple comparisons test. Two-way ANOVA followed by Dunnett’s multiple comparison test was used to compare dose-response curves. Statistical significance was set at p<0.05.
3.Results
3-1. Effect of LCZ696 on the Metabolic Parameters
The blood glucose and lipid levels significantly increased in STZ-induced diabetic mice. After 3 weeks of administration, systolic BP was lower in the LCZ696 and valsartan groups than in the STZ group, whereas there was no difference between the LCZ696 and valsartan groups ( Table 1 ) .
Table 1. Metabolic parameters after 3 weeks of treatment.
| CTRL | STZ | LCZ | VAL | |
|---|---|---|---|---|
| Blood glucose, mg/dl | 144.3±4.7*** | 723.3±23.5 | 654.8±26.5 | 691.9±48.3 |
| Heart rate, bpm | 691±11*** | 474±23 | 536±19* | 479±15 |
| Systolic blood pressure, mmHg | 103.3±1.8 | 103.3±4.2 | 91.0±2.4* | 93.0±3.0* |
| Diastolic blood pressure, mmHg | 69.4 ±2.0** | 54.6±3.2 | 45.1±2.9 | 48.8±4.6 |
| Total-cholesterol, mg/dl | 110.9±2.4*** | 354.8±20.5 | 383.4±46.0 | 392.5±34.7 |
| Triglycerides, mg/dl | 122.7±11.0* | 320.2±57.7 | 415.9±92.5 | 384.0±117.2 |
| HDL-cholesterol, mg/dl | 60.4±1.5*** | 128.8±7.4 | 98.5±12.2* | 127.5±10.7 |
CTRL, non-diabetic control; STZ, non-treated diabetic group; LCZ, LCZ696-treated group. VAL, valsartan-treated group; HDL, high-density lipoprotein. *; P<0.05, **; P<0.01, and ***; P<0.001 vs. STZ group. All values are presented as mean±SEM.
3.2. LCZ696 Attenuated Endothelial Dysfunction in Diabetic Mice
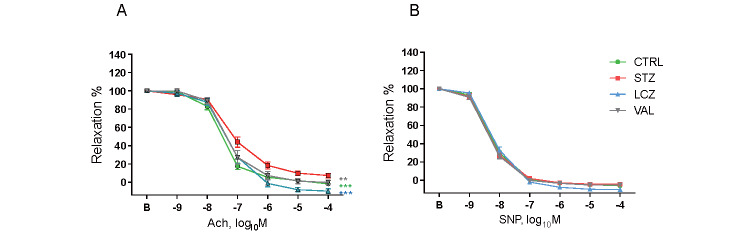
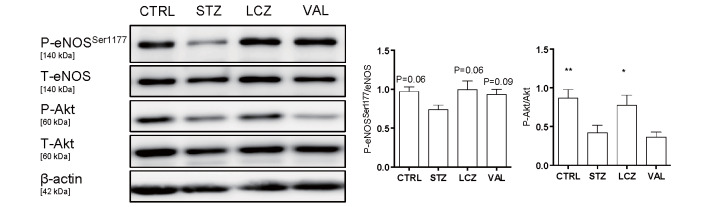
The induction of diabetes by STZ impaired endothelial relaxation in response to Ach compared to that in the non-diabetic control group (P<0.001). However, the administration of LCZ696 (P<0.001) and valsartan (P<0.01) ameliorated endothelium-dependent vascular dysfunction ( Fig.1A ) , whereas endothelium-independent vasodilation in response to SNP did not differ among the groups ( Fig.1B ) . As shown in Fig.2 , Akt phosphorylation in the aorta was reduced in diabetic mice (P<0.01). The administration of LCZ696 restored Akt phosphorylation (P<0.05); however, valsartan did not. A similar tendency was observed for the phosphorylation of eNOSSer1177; however, no significant difference was observed.
Fig.1. LCZ696 administration ameliorated endothelial dysfunction in diabetic mice.
Endothelium-dependent or -independent vascular relaxation in response to Ach (A) and SNP (B), respectively, was determined in the aortic segments of non-diabetic mice and diabetic mice treated with LCZ696, valsartan, or vehicle. (A) The induction of diabetes by STZ injection impaired endothelium-dependent vascular relaxation compared to non-diabetic mice. Treatment with LCZ696 and valsartan ameliorated this response. (B) There was no difference in the endothelium-independent vascular response among the four groups (n=12-19, per group). **P<0.01 and ***P<0.001 vs. STZ group. CTRL: non-diabetic control. STZ: untreated diabetic group. LCZ: LCZ696-treated group. VAL: Valsartan-treated group. All values are presented as the mean±SEM.
Fig.2. LCZ696 administration restored eNOSSer1177 phosphorylation in diabetic mice .
A western blot analysis demonstrated that the induction of diabetes by STZ decreased eNOSSer1177 and Akt phosphorylation in the aorta, whereas LCZ696 treatment restored such phosphorylation. (n=12-18, per group). *P<0.05 and **P<0.01 vs. STZ group. CTRL: non-diabetic control. STZ: untreated diabetic group. LCZ: LCZ696-treated group. VAL: Valsartan-treated group. All values are presented as the mean±SEM.
3.3. ANP Improved the Phosphorylation of eNOS and Akt in MGO-Treated HUVEC
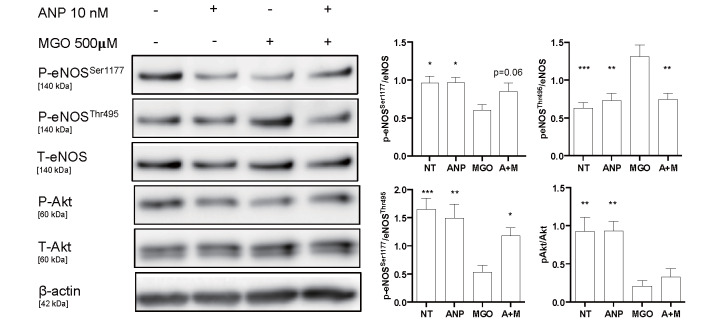
Based on the findings of the in vivo experiments, we treated HUVEC with ANP and MGO, a major cell-permeant precursor of AGEs, to investigate the mechanism by which LCZ696 ameliorated endothelial dysfunction. We next focused on the effect of the increased bioavailability of ANP that is expected by LCZ696 treatment. Incubation with MGO for 30 min resulted in a reduced phosphorylation of eNOSSer1177 (P<0.05) and Akt (P<0.01), while the phosphorylation of eNOSThr495 increased (P<0.001). Therefore, the eNOSSer1177/eNOSThr495 ratio decreased in the MGO-treated group. The presence of ANP inhibited MGO-induced elevation of eNOSThr495 (P<0.01) and a decrease in eNOSSer1177 (P=0.06). As a result, the ratio of eNOSSer1177/eNOSThr495 increased in the ANP-treated group in the presence of MGO (P<0.05), as shown in Fig.3 .
Fig.3. ANP ameliorated eNOS phosphorylation in MGO-treated HUVEC.
Incubation with MGO attenuated eNOSSer1177 and Akt phosphorylation and promoted eNOSThr495 phosphorylation in HUVEC. Therefore, the eNOSSer1177/eNOSThr495 phosphorylation ratio decreased by MGO treatment (n=7 per group). However, ANP tended to increase eNOSSer1177 phosphorylation and significantly decrease the eNOSThr495 phosphorylation induced by MGO. Therefore, the eNOSSer1177/ eNOSThr495 phosphorylation ratio was significantly recovered in the presence of ANP (n=7 per group). *; P<0.05, **; P<0.01, and ***; P<0.001 vs. MGO-treated group. ANP, ANP-treated group; MGO, MGO-treated group; A+M, combination treatment group with ANP and MGO. All values are presented as the mean±SEM.
3.4. ANP Improved Endothelium-Dependent Relaxation in the MGO-Treated Aortic Segments
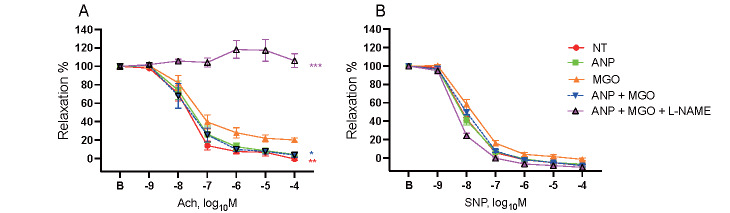
To assess the impact of ANP on the vascular function, aortic segments were stimulated with MGO in the presence or absence of ANP and examined for vascular reactivity. MGO impaired endothelium-dependent relaxation, as evidenced by the reduced response to Ach compared with the non-treated group (P<0.01). However, this impairment was reversed by the presence of ANP (P<0.05). In addition, the beneficial effect of ANP on endothelium-dependent relaxation was completely abrogated in the presence of L-NAME (P<0.001), as shown in Fig.4A . Nonetheless, ANP, MGO, and L-NAME did not affect the endothelium-independent vascular function, which was examined in response to SNP, as shown in Fig.4B .
Fig.4. ANP ameliorated endothelium-dependent vascular relaxation impaired by MGO.
(A) MGO significantly impaired the endothelium-dependent vascular relaxation in the aortic segments obtained from C57BL/6 mice. Incubation with ANP ameliorated MGO-induced endothelial dysfunction. However, in the presence of L-NAME, the effect of ANP was abrogated (n=7, per group). (B) Both ANP and MGO did not affect the vascular response to SNP (n=7, per group). *; P<0.05, **; P<0.01, and ***; P<0.001 vs. MGO group. NT: non-treated group, ANP: ANP-treated group, MGO: MGO-treated group, ANP+MGO: combination treatment of ANP and MGO, ANP+MGO+L-NAME: combination treatment of ANP, MGO, and L-NAME. All values are presented as the mean±SEM.
4.Discussion
It is well established that endothelial dysfunction is a primary contributor to the development of cardiovascular disease and it also affects the prognosis of diabetic patients. In this study, the induction of diabetes by STZ injection impaired endothelium-dependent vascular relaxation in response to Ach, along with a decreased phosphorylation of eNOSSer1177 and Akt in the aorta compared to the non-diabetic control group. Although both LCZ696 and valsartan treatment improved endothelial relaxation in response to Ach, LCZ696 showed a greater effect than valsartan. The observed blood pressure reduction was similar for both LCZ696 and valsartan. There was no difference in the response to SNP among the groups. In addition, LCZ696 treatment tended to ameliorate eNOSSer1177 phosphorylation and significantly ameliorated Akt phosphorylation in the aorta of diabetic mice compared to the STZ group, whereas valsartan failed to achieve this. Consistent with our findings, a previous study demonstrated that four weeks of treatment with LCZ696 attenuated high-salt-diet-induced endothelial dysfunction in spontaneously hypertensive rats, whereas valsartan alone failed to ameliorate it. That study also demonstrated no difference in endothelium-independent relaxation with SNP 14) . Moreover, Trivedi et al. demonstrated that LCZ696 showed a time-dependent superiority over valsartan in the vascular relaxation responses to both Ach and SNP, which correlated with increased NO bioavailability in both the circulation and myocardium 18) . Thus, these findings suggest that the elevated bioavailability of NPs by neprilysin inhibition during treatment with LCZ696 may have a beneficial impact on NO synthesis and exert superior effects on preventing cardiovascular complications compared with stand-alone ARBs. One clinical study also demonstrated that LCZ696 improved the endothelial function in patients with chronic heart failure with a reduced ejection fraction 25) . In addition, ex vivo and in vitro experiments that had been conducted in normoglycemic conditions strengthened the concept that the combination of neprilysin inhibitor and ARB treatment has potential benefits in enhancing endothelial NO synthesis as a result of reducing oxidative stress, inflammation, or inhibiting vasoconstrictors such as endothelin-1 and angiotensin II 14 - 21 , 26 , 27) . The results of these studies suggested that LCZ696 may have a beneficial effect on the endothelial function not only in diabetic conditions, but also in non-diabetic conditions.
In addition to its role in the degradation of NPs, neprilysin is also responsible for the inactivation of other circulating vasodilating mediators, such as substance P and bradykinin 28) . It is well documented that bradykinin and substance P can increase the activity of eNOS 29 , 30) . Moreover, NPs (ANP, BNP, and CNP) and their receptors (NPR-A, NPR-B, and NPR-C) mediate various benefits on blood pressure homeostasis, cardiac hypertrophy and remodeling, vascular relaxation, and RAAS. Most of these effects are mediated by increased cGMP via the interaction of NPs with the NPR-A and NPR-B receptors 31) . Specifically, chronic ANP treatment almost completely restored endothelium-dependent relaxation in response to Ach, whereas no difference was observed among the groups in response to SNP in a rabbit model 19) .
To clarify the underlying mechanism, in vitro and ex vivo experiments were performed using ANP, MGO, and L-NAME. MGO is a highly reactive a-dicarbonyl compound that is generated as an end-product of glycolysis; therefore, MGO is elevated in both type 1 and type 2 diabetic patients 32) . Our in vitro experiment showed that MGO decreases the phosphorylation of eNOSSer1177 and Akt, and, on the other hand, enhances the phosphorylation of eNOSThr495 in HUVEC 33 , 34) . However, incubation with ANP tended to increase the phosphorylation of eNOSSer1177 and Akt, and significantly decreased eNOSThr495 phosphorylation. These results suggested that ANP promoted eNOS activation in the presence of MGO. To confirm this effect from the point of view of vascular relaxation, we performed an ex vivo experiment. The findings clearly demonstrated that ANP ameliorates the endothelium-dependent vascular relaxation induced by MGO. L-NAME, a well-known inhibitor of NO synthesis, completely abolished the effects of ANP. These results suggest that ANP may have beneficial effects on the endothelial function by enhancing eNOS activity under diabetic conditions, at least partially. These results are consistent with those of previous studies demonstrating that ANP promotes NOS activity in the endothelium of L-NAME-injected rats 35) .
In conclusion, our findings demonstrate that LCZ696 ameliorates diabetes-induced endothelial dysfunction at least partially by enhancing the eNOS activity. Furthermore, ANP ameliorated MGO-induced vascular dysfunction, and this effect correlated with an increase in eNOS phosphorylation. These results provide insight into the superior effects of LCZ696 over valsartan on the endothelial function, which is partially due to the increased bioavailability of NPs and NO. This comparative analysis not only provides valuable insights into the pharmacological actions of LCZ696 but it also suggests its potential advantages over traditional ARBs in preserving endothelial health. The findings of this study may have implications in optimizing the therapeutic strategies targeting endothelial dysfunction and various types of cardiovascular intervention. However, future studies should aim to investigate the mechanisms by which NPs improve the eNOS activity and endothelial function under diabetic conditions.
Acknowledgments
The authors thank Etsuko Uematsu (Tokushima University) for technical assistance.
Competing Interests
The authors declare that they have no conflict of interest.
Sources of Funding
This work was partially supported by JSPS Kakenhi Grants (Number 22H03069 to M.S.), Bristol-Myers Squibb Research Grants (D.F.), Bayer Vascular Frontiers Research Grant (M.S. and D.F.), and Japan Agency for Medical Research and Development (M.S.). The funders had no role in the study design, data collection and analysis, or manuscript preparation.
References
- 1).Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW and Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract, 2018; 138: 271-281 [DOI] [PubMed] [Google Scholar]
- 2).Forbes JM and Fotheringham AK. Vascular complications in diabetes: old messages, new thoughts. Diabetologia, 2017; 60: 2129-2138 [DOI] [PubMed] [Google Scholar]
- 3).Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K and Martin SS. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation, 2022; 145: e153-e639 [DOI] [PubMed] [Google Scholar]
- 4).Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA and Sowers JR. The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol (Lausanne), 2013; 4: 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Jin RC and Loscalzo J. Vascular Nitric Oxide: Formation and Function. J Blood Med, 2010; 2010: 147-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).McFarlane SI, Winer N and Sowers JR. Role of the natriuretic peptide system in cardiorenal protection. Arch Intern Med, 2003; 163: 2696-2704 [DOI] [PubMed] [Google Scholar]
- 7).Menendez JT. The Mechanism of Action of LCZ696. Card Fail Rev, 2016; 2: 40-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Kostis JB, Packer M, Black HR, Schmieder R, Henry D and Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens, 2004; 17: 103-111 [DOI] [PubMed] [Google Scholar]
- 9).Messerli FH and Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet, 2000; 356: 608-609 [DOI] [PubMed] [Google Scholar]
- 10).Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W and Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol, 2010; 50: 401-414 [DOI] [PubMed] [Google Scholar]
- 11).Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray JJ, Packer M, Investigators P-H and Committees. Risk Related to Pre-Diabetes Mellitus and Diabetes Mellitus in Heart Failure With Reduced Ejection Fraction: Insights From Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial. Circ Heart Fail, 2016; 9: e002560 [Google Scholar]
- 12).McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P-H and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med, 2014; 371: 993-1004 [DOI] [PubMed] [Google Scholar]
- 13).Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ and Prospective comparison of AwARBoMOhfwpefI. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet, 2012; 380: 1387-1395 [DOI] [PubMed] [Google Scholar]
- 14).Kusaka H, Sueta D, Koibuchi N, Hasegawa Y, Nakagawa T, Lin B, Ogawa H and Kim-Mitsuyama S. LCZ696, Angiotensin II Receptor-Neprilysin Inhibitor, Ameliorates High-Salt-Induced Hypertension and Cardiovascular Injury More Than Valsartan Alone. Am J Hypertens, 2015; 28: 1409-1417 [DOI] [PubMed] [Google Scholar]
- 15).Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E and Saku K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail, 2016; 18: 386-393 [DOI] [PubMed] [Google Scholar]
- 16).von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D and Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail, 2015; 8: 71-78 [DOI] [PubMed] [Google Scholar]
- 17).Ishii M, Kaikita K, Sato K, Sueta D, Fujisue K, Arima Y, Oimatsu Y, Mitsuse T, Onoue Y, Araki S, Yamamuro M, Nakamura T, Izumiya Y, Yamamoto E, Kojima S, Kim-Mitsuyama S, Ogawa H and Tsujita K. Cardioprotective Effects of LCZ696 (Sacubitril/Valsartan) After Experimental Acute Myocardial Infarction. JACC Basic Transl Sci, 2017; 2: 655-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Trivedi RK, Polhemus DJ, Li Z, Yoo D, Koiwaya H, Scarborough A, Goodchild TT and Lefer DJ. Combined Angiotensin Receptor-Neprilysin Inhibitors Improve Cardiac and Vascular Function Via Increased NO Bioavailability in Heart Failure. J Am Heart Assoc, 2018; 7: e008268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Barber MN, Gaspari TA, Kairuz EM, Dusting GJ and Woods RL. Atrial natriuretic peptide preserves endothelial function during intimal hyperplasia. J Vasc Res, 2005; 42: 101-110 [DOI] [PubMed] [Google Scholar]
- 20).Mustafa NH, Jalil J, Zainalabidin S, Saleh MSM, Asmadi AY and Kamisah Y. Molecular mechanisms of sacubitril/valsartan in cardiac remodeling. Front Pharmacol, 2022; 13: 892460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Quaschning T, Galle J and Wanner C. Vasopeptidase inhibition: a new treatment approach for endothelial dysfunction. Kidney Int Suppl, 2003: S54-S57 [DOI] [PubMed] [Google Scholar]
- 22).Croteau D, Qin F, Chambers JM, Kallick E, Luptak I, Panagia M, Pimentel DR, Siwik DA and Colucci WS. Differential Effects of Sacubitril/Valsartan on Diastolic Function in Mice With Obesity-Related Metabolic Heart Disease. JACC Basic Transl Sci, 2020; 5: 916-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Esser N, Schmidt C, Barrow BM, Cronic L, Hackney DJ, Mongovin SM, Hogan MF, Templin AT, Castillo JJ, Hull RL and Zraika S. Insulinotropic Effects of Neprilysin and/or Angiotensin Receptor Inhibition in Mice. Front Endocrinol (Lausanne), 2022; 13: 888867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Pham PT, Bavuu O, Kim-Kaneyama JR, Lei XF, Yamamoto T, Otsuka K, Suto K, Kusunose K, Yagi S, Yamada H, Soeki T, Shimabukuro M, Barber GN, Sata M and Fukuda D. Innate Immune System Regulated by Stimulator of Interferon Genes, a Cytosolic DNA Sensor, Regulates Endothelial Function. J Am Heart Assoc, 2023; 12: e030084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Li BH, Fang KF, Lin PH, Zhang YH, Huang YX and Jie H. Effect of sacubitril valsartan on cardiac function and endothelial function in patients with chronic heart failure with reduced ejection fraction. Clin Hemorheol Microcirc, 2021; 77: 425-433 [DOI] [PubMed] [Google Scholar]
- 26).Bai W, Huo T, Chen X, Song X, Meng C, Dang Y, Rong C, Dou L and Qi X. Sacubitril/valsartan inhibits ox‑LDL‑induced MALAT1 expression, inflammation and apoptosis by suppressing the TLR4/NF‑kappaB signaling pathway in HUVECs. Mol Med Rep, 2021; 23: 402 [DOI] [PubMed] [Google Scholar]
- 27).Gao A, Wang Y, Gao X and Tian W. LCZ696 ameliorates lipopolysaccharide-induced endothelial injury. Aging (Albany NY), 2021; 13: 9582-9591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Howell EH and Cameron SJ. Neprilysin inhibition: A brief review of past pharmacological strategies for heart failure treatment and future directions. Cardiol J, 2016; 23: 591-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Searles CD and Harrison DG. The interaction of nitric oxide, bradykinin, and the angiotensin II type 2 receptor: lessons learned from transgenic mice. J Clin Invest, 1999; 104: 1013-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P and Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest, 1994; 94: 2036-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Potter LR, Yoder AR, Flora DR, Antos LK and Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol, 2009: 341-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Wang Y, Hall LM, Kujawa M, Li H, Zhang X, O’Meara M, Ichinose T and Wang JM. Methylglyoxal triggers human aortic endothelial cell dysfunction via modulation of the K(ATP)/MAPK pathway. Am J Physiol Cell Physiol, 2019; 317: C68-C81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Nigro C, Raciti GA, Leone A, Fleming TH, Longo M, Prevenzano I, Fiory F, Mirra P, D’Esposito V, Ulianich L, Nawroth PP, Formisano P, Beguinot F and Miele C. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia, 2014; 57: 1485-1494 [DOI] [PubMed] [Google Scholar]
- 34).Sessa WC. eNOS at a glance. J Cell Sci, 2004; 117: 2427-2429 [DOI] [PubMed] [Google Scholar]
- 35).Costa MD, Bosc LV, Majowicz MP, Vidal NA, Balaszczuk AM and Arranz CT. Atrial natriuretic peptide modifies arterial blood pressure through nitric oxide pathway in rats. Hypertension, 2000; 35: 1119-1123 [DOI] [PubMed] [Google Scholar]