Abstract
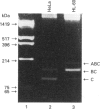
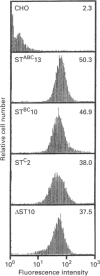
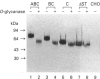
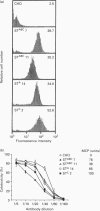
Three major membrane cofactor protein (MCP) phenotypes with different serine-threonine (ST)-rich regions, namely STc (L-phenotype), STBC (H or U phenotype) and STABC, and the MCP without the ST domain (delta ST) were expressed in Chinese hamster ovary (CHO) cells by transfecting the respective cDNAs. The expressed molecules migrated with a larger molecular mass on SDS/PAGE than those expected from their amino acid sequences. O-Glycanase digestion showed that this was due to O-linked sugar chains. The apparent sugar contents in each ST segment were compatible with their serine and threonine contents in the ST regions. The functional properties of these phenotypes as inhibitors of human complement (C) and receptors of measles virus (MV) were compared. The classical pathway-dependent CHO cell lysis by human C was more effectively suppressed by the expressed delta ST and STC than by the STABC and STBC phenotypes, although the difference was not so prominent. In contrast, alternative C pathway-dependent CHO-cell lysis was most effectively suppressed by the STABC phenotype and was only slightly blocked by the ST-deleted mutant. MV infection occurred with all of the phenotypes, but the infectious dose required to cause the same level of syncytium formation was 100-times higher in large ST (STABC and STBC) than in small ST (STC and delta ST) phenotypes. Thus, the ST domain serves as a functional modulator in MCP: MCP with a large ST domain having high O-linked sugar contents is favourable to the effective suppression of both the alternative C pathway-mediated cytolysis and MV infection, whereas MCP with a small ST domain is favourable to the suppression of the classical C pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. M., Brown M. C., Nunge M., Krych M., Atkinson J. P. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991 Nov 1;147(9):3005–3011. [PubMed] [Google Scholar]
- Ballard L., Seya T., Teckman J., Lublin D. M., Atkinson J. P. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45-70). J Immunol. 1987 Jun 1;138(11):3850–3855. [PubMed] [Google Scholar]
- Chan A. C., Atkinson J. P. Oligosaccharide structure of human C4. J Immunol. 1985 Mar;134(3):1790–1798. [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne K. E., Hall S. E., Thompson S., Arce M. A., Kinoshita T., Fujita T., Anstee D. J., Rosse W., Lublin D. M. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992 Nov 1;149(9):2906–2913. [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Giraudon P., Wild T. F. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985 Jul 15;144(1):46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Iwata K., Seya T., Ariga H., Nagasawa S. Expression of a hybrid complement regulatory protein, membrane cofactor protein decay accelerating factor on Chinese hamster ovary. Comparison of its regulatory effect with those of decay accelerating factor and membrane cofactor protein. J Immunol. 1994 Apr 1;152(7):3436–3444. [PubMed] [Google Scholar]
- Johnstone R. W., Russell S. M., Loveland B. E., McKenzie I. F. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993 Oct;30(14):1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- Kojima A., Iwata K., Seya T., Matsumoto M., Ariga H., Atkinson J. P., Nagasawa S. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol. 1993 Aug 1;151(3):1519–1527. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Post T. W., Atkinson J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Coyne K. E. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J Exp Med. 1991 Jul 1;174(1):35–44. doi: 10.1084/jem.174.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin D. M., Liszewski M. K., Post T. W., Arce M. A., Le Beau M. M., Rebentisch M. B., Lemons L. S., Seya T., Atkinson J. P. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988 Jul 1;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Seya T., Nagasawa S. Polymorphism and proteolytic fragments of granulocyte membrane cofactor protein (MCP, CD46) of complement. Biochem J. 1992 Jan 15;281(Pt 2):493–499. doi: 10.1042/bj2810493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Wild T. F., Rabourdin-Combe C., Gerlier D. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J Gen Virol. 1993 Jun;74(Pt 6):1073–1079. doi: 10.1099/0022-1317-74-6-1073. [DOI] [PubMed] [Google Scholar]
- Post T. W., Liszewski M. K., Adams E. M., Tedja I., Miller E. A., Atkinson J. P. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991 Jul 1;174(1):93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Sparrow R. L., McKenzie I. F., Purcell D. F. Tissue-specific and allelic expression of the complement regulator CD46 is controlled by alternative splicing. Eur J Immunol. 1992 Jun;22(6):1513–1518. doi: 10.1002/eji.1830220625. [DOI] [PubMed] [Google Scholar]
- Seya T., Atkinson J. P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989 Dec 1;264(2):581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Ballard L. L., Bora N. S., Kumar V., Cui W., Atkinson J. P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988 Aug;18(8):1289–1294. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990 Jul 1;145(1):238–245. [PubMed] [Google Scholar]
- Seya T., Okada M., Matsumoto M., Hong K. S., Kinoshita T., Atkinson J. P. Preferential inactivation of the C5 convertase of the alternative complement pathway by factor I and membrane cofactor protein (MCP). Mol Immunol. 1991 Oct;28(10):1137–1147. doi: 10.1016/0161-5890(91)90029-j. [DOI] [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kusumoto H., Deyashiki Y., Nishioka J., Maruyama I., Zushi M., Kawahara S., Honda G., Yamamoto S., Horiguchi S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987 Jul;6(7):1891–1897. doi: 10.1002/j.1460-2075.1987.tb02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild T. F., Malvoisin E., Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991 Feb;72(Pt 2):439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- Wong T. C., Ayata M., Ueda S., Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991 May;65(5):2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]