Abstract
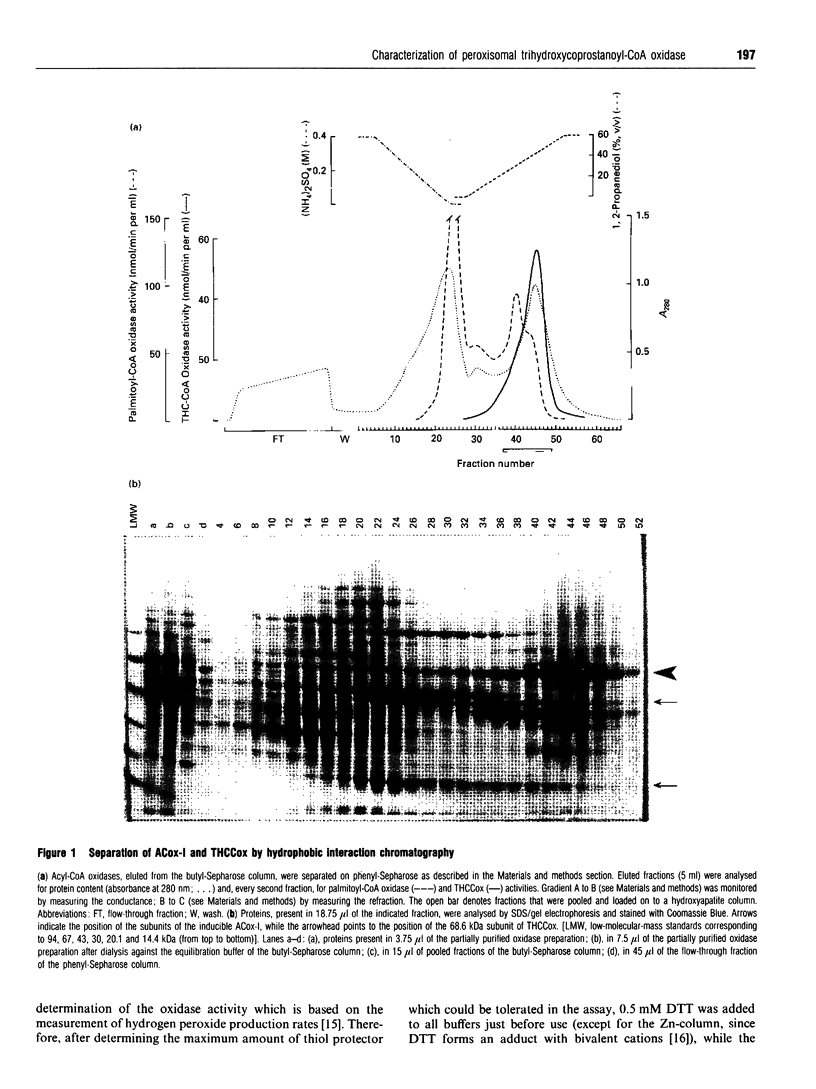
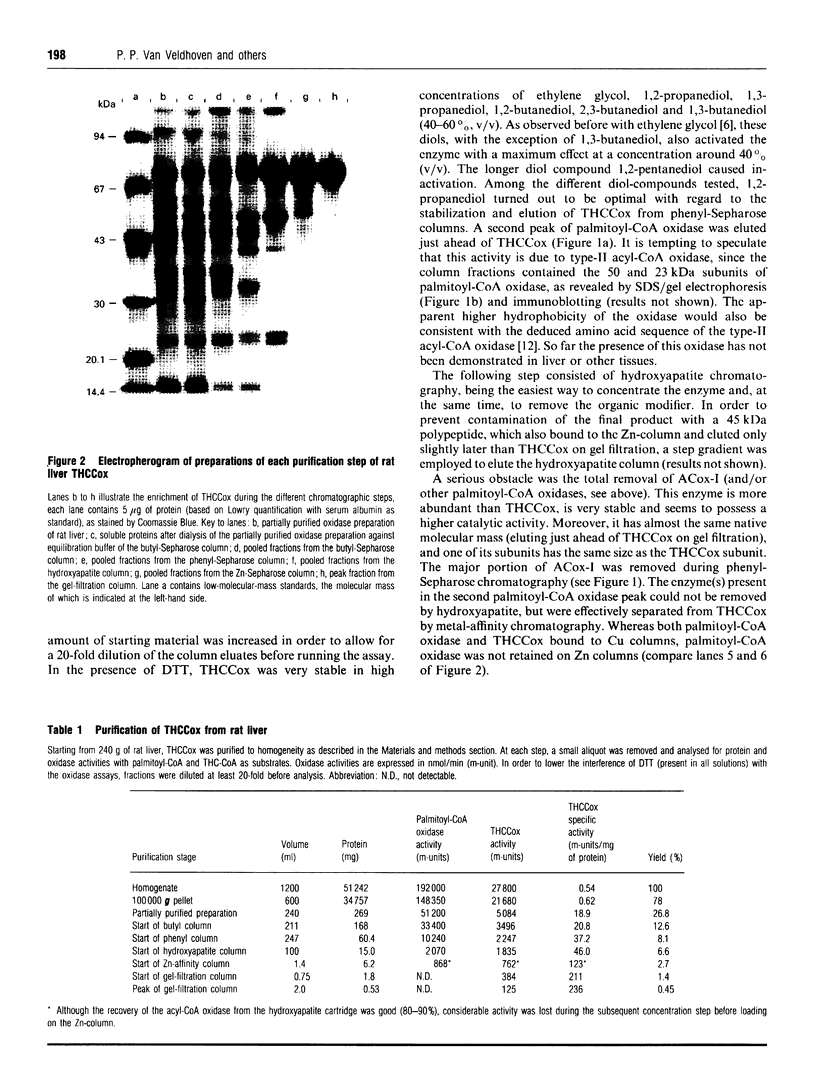
The acyl-CoA oxidase, catalysing the peroxisomal desaturation of the CoA-ester of trihydroxycoprostanic acid, a bile acid intermediate, has been purified to homogeneity from rat liver. Its native molecular mass, as determined by gel filtration and native gel electrophoresis, was 120 and 175 kDa respectively, suggesting a homodimeric protein consisting of 68.6 kDa subunits. If isolated in the presence of FAD, the enzyme showed a typical flavoprotein spectrum and contained most likely 2 mol of FAD per mol of enzyme. The cofactor, however, was loosely bound. The enzyme acted exclusively on 2-methyl-branched compounds, including pristanoyl-CoA and 2-methylhexanoyl-CoA if albumin was present. Important parameters to obtain a pure and active enzyme were the following: (1) using chromatographic separations like hydrophobic interaction and metal affinity, which allow the presence of high salt concentrations, conditions which stabilize the oxidase; (2) avoiding dialysis and (NH4)2SO4 precipitation; (3) including, when appropriate, FAD, dithiothreitol and a diol-compound in the solvents; and (4) carefully monitoring the removal of other acyl-CoA oxidases which possess the same native molecular mass and subunit size.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casteels M., Schepers L., Van Eldere J., Eyssen H. J., Mannaerts G. P. Inhibition of 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acid oxidation and of bile acid secretion in rat liver by fatty acids. J Biol Chem. 1988 Apr 5;263(10):4654–4661. [PubMed] [Google Scholar]
- Cornell N. W., Crivaro K. E. Stability constant for the zinc-dithiothreitol complex. Anal Biochem. 1972 May;47(1):203–208. doi: 10.1016/0003-2697(72)90293-x. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Bronfman M., Leighton F. Purification of the peroxisomal fatty acyl-CoA oxidase from rat liver. Biochem Biophys Res Commun. 1980 Jul 16;95(1):7–12. doi: 10.1016/0006-291x(80)90696-8. [DOI] [PubMed] [Google Scholar]
- Mannaerts G. P., Van Veldhoven P. P. Metabolic pathways in mammalian peroxisomes. Biochimie. 1993;75(3-4):147–158. doi: 10.1016/0300-9084(93)90072-z. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Hayashi H., Hijikata M., Ishii N., Furuta S., Kagamiyama H., Osumi T., Hashimoto T. Complete nucleotide sequence of cDNA and predicted amino acid sequence of rat acyl-CoA oxidase. J Biol Chem. 1987 Jun 15;262(17):8131–8137. [PubMed] [Google Scholar]
- Osumi T., Hashimoto T., Ui N. Purification and properties of acyl-CoA oxidase from rat liver. J Biochem. 1980 Jun;87(6):1735–1746. doi: 10.1093/oxfordjournals.jbchem.a132918. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I. Peroxisomal oxidation of the steroid side chain in bile acid formation. Biochimie. 1993;75(3-4):159–165. doi: 10.1016/0300-9084(93)90073-2. [DOI] [PubMed] [Google Scholar]
- Prydz K., Kase B. F., Björkhem I., Pedersen J. I. Subcellular localization of 3 alpha, 7 alpha-dihydroxy- and 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoyl-coenzyme A ligase(s) in rat liver. J Lipid Res. 1988 Aug;29(8):997–1004. [PubMed] [Google Scholar]
- Schepers L., Casteels M., Verheyden K., Parmentier G., Asselberghs S., Eyssen H. J., Mannaerts G. P. Subcellular distribution and characteristics of trihydroxycoprostanoyl-CoA synthetase in rat liver. Biochem J. 1989 Jan 1;257(1):221–229. doi: 10.1042/bj2570221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers L., Van Veldhoven P. P., Casteels M., Eyssen H. J., Mannaerts G. P. Presence of three acyl-CoA oxidases in rat liver peroxisomes. An inducible fatty acyl-CoA oxidase, a noninducible fatty acyl-CoA oxidase, and a noninducible trihydroxycoprostanoyl-CoA oxidase. J Biol Chem. 1990 Mar 25;265(9):5242–5246. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Just W. W., Mannaerts G. P. Permeability of the peroxisomal membrane to cofactors of beta-oxidation. Evidence for the presence of a pore-forming protein. J Biol Chem. 1987 Mar 25;262(9):4310–4318. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Van Rompuy P., Fransen M., De Béthune B., Mannaerts G. P. Large-scale purification and further characterization of rat pristanoyl-CoA oxidase. Eur J Biochem. 1994 Jun 15;222(3):795–801. doi: 10.1111/j.1432-1033.1994.tb18926.x. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Vanhove G., Assselberghs S., Eyssen H. J., Mannaerts G. P. Substrate specificities of rat liver peroxisomal acyl-CoA oxidases: palmitoyl-CoA oxidase (inducible acyl-CoA oxidase), pristanoyl-CoA oxidase (non-inducible acyl-CoA oxidase), and trihydroxycoprostanoyl-CoA oxidase. J Biol Chem. 1992 Oct 5;267(28):20065–20074. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Vanhove G., Vanhoutte F., Dacremont G., Parmentier G., Eyssen H. J., Mannaerts G. P. Identification and purification of a peroxisomal branched chain fatty acyl-CoA oxidase. J Biol Chem. 1991 Dec 25;266(36):24676–24683. [PubMed] [Google Scholar]