Abstract
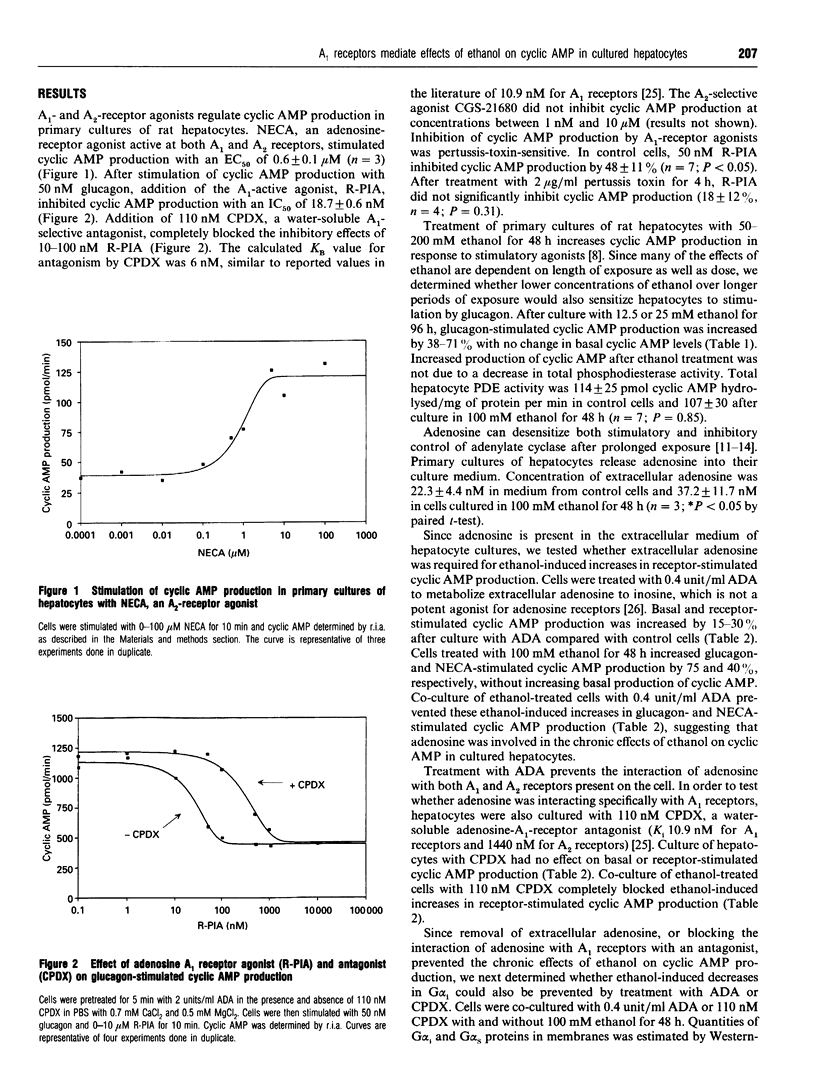
Cellular responses to adenosine depend on the distribution of the two adenosine receptor subclasses. In primary cultures of rat hepatocytes, adenosine receptors were coupled to adenylate cyclase via A1 and A2 receptors which inhibit and stimulate cyclic AMP production respectively. R-(-)-N6-(2-phenylisopropyl)-adenosine (R-PIA), the adenosine A1 receptor-selective agonist, inhibited glucagon-stimulated cyclic AMP production with an IC50 of 19 nM. This inhibition was blocked by the A1-specific antagonist 8-cyclopentyl-1,3-dimethylxanthine (CPDX). 5'-N- Ethylcarboxamidoadenosine (NECA), an agonist which stimulates A2 receptors, increased cyclic AMP production with an EC50 of 0.6 microM. Treatment of primary cultures of rat hepatocytes with 100 mM ethanol for 48 h decreases the quantity and function of the inhibitory guanine-nucleotide regulatory protein (G(i)), resulting in a sensitization of receptor-stimulated cyclic AMP production [Nagy and deSilva (1992) Biochem. J. 286, 681-686]. When cells were cultured with 2 units/ml adenosine deaminase, to degrade extracellular adenosine, ethanol-induced increases in cyclic AMP production were completely prevented. Moreover, the specific A1-receptor antagonist, CPDX, also blocked the chronic effects of ethanol on receptor-stimulated cyclic AMP production. Treatment with adenosine deaminase or CPDX also prevented the decrease in quantity of the alpha subunit protein of G(i) observed in hepatocytes after chronic treatment with ethanol. Taken together, these results suggest that activation of adenosine A1 receptors on primary cultures of hepatocytes is involved in the development of chronic ethanol-induced sensitization of receptor-stimulated cyclic AMP production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartrons R., Van Schaftingen E., Hers H. G. The ability of adenosine to decrease the concentration of fructose 2,6-bisphosphate in isolated hepatocytes. A cyclic AMP-mediated effect. Biochem J. 1984 Feb 15;218(1):157–163. doi: 10.1042/bj2180157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R. S., Flinn I. W., Proske O., Jackson D. G., Tena R. G., Mitchell M. C., Feldman A. M. Effects of chronic ethanol exposure on cardiac receptor-adenylyl cyclase coupling: studies in cultured embryonic chick myocytes and ethanol fed rats. Alcohol Clin Exp Res. 1991 Dec;15(6):1077–1083. doi: 10.1111/j.1530-0277.1991.tb05215.x. [DOI] [PubMed] [Google Scholar]
- Bruns R. F. Adenosine antagonism by purines, pteridines and benzopteridines in human fibroblasts. Biochem Pharmacol. 1981 Feb 15;30(4):325–333. doi: 10.1016/0006-2952(81)90062-9. [DOI] [PubMed] [Google Scholar]
- Chern Y., Lai H. L., Fong J. C., Liang Y. Multiple mechanisms for desensitization of A2a adenosine receptor-mediated cAMP elevation in rat pheochromocytoma PC12 cells. Mol Pharmacol. 1993 Nov;44(5):950–958. [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Dar M. S., Mustafa S. J., Wooles W. R. Possible role of adenosine in the CNS effects of ethanol. Life Sci. 1983 Oct 3;33(14):1363–1374. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- Diamond I., Wrubel B., Estrin W., Gordon A. Basal and adenosine receptor-stimulated levels of cAMP are reduced in lymphocytes from alcoholic patients. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1413–1416. doi: 10.1073/pnas.84.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz A., Guinzberg R., Uribe S., Piña E. Metabolic responses of isolated hepatocytes to adenosine; dependence on external calcium. Life Sci. 1991;49(7):505–510. doi: 10.1016/0024-3205(91)90067-l. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Shepherd R. E. Adenosine, cyclic AMP metabolism, and glycogenolysis in rat liver cells. J Biol Chem. 1977 Nov 25;252(22):8066–8070. [PubMed] [Google Scholar]
- Gordon A. S., Collier K., Diamond I. Ethanol regulation of adenosine receptor-stimulated cAMP levels in a clonal neural cell line: an in vitro model of cellular tolerance to ethanol. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2105–2108. doi: 10.1073/pnas.83.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1987 Nov 15;262(32):15702–15707. [PubMed] [Google Scholar]
- Green R. D. Release of adenosine by C1300 neuroblastoma cells in tissue culture. J Supramol Struct. 1980;13(2):175–182. doi: 10.1002/jss.400130205. [DOI] [PubMed] [Google Scholar]
- Hoffer L. J., Lowenstein J. M. Effects of adenosine and adenosine analogues on glycogen metabolism in isolated rat hepatocytes. Biochem Pharmacol. 1986 Dec 15;35(24):4529–4536. doi: 10.1016/0006-2952(86)90775-6. [DOI] [PubMed] [Google Scholar]
- Kenimer J. G., Nirenberg M. Desensitization of adenylate cyclase to prostaglandin E1 or 2-chloroadenosine. Mol Pharmacol. 1981 Nov;20(3):585–591. [PubMed] [Google Scholar]
- Kiyokawa H., Fukui H., Mizuguchi H., Kubo A., Kono N., Tarui S., Wada H. Adenosine induces System A amino acid transport in cultured rat hepatocytes. J Biochem. 1991 Jul;110(1):9–11. doi: 10.1093/oxfordjournals.jbchem.a123548. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991 Sep;5(12):2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh J. P., Didsbury J., Spiegel A., Stiles G. L. Modification of the rat adipocyte A1 adenosine receptor-adenylate cyclase system during chronic exposure to an A1 adenosine receptor agonist: alterations in the quantity of GS alpha and Gi alpha are not associated with changes in their mRNAs. Mol Pharmacol. 1989 Nov;36(5):681–688. [PubMed] [Google Scholar]
- Luthin G. R., Tabakoff B. Activation of adenylate cyclase by alcohols requires the nucleotide-binding protein. J Pharmacol Exp Ther. 1984 Mar;228(3):579–587. [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D., Chang F. H., Cheever L., Kim M., Diamond I., Gordon A. S. Chronic ethanol causes heterologous desensitization of receptors by reducing alpha s messenger RNA. Nature. 1988 Jun 30;333(6176):848–850. doi: 10.1038/333848a0. [DOI] [PubMed] [Google Scholar]
- Nagy L. E., DeSilva S. E. Ethanol increases receptor-dependent cyclic AMP production in cultured hepatocytes by decreasing G(i)-mediated inhibition. Biochem J. 1992 Sep 15;286(Pt 3):681–686. doi: 10.1042/bj2860681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L. E., Diamond I., Collier K., Lopez L., Ullman B., Gordon A. S. Adenosine is required for ethanol-induced heterologous desensitization. Mol Pharmacol. 1989 Nov;36(5):744–748. [PubMed] [Google Scholar]
- Nagy L. E., Diamond I., Gordon A. Cultured lymphocytes from alcoholic subjects have altered cAMP signal transduction. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6973–6976. doi: 10.1073/pnas.85.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L. E. Ethanol metabolism and inhibition of nucleoside uptake lead to increased extracellular adenosine in hepatocytes. Am J Physiol. 1992 May;262(5 Pt 1):C1175–C1180. doi: 10.1152/ajpcell.1992.262.5.C1175. [DOI] [PubMed] [Google Scholar]
- Newman M. E., Levitzki A. Desensitization of normal rat kidney cells to adenosine. Biochem Pharmacol. 1983 Jan 1;32(1):137–140. doi: 10.1016/0006-2952(83)90665-2. [DOI] [PubMed] [Google Scholar]
- Orrego H., Carmichael F. J., Saldivia V., Giles H. G., Sandrin S., Israel Y. Ethanol-induced increase in portal blood flow: role of adenosine. Am J Physiol. 1988 Apr;254(4 Pt 1):G495–G501. doi: 10.1152/ajpgi.1988.254.4.G495. [DOI] [PubMed] [Google Scholar]
- Parsons W. J., Stiles G. L. Heterologous desensitization of the inhibitory A1 adenosine receptor-adenylate cyclase system in rat adipocytes. Regulation of both Ns and Ni. J Biol Chem. 1987 Jan 15;262(2):841–847. [PubMed] [Google Scholar]
- Premont R. T., Jacobowitz O., Iyengar R. Lowered responsiveness of the catalyst of adenylyl cyclase to stimulation by GS in heterologous desensitization: a role for adenosine 3',5'-monophosphate-dependent phosphorylation. Endocrinology. 1992 Dec;131(6):2774–2784. doi: 10.1210/endo.131.6.1332848. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Behavioral sensitivity to purinergic drugs parallels ethanol sensitivity in selectively bred mice. Science. 1984 May 4;224(4648):519–521. doi: 10.1126/science.6324348. [DOI] [PubMed] [Google Scholar]
- Rabin R. A., Fiorella D., Van Wylen D. G. Role of extracellular adenosine in ethanol-induced desensitization of cyclic AMP production. J Neurochem. 1993 Mar;60(3):1012–1017. doi: 10.1111/j.1471-4159.1993.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Rabin R. A., Molinoff P. B. Multiple sites of action of ethanol on adenylate cyclase. J Pharmacol Exp Ther. 1983 Dec;227(3):551–556. [PubMed] [Google Scholar]
- Saito T., Lee J. M., Hoffman P. L., Tabakoff B. Effects of chronic ethanol treatment on the beta-adrenergic receptor-coupled adenylate cyclase system of mouse cerebral cortex. J Neurochem. 1987 Jun;48(6):1817–1822. doi: 10.1111/j.1471-4159.1987.tb05741.x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Stanley J. C., Markovic J., Gutknecht A. M., Lozeman F. J. Stimulation of glycogenolysis in isolated hepatocytes by adenosine and one of its analogues is inhibited by caffeine. Biochem J. 1987 Nov 1;247(3):779–783. doi: 10.1042/bj2470779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. M., Vagelos R., Hoffman B. B. Decreased cyclic AMP degradation in NG 108-15 neuroblastoma X glioma hybrid cells and S49 lymphoma cells chronically treated with drugs that inhibit adenylate cyclase. J Neurochem. 1990 Feb;54(2):402–410. doi: 10.1111/j.1471-4159.1990.tb01887.x. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]