Abstract
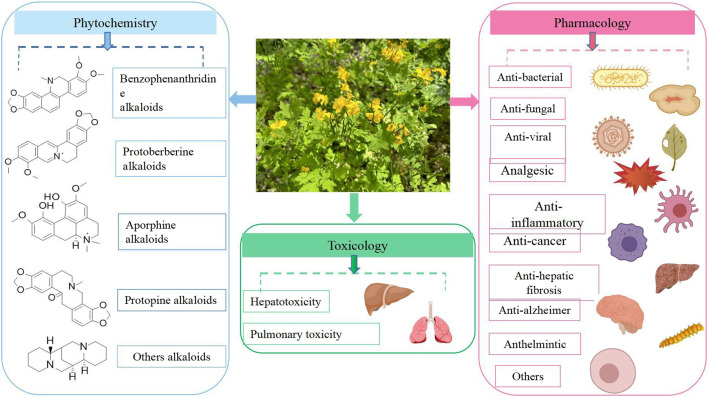
Chelidonium majus L. (C. majus), commonly known as “Bai Qu Cai” in China, belongs to the genus Chelidonium of the Papaveraceae family. It has rich medicinal value, such as alleviating coughs, asthma, spasms and pain. Recent studies have demonstrated that C. majus is abundant in various alkaloids, which are the primary components of C. majus and have a range of pharmacological effects, including anti-microbial, anti-inflammatory, anti-viral, and anti-tumor effects. So far, 94 alkaloids have been isolated from C. majus, including benzophenanthridine, protoberberine, aporphine, protopine and other types of alkaloids. This paper aims to review the research progress in phytochemistry, pharmacology and toxicology of C. majus alkaloids, in order to provide a theoretical basis for the application of C. majus in the field of medicinal chemistry and to afford reference for further research and development efforts.
Keywords: Chelidonium majus L., alkaloids, phytochemistry, pharmacological effect, toxicity
Graphical Abstract
1 Introduction
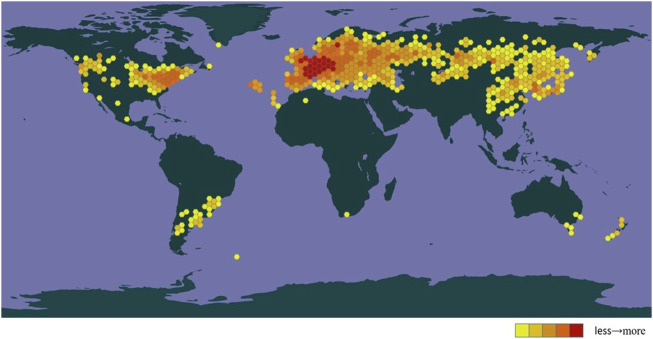
Chelidonium majus L., a traditional medicinal plant from the Chelidonium genus of the Papaveraceae family, is a perennial herb extensively distributed in Europe, Asia, and Africa (Figure 1). It has been widely used as a traditional Chinese ethnic medicine for centuries and was first documented in the “Herbal for Relief of Famines.” Known by various names in folklore, such as great celandine, swallow-wort, rock poppy, bai qu cai, tuhuanglian (土黄连) and dunchangcao (断肠草), it mostly thrives on hillsides, valley forest edges, grasslands, roadsides, and rock crevices (Wei et al., 2009; Gilca et al., 2010). According to the online records of China’s flora (http://www.cn-flora.ac.cn/index.html), the height of C. majus is approximately 30–60 (−100) cm. The stems are erect, multi-branched, and the branches are frequently covered with small hairs and may exude yellow latex when broken. The leaf blade is obovate-oblong or broadly obovate, 8–20 cm long. They are pinnatisect and divided into 2–4 pairs of lobes with irregularly parted or lobed crenate margins, appearing glaucous abaxially and green adaxially. Additionally, the blade is sparsely pubescent abaxially and glabrous adaxially. Flower buds are oval, with a diameter of 5–8 mm. The sepals are also oval and cymbiform, with a length of 5–8 mm. They may be glabrous or sparsely pubescent, and tend to be caducous. Petals are yellow, obovate, and entire, approximately 1 cm long. The capsule is narrowly terete at 2–5 cm × 2-3 mm, with a pedicel usually shorter than the fruit. The seeds are dark brown, ovoid, about 1 mm long or shorter, and have a shiny, alveolate appearance. The flowering and fruiting period is from April to September.
FIGURE 1.
The global distributions of Chelidonium majus L. (https://www.gbif.org/species).
In traditional Chinese medicine (TCM), C. majus is classified as a heat-clearing herb (Gilca et al., 2010). The 2020 edition of the Pharmacopoeia of the People’s Republic of China describes C. majus as bitter, cool, and toxic, returning to the lung and stomach meridians. The whole herb has the effect of relieving spasms and pain, coughs, and asthma. As extraction techniques have evolved and advanced, significant progress in research on C. majus has been made. Various active ingredients of C. majus have been isolated and purified, including alkaloids, flavonoids, saponins, volatile oils, vitamin C, and other components (Kwasniewski, 1958; Colombo and Bosisio, 1996; Bai and Zhang, 2009). Pharmacological studies have shown that its active components exhibit wide-ranging pharmacological activities, such as antibacterial, antifungal, anti-inflammatory, antiviral, and antitumor effects (Hong et al., 2022). Further studies have identified that the major active compounds of C. majus are isoquinoline alkaloids (Colombo and Bosisio, 1996), including benzophenanthridine, protoberberine, aporphine, protopine, and other types (Tomè and Colombo, 1995). As a hemicryptophyte, the concentration of alkaloids in C. majus continues to accumulate with changes in light (Tomè and Colombo, 1995). These alkaloids are found in concentrations ranging from 0.27% to 2.25% in aerial parts and 3%–4% in the roots (Maji and Banerji, 2015), with varying alkaloid content across different plant organs. Compared to the aerial parts and underground parts, the total content of alkaloids in the leaves is lower, while the content in latex is 32 times higher than in leaves and 9 times higher than in roots (Tomè and Colombo, 1995). These findings suggest that the alkaloid content in plant organs is influenced by the number of laticifers where they are stored (Zielińska et al., 2018). Despite extensive literature reviews, we have not yet found any article that provides a comprehensive and detailed review of the alkaloids of C. majus. Therefore, this paper focuses on the alkaloids of C. majus, reviewing research progress in phytochemistry, pharmacological effects, and toxicology. The aim is to provide a reference for the application of C. majus in medicinal drugs, which is extremely significant for the further advancement of traditional ethnic medicine.
2 Methodology
To comprehensively understand the research status of C. majus, we conducted a thorough literature search using various electronic databases, including Web of Science, PubMed, Google Scholar, and China National Knowledge Infrastructure (CNKI). Additionally, we referred to other literature sources, such as Pharmacopoeia of the People’s Republic of China, to obtain relevant information about the alkaloids in C. majus. This article exclusively utilizes Chinese and English texts. The keywords employed were C. majus L., alkaloids, phytochemistry, pharmacological effects, and toxicity. As of May 2024, a total of 915 relevant literature sources were retrieved. To ensure the accuracy and relevance of the review, we conducted screening based on the title, abstract, and full text of the article. Duplicate articles, conference abstracts, and unavailable articles have been excluded. Additionally, articles with research purposes not relevant to the topic of this review, as well as non-English and non-Chinese articles, have also been excluded. Finally, 166 eligible articles were included.
3 Phytochemistry
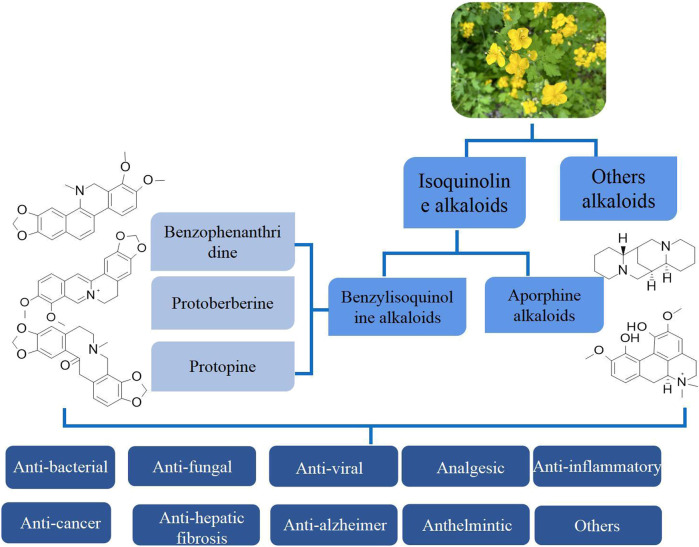
The chemical composition of C. majus is complex, with isoquinoline alkaloids being recognized as the main active ingredients. In addition, some scholars have reported that C. majus also contains flavonoids, triterpenoids, volatile oils, and other components. Isoquinoline alkaloids are a class of alkaloids derived from phenylalanine or tyrosine, which are abundant in quantity and complex in structure, providing a rich material basis for the pharmacological effects of C. majus. At present, 94 alkaloids have been isolated and identified from C. majus, which can be categorized into benzophenanthridines, protoberberines, aporphines, protopines, and other alkaloids based on their carbon skeletons (Figure 2) (Gerenčer et al., 2006; Wei et al., 2009). Among them, the three main alkaloid groups, including benzophenanthridines, protoberberines, and protopines, belong to benzylisoquinoline alkaloids, and aporphines belong to isoquinoline alkaloids, which are considered to be the active ingredients of C. majus and exhibit significant pharmacological activity (Tuzimski and Petruczynik, 2023; Zwerger et al., 2024). The concentrations of these alkaloids differ according to the plant parts and growth conditions, but they generally have high medicinal value. This section provides information on the types, molecular formulas, plant parts, and references of these alkaloids isolated from C. majus.
FIGURE 2.
The alkaloids groups of alkaloids and pharmacological effects of Chelidonium majus L.
3.1 Benzophenanthridine alkaloids
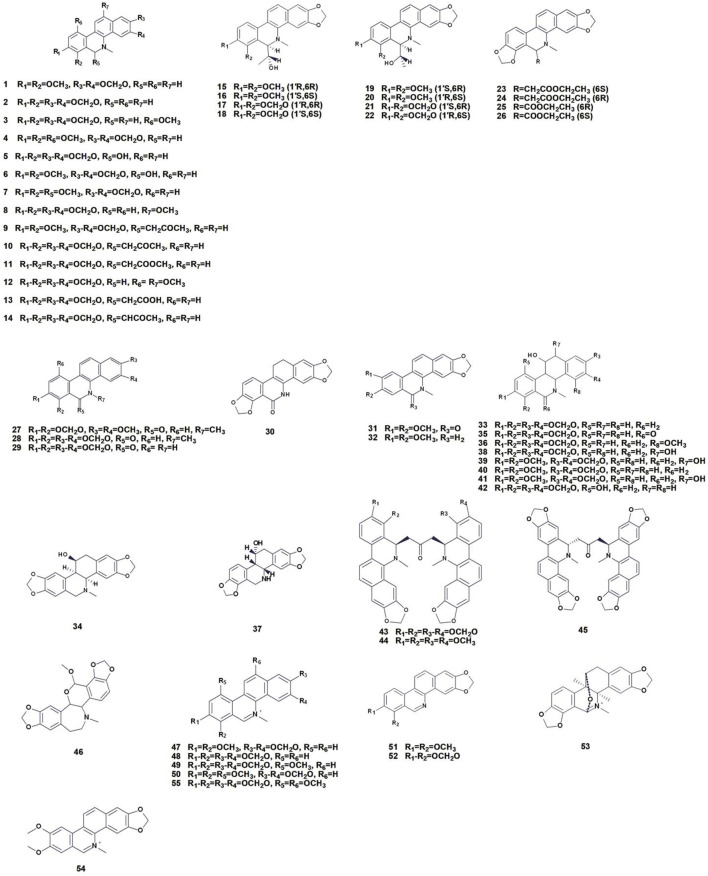
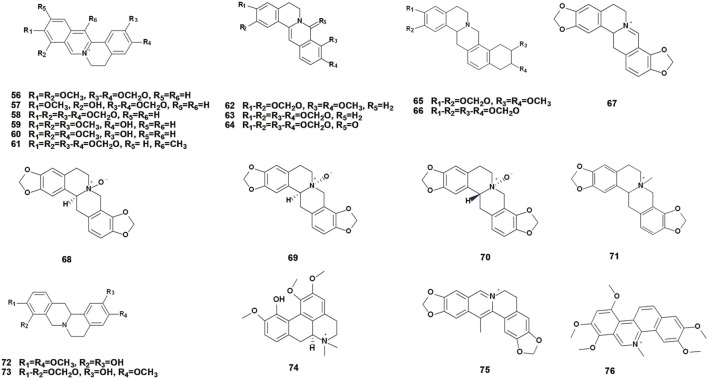
Benzophenanthridine alkaloids are classified as isoquinoline alkaloids, characterized by a tetracyclic structural motif, and represent an important category of nitrogen-containing small molecules (Bisai et al., 2019). These alkaloids are a common in the Papaveraceae family and are the most abundant and important components in C. majus. Currently, 55 alkaloids (1–55) have been identified and extracted from C. majus. The skeletal structure consists of one isoquinoline nucleus and two benzene rings. The benzophenanthridine alkaloids isolated and identified from C. majus can be further divided into four structural types according to the degree of unsaturated skeleton: dihydrobenzophenthridine, hexahydrobenzophenthridine, dimindihydrobenzophenthridine and benzophenanthrine quaternary amine (Table 1; Figure 3) (Wei et al., 2009; Bisai et al., 2019; Laines-Hidalgo et al., 2022). These alkaloids are structurally variable due to their N-atom content and have been reported to have a wide range of pharmacological activities (Wei et al., 2021), with significant anti-inflammatory, analgesic, and antitumor activities (Han et al., 2016).
TABLE 1.
Benzophenanthridine alkaloids compounds isolated from Chelidonium majus L.
| No. | Compound name | Formula | Part of the plant | Ref. |
|---|---|---|---|---|
| Dihydrobenzophen-anthridine alkaloids | ||||
| 1 | Dihydrochelerythrine | C21H19NO4 | Whole plant | Kadan et al. (1990), Oechslin et al. (1991) |
| 2 | Dihydrosanguinarine | C20H15NO4 | Whole plant | Slavík and Slavíková (1977), Kadan et al. (1990) |
| 3 | Dihydrochelirubine | C21H17NO5 | Root | Tanahashi and Zenk (1990), Táborská et al. (1994) |
| 4 | Dihydrochelilutine | C22H21NO5 | Aerial part | Hanaoka et al. (1991), Deng et al. (2016) |
| 5 | 8-Hydroxydihydrosanguinarine | C20H15NO5 | Aerial part | Zuo et al. (2008) |
| 6 | 8-Hydroxydihydrochelerythrine | C21H19NO5 | Aerial part | Zuo et al. (2008) |
| 7 | 6-Methoxydihydrochelerythine/Angoline | C22H21NO5 | Whole plant | Zhou et al. (1989) |
| 8 | 6-Methoxydihydrosanguinarine | C21H17NO5 | Whole plant | Zhou et al. (1989) |
| 9 | 8-Acetonyldihydrochelerythrine | C24H23NO5 | Whole plant | Kadan et al. (1990) |
| 10 | 8-Acetonyldihydrosanguinarine | C23H19NO5 | Whole plant | Kadan et al. (1990) |
| 11 | Methyl-2'-(7,8-dihydrosanguinarine-8-yl) acetate | C23H19NO6 | Park et al. (2011) | |
| 12 | Dihydromacarpine | C22H19NO6 | Aerial part | Tanahashi and Zenk (1990), Deng et al. (2016) |
| 13 | Spallidamine | C18H19NO4 | Aerial part | Kim et al. (2015a) |
| 14 | 6-Ketenesanguinarine | C23H17NO5 | Aerial part | Zhang et al. (2014) |
| 15 | (1′R,6 R) -1-(Dihydrochelerythrine-6-yl) ethanol | C23H24NO5 | Aerial part | Deng et al. (2017) |
| 16 | (1′S,6 S) -1-(Dihydrochelerythrine-6-yl) ethanol | C23H24NO5 | Aerial part | Deng et al. (2017) |
| 17 | (1′R,6 R) -1-(Dihydrosanguinarine-6-yl) ethanol | C22H20NO5 | Aerial part | Deng et al. (2017) |
| 18 | (1′S,6 S) -1-(Dihydrosanguinarine-6-yl) ethanol | C22H20NO5 | Aerial part | Deng et al. (2017) |
| 19 | (1′S,6 R) -1-(Dihydrochelerythrine-6-yl) ethanol | C23H24NO5 | Aerial part | Deng et al. (2017) |
| 20 | (1′R,6 S) -1-(Dihydrochelerythrine-6-yl) ethanol | C23H24NO5 | Aerial part | Deng et al. (2017) |
| 21 | (1′S,6 R) -1-(Dihydrosanguinarine-6-yl) ethanol | C22H20NO5 | Aerial part | Deng et al. (2017) |
| 22 | (1′R,6 S) -1-(Dihydrosanguinarine-6-yl) ethanol | C22H20NO5 | Aerial part | Deng et al. (2017) |
| 23 | (6 S) -Ethyl 2-(dihydrosanguinarine-6-yl) acetate | C24H22NO6 | Aerial part | Deng et al. (2017) |
| 24 | (6 R) -Ethyl 2-(dihydrosanguinarine-6-yl) acetate | C24H22NO6 | Aerial part | Deng et al. (2017) |
| 25 | (6 R) -Ethyl-dihydrosanguinarine-6-carboxylate | C23H20NO6 | Aerial part | Deng et al. (2017) |
| 26 | (6 S) -Ethyl dihydrosanguinarine-6-carboxylate | C23H20NO6 | Aerial part | Deng et al. (2017) |
| 27 | Oxychelerythrine | C21H17NO5 | Root | Táborská et al. (1994) |
| 28 | Oxysanguinarine | C20H13NO5 | Whole plant | Kadan et al. (1990), Táborská et al. (1994) |
| 29 | N-Demethyloxysanguinarine | C19H11NO5 | Chang et al. (2003), Wei et al. (2009) | |
| 30 | N-dimethyl-9,10-dihydroxysanguinarine | C19H13NO5 | Root | Wei et al. (2009), Kopyt’ko et al. (2005) |
| 31 | Oxynitidine | C21H17NO5 | Maji and Banerji (2015) | |
| 32 | Dihydronitidine | C21H19NO4 | Root | Maji and Banerji (2015), Kopyt’ko et al. (2005) |
| Hexahydrobenzophenthridinealkaloids | ||||
| 33 | Chelidonine | C20H19NO5 | Whole plant, latex | Bugatti et al. (1987), Zhao et al. (2020), Warowicka et al. (2021) |
| 34 | Isochelidonine | C20H19NO5 | Aerial part | Rosa and Vincenzo (1992) |
| 35 | Oxychleidonine | C20H17NO6 | Root | Kaczmarek and Malek (1959) |
| 36 | Methoxychelidonine | C21H21NO6 | Root | Kaczmarek and Malek (1959) |
| 37 | Norchelidonine | C19H17NO5 | Whole plant | Kadan et al. (1992) |
| 38 | Chelamine | C20H19NO6 | Whole plant | Slavík and Slavíková (1977), Táborská et al. (1994) |
| 39 | Chelamidine | C21H23NO6 | Whole plant | Slavík and Slavíková (1977), Táborská et al. (1994) |
| 40 | (+)-Homochelidonine | C21H23NO5 | Whole plant | Kadan et al. (1990) |
| 41 | 10-Hydroxyhomochelidonine | C21H24NO6 | Root | Slavík and Slavíková (1977) |
| 42 | 10-Hydroxychelidonine | C20H19NO6 | Root | Slavík and Slavíková (1977) |
| Dimindihydrobenz-ophenthridine alkaloids | ||||
| 43 | Chelidimerine | C43H32N2O9 | Whole plant | Tin-wa et al. (1972b), Kadan et al. (1990) |
| 44 | Chelerythridimerine | C45H40N2O9 | MacLean et al. (1969), Wei et al. (2009) | |
| 45 | Sanguidimerine | C45H40N2O9 | Tin-wa et al. (1972a), Wei et al. (2009) | |
| 46 | Rhoeadine | C21H21NO6 | Root | Pfeifer et al. (1965), Yang et al. (2024b) |
| Benzophenanthrine quaternary amine alkaloids | ||||
| 47 | Chelerythrine | C21H18NO4 + | Whole plant, latex | Bugatti et al. (1987), Warowicka et al. (2019), Zhao et al. (2020) |
| 48 | Sanguinarine | C20H14NO4 + | Whole plant, latex | Kaczmarek and Malek (1959), Warowicka et al. (2019), Zhao et al. (2020) |
| 49 | Chelirubine | C21H16NO5 + | Root | Kaczmarek and Malek (1959) |
| 50 | Chelilutine | C22H21NO5 | Root | Slavík and Slavíková (1977), Táborská et al. (1994) |
| 51 | Demethylchelerythrine | C20H15NO4 | Aerial part | Zhang et al. (2014) |
| 52 | Demethylsanguinarine | C19H11NO4 | Aerial part | Zhang et al. (2014) |
| 53 | Didehydrochelidonine | C20H20NO5 | Maji and Banerji (2015) | |
| 54 | Nitidine | C21H18NO4 + | Maji and Banerji (2015) | |
| 55 | Macarpine | C22H18NO6 + | Root | Táborská et al. (1994), Maji and Banerji (2015) |
FIGURE 3.
Chemical structures of benzophenanthridine alkaloids isolated from Chelidonium majus L. (The numbers in Figure 3 refer to the numbers of alkaloids present in Table 1).
3.2 Protoberberine alkaloids
Protoberberine alkaloids are widely distributed and represent one of the largest categories of isoquinoline alkaloids. They are synthesized in plants through a series of complex enzymatic reactions using tyrosine as a substrate (Liu et al., 2023). This type of alkaloid is composed of two fused isoquinoline rings, primarily in the form of hydrochloride. Protoberberine alkaloids are abundantly present in nature, with C. majus containing a relatively high content of these compounds. Currently, 21 protoberberine compounds (56–76) have been isolated from this plant (Table 2; Figure 4). In clinical practice, this class of alkaloids demonstrates a range of biological activities, including antimicrobial and anti-inflammatory properties. These alkaloids exhibit various beneficial effects in the field of medicine.
TABLE 2.
Protoberberine alkaloids compounds isolated from Chelidonium majus L.
| No. | Compound name | Formula | Part of the plant | Ref. |
|---|---|---|---|---|
| 56 | Berberine | C20H18NO4 + | Whole plant, latex | Bugatti et al. (1987), Zhao et al. (2020), Warowicka et al. (2021) |
| 57 | Berberrubine | C19H16NO4 + | Whole plant | Yang et al. (2017), Yang et al. (2024b) |
| 58 | Coptisine | C19H14NO4 + | Whole plant, latex | Sárközi et al. (2006), Zhao et al. (2020), Warowicka et al. (2021) |
| 59 | Jatrorrhizine | C20H20NO4 + | Whole plant | Yang et al. (2017), Yang et al. (2024b) |
| 60 | Columbamine | C20H20NO4 + | Whole plant | Yang et al. (2017), Yang et al. (2024b) |
| 61 | Corysamine | C20H16NO4 + | Whole plant | Golkiewicz and Gadzikowska (1999), Zhao et al. (2020) |
| 62 | Dihydroberberine | C20H19NO4 | Root | Kopyt’ko et al. (2005) |
| 63 | Dihydrocoptisine | C19H15NO4 | Root | Kopyt’ko et al. (2005) |
| 64 | 8-Oxycoptisine | C19H13NO5 | Whole plant | Zhou et al. (1989) |
| 65 | Tetrahydroberberine/Canadine | C20H21NO4 | Aerial part | Ruizhi et al. (1985), Bozhadze et al. (2013) |
| 66 | Stylopine/Tetrahydrocoptisine | C19H17NO4 | Whole plant | Kadan et al. (1990), Sárközi et al. (2006) |
| 67 | 13,14-Dihydrocoptisine | C19H16NO4 + | Whole plant | Paulsen et al. (2015), Yang et al. (2024b) |
| 68 | Tetrahydrocoptisine N-oxide | C19H18NO6 | Aerial part | Huang et al. (2019) |
| 69 | 7 R,14 S-cis-tetrahydrocoptisine N-oxide | C19H17NO5 | Aerial part | Le et al. (2021) |
| 70 | 7 R,14 R-trans-tetrahydrocoptisine N-oxide | C19H17NO5 | Aerial part | Le et al. (2021) |
| 71 | (S)-N-Methylstylopine | C20H20NO4 + | Whole plant | Zhao et al. (2020) |
| 72 | Scoulerine | C19H21NO4 | Root, leaf | Schrittwieser et al. (2011), Yahyazadeh et al. (2017) |
| 73 | Cheilanthifoline | C19H19NO4 | Root, leaf | Yahyazadeh et al. (2017) |
| 74 | Menisperine | C21H26NO4 + | Root | Tomita and Kikuchi (1955), Yang et al. (2024b) |
| 75 | Worenine | C20H16NO4 + | Root | Zhang et al. (2011a), Yang et al. (2024b) |
| 76 | Sanguilutine | C23H24NO5 + | Root | Táborská et al. (1994) |
FIGURE 4.
Chemical structures of protoberberine alkaloids isolated from Chelidonium majus L. (The numbers in Figure 4 refer to the numbers of alkaloids present in Table 2).
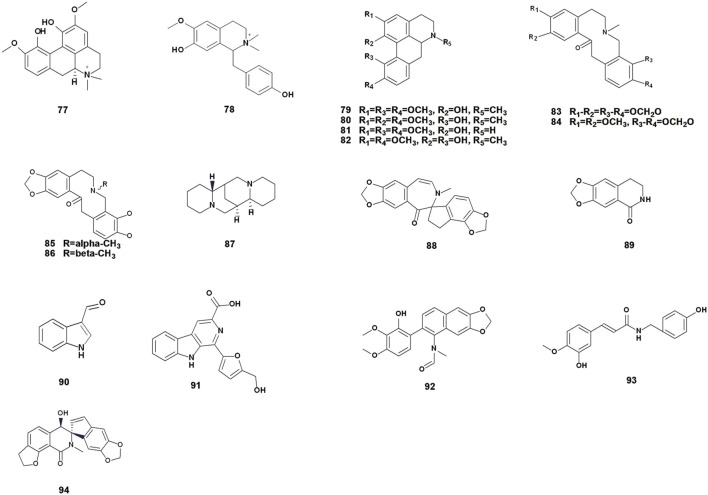
3.3 Aporphine alkaloids
Aporphine alkaloids are natural compounds that are widely distributed in nature and have important biological activities. This group of alkaloids belongs to isoquinoline alkaloids, which is an important type of natural alkaloids (Lin et al., 2020). These alkaloids are composed of four fused hexagonal rings, formed by connecting the C-2 position of the benzyl part of the benzylisoquinoline and the C-8 position of the isoquinoline part and eliminating one hydrogen molecule. A total of 6 species of aporphine alkaloids have been isolated and identified (Table 3; Figure 5). Aporphine alkaloids exhibit various pharmacological effects, including antioxidant, antiviral, and antitumor activities.
TABLE 3.
Aporphine, protopine and other alkaloids compounds isolated from Chelidonium majus L.
| No. | Compound name | Formula | Part of the plant | Ref. |
|---|---|---|---|---|
| Aporphine alkaloids | ||||
| 77 | Magnoflorine | C20H24NO4 + | Root | Slavík and Slavíková (1977) |
| 78 | Magnocurarine | C19H24NO3 + | Whole plant | Yang et al. (2017), Yang et al. (2024b) |
| 79 | Corydine | C20H23NO4 | Root | Shafiee and Jafarabadi (1998) |
| 80 | Isocorydine | C20H23NO4 | Root | Jeong and Lim (2017), Yang et al. (2024b) |
| 81 | Norcorydine | C19H21NO4 | Root | Shafiee and Jafarabadi (1998) |
| 82 | Corytuberine | C19H21NO4 | Aerial part | Táborská et al. (1994) |
| Protopine alkaloids | ||||
| 83 | Protopine | C20H19NO5 | Whole plant | Kadan et al. (1990), Kim et al. (1999) |
| 84 | Cryptopine | C21H23NO5 | Whole plant | Seger et al. (2004), Zhao et al. (2020) |
| 85 | α-Allocryptopine | C21H23NO5 | Root | Kaczmarek and Malek (1959), Marek et al. (1998) |
| 86 | β-Allocryptopine | C21H23NO5 | Root | Kadan et al. (1990), Marek et al. (1998) |
| Others alkaloids | ||||
| 87 | Sparteine | C15H26N2 | Seed, Root, Aerial part | Kaczmarek and Malek (1959), Kopyt’ko et al. (2005) |
| 88 | (−)-Turkiyenine | C20H15NO6 | Whole plant | Kadan et al. (1990) |
| 89 | Noroxyhydrastinine | C10H9NO3 | Whole plant | Yuan et al. (2022) |
| 90 | Indole-3-carboxaldehyde | C9H7NO | Whole plant | Yuan et al. (2022) |
| 91 | Flazin | C17H12N2O4 | Whole plant | Yuan et al. (2022) |
| 92 | Arnottianamide | C21H19NO6 | Aerial part | Kim et al. (2015a) |
| 93 | N-Trans-feruloyltyramine | C18H19NO4 | Aerial part | Kim et al. (2015a) |
| 94 | Chelidoniumine | C20H15NO6 | Aerial part | Huang et al. (2019) |
FIGURE 5.
Chemical structures of aporphine, protopine and other alkaloids isolated from Chelidonium majus L. (The numbers in Figure 5 refer to the numbers of alkaloids present in Table 3).
3.4 Protopine alkaloids
Protopine alkaloids are a class of isoquinoline alkaloids with ten-membered nitrogen heterocycles formed by protoberberine alkaloids through N- methylation and ring splitting in the biosynthetic pathway. The most noticeable characteristic of these compounds is C-14 carbonylation. These natural isoquinoline alkaloids possess a fundamental three-ring structure, comprising two benzene rings (A and C rings) and one ten-membered nitrogen heterocyclic ring (B ring) (Paul and Maurer, 2003). Currently, 4 species of protopine alkaloids have been isolated from C. majus (83–86) (Table 3; Figure 5).
3.5 Other alkaloids
In addition to the above four groups of alkaloids, other alkaloids have been reported. For example, Kaczmarek and Malek isolated sparteine by paper chromatography (Kaczmarek and Malek, 1959); Kadan G et al. isolated (−)-turkiyenine from the dried whole plant (Kadan et al., 1990); Yuan et al. obtained noroxyhydrastinine, indole-3-carboxaldehyde, and flazin (Yuan et al., 2022); Kim et al. identified N-trans-feruloyltyramine and arnottianamide (Kim et al., 2015); and Huang et al. extracted chelidoniumine from the aerial part of C. majus. Although the concentrations of these alkaloids are low, they may have unique pharmacological activities that merit further research and development (Table 3; Figure 5).
4 Pharmacology
To date, C. majus has demonstrated a diverse range of pharmacological effects, with its aerial parts and roots being rich in various alkaloids. This paper explores the alkaloids of C. majus, which are widely found in numerous plants and significantly impact human health. As research on C. majus deepens, its alkaloid components have been found to interact with multiple biological targets, exerting therapeutic effects on various diseases. These include antibacterial, antifungal, anti-tumor, anti-inflammatory, analgesic, expectorant, anti-cough, anti-asthma, and anti-liver fibrosis activities.
4.1 Anti-bacterial effect
Studies conducted in the past few years have shown that the alkaloids in C. majus have broad-spectrum antibacterial effects. Chelerythrine (CHE), extracted from C. majus, exhibits a strong antibacterial effect on Streptococcus mutans, the main caries-causing bacterium in the oral cavity. It effectively reduces the adhesion ability of S. mutans, suggesting its potential use as a preventative treatment for dental caries (Chen et al., 2011). Staphylococcus aureus (S. aureus) and methicillin-resistant S. aureus (MRSA) are common clinical pathogens. 8-hydroxydihydrosanguinarine (HHS) and 8-hydroxydihydrochelerythrine (HHC), solated from C. majus, have shown significant inhibition against MRSA strains, with minimal inhibitory concentrations/minimal bactericidal concentrations (MIC/MBC) of MRSA strains ranging from 0.49–15.63/1.95–62.50 μg/mL (Zuo et al., 2008). Sanguinarine (SNG) disrupts the cytoplasmic membrane, causing cell lysis, and is effective against MRSA, with MIC values between 3.12 μg/mL and 1.56 μg/mL, and the activity range was found to be between 3.12 μg/mL and 6.25 μg/mL (Obiang-Obounou et al., 2011). Remarkably, a study confirmed that SNG, CHE, and their derivatives exhibit robust antibacterial effects against S. aureus, Escherichia coli (E. coli), and Aeromonas hydrophila (Miao et al., 2011). Another study evaluated the antimicrobial potential of the major alkaloids in C. majus and found that C. majus was most effective against Pseudomonas aeruginosa (MIC of 1.9 mg/L), while SNG showed effectiveness against S. aureus (MIC of 1.9 mg/L) (Zielińska et al., 2019). Moreover, chelidonine (CHLD), SNG, and CHE also demonstrated inhibitory effects on E. coli (Móricz et al., 2015). These antibacterial effects have been summarized in Table 4.
TABLE 4.
The anti-bacterial and anti-fungal effects of alkaloids from Chelidonium majus L.
| Compounds | Models | Positive control | Results | Ref. |
|---|---|---|---|---|
| Anti-bacterial | ||||
| Chelerythrine | Streptococcus mutans | Exhibited inhibitory activity | Chen et al. (2011) | |
| Pseudomonas aeruginosa | MIC = 1.9 mg/L | Zielińska et al. (2019) | ||
| S. aureus, E. coli, Aeromonas hydrophila | Penicillin sodium Ceftriaxone sodium |
Exhibited inhibitory activity | Miao et al. (2011) | |
| Sanguinarine | S. aureus, E. coli, Aeromonas hydrophila | Penicillin sodium Ceftriaxone sodium |
Exhibited inhibitory activity | Miao et al. (2011) |
| Sanguinarine | S. aureus | MIC = 1.9 mg/L | Zielińska et al. (2019) | |
| Sanguinarine | MRSA | Ampicillin Ciprofloxacin |
MIC = 3.12–6.25 μg/mL | Obiang-Obounou et al. (2011) |
| Sanguinarine | E. coli | Exhibited inhibitory activity | Móricz et al. (2015) | |
| Chelidonine | E. coli | Exhibited inhibitory activity | Móricz et al. (2015) | |
| HHS | MRSA | Vancomycin | MIC/MBC = 0.49–15.63 μg/mL | Zuo et al. (2008) |
| HHC | MRSA | Vancomycin | MIC/MBC = 1.95–62.50 μg/mL | Zuo et al. (2008) |
| Anti-fungal | ||||
| Chelerythrine | Candida albicans | Penicillin sodium | MIC = 2–16 μg/mL | Gong et al. (2019) |
| Ustilaginoidea virens | EC50 = 6.53 × 10−3 mg/mL | Wei et al. (2020) | ||
| Cochliobolus miyabeanus | EC50 = 5.62 × 10−3 mg/mL | Wei et al. (2020) | ||
4.2 Anti-fungal effect
The alkaloids and derivatives in C. majus exhibit significant antifungal activity, and the mechanism of action has gradually been revealed. This provides innovative insights and approaches for the development of new antifungal agents and the control of agricultural diseases. CHE induces the accumulation of reactive oxygen species (ROS) by increasing the intracellular calcium concentration in mycelium, thus inhibiting the growth of Candida albicans mycelium, with MIC values ranging from 2 to 16 μg/mL (Gong et al., 2019). Furthermore, Wei QH’s experiment studied the in vitro antifungal activity of CHE against five rice pathogenic fungi. The results indicated that the EC50 values for Ustilaginoidea virens and Cochliobolus miyabeanus were 6.53 × 10−3 mg/mL and 5.62 × 10−3 mg/mL, respectively (Wei et al., 2020). These antifungal effects have been summarized in Table 4.
4.3 Anti-viral effect
The yellow latex of C. majus is widely used in folk medicine to treat human papillomavirus (HPV) because of its antiviral properties. Recent studies have found that its antiviral effect is mainly attributed to the alkaloid and protein components contained in the latex, which can target different stages of the virus replication cycle, effectively reducing HPV infection, and suppressing the expression of the viral oncogenes (E6, E7) at the mRNA and protein levels (Musidlak et al., 2022). Moreover, CHE can directly target the gB and gD glycoproteins on the surface of HSV-1, thereby inhibiting HSV-1 infection by preventing the binding of the virus to cells (Hu et al., 2023). In addition to its effect on human viruses, C. majus can also be employed for the prevention and control of agricultural plant virus es. Three alkaloids isolated from C. majus have shown activity against Tobacco mosaic virus (TMV). CHE and CHLD significantly inhibit TMV, while SNG moderately reduces TMV infection, thereby mitigating virus-induced damage in plants (Guo et al., 2021).
4.4 Analgesic effect
The alkaloids in C. majus exhibit significant analgesic effects on inflammatory pain, cancer-related pain, peripheral neuralgia and other types of pain, demonstrating high clinical application value. At present, C. majus is used as the main ingredient in clinical analgesic drugs. For example, Weitongshu capsules alleviate pain associated with gastric ulcers, and the compound Chinese medicine Tongan injection treats cancer pain caused by radiotherapy and chemotherapy or non-radiotherapy and chemotherapy. The analgesic mechanism of C. majus extract is different from that of morphine, indicating that it does not act as a narcotic analgesic and is devoid of side effects such as addiction, showing peripheral analgesic effects that are highly valued in clinical application (Li et al., 2013). CHE is one of the primary constituents of C. majus, and progress has been made in understanding its analgesic mechanism. It mitigates the occurrence of neuropathic pain by inhibiting the activation of PKC and spinal cord astrocytes (Chen et al., 2014). In addition, for chronic pain such as functional abdominal pain, a combination of network pharmacology and molecular docking technology revealed that alkaloids in C. majus mainly induce central analgesia through a network mode of multiple target interventions, targeting SRC, AKT1, EGFR, CASP3, and MAPK3, resulting in anti-functional abdominal pain effects (Zhang et al., 2023).
4.5 Anti-inflammatory effect
Inflammation is common in clinical practice, making the study of anti-inflammatory drugs essential. CHE can inhibit the production of PGE2 by modulating COX-2, a crucial enzyme in the response to inflammation, thus exerting an anti-inflammatory effect (Niu et al., 2011). The tumor necrosis factor (TNF)-induced nuclear factor-kappa B (NF-κB) signaling pathway has been discovered in some types of inflammation, among which the TNF-α/NF-κB pathway is a well-studied typical inflammatory signaling pathway and the core of coordinating inflammatory immune response. CHE was found to mitigate the inflammatory response of lipopolysaccharide (LPS)-induced serum levels of TNF-α and NO production in mouse models of endotoxin shock (Li et al., 2012). Another study found that CHE protects against LPS-induced acute lung injury, inhibits the production of inflammatory factors such as TNF-α, IL-6, and IL-1β, and reduces pulmonary edema and neutrophil infiltration. The mechanism may be related to the inhibition of NF-κB activation and interference with the nuclear translocation of Nrf2 protein (Fan et al., 2018). CHE also significantly reduces the gastric ulcer index, inhibits NO concentration, IL-6 and TNF-α levels in serum and gastric mucosa of mice with gastric ulcers, while markedly attenuating the overexpression of NF-κB in the gastric mucosa to exert anti-inflammatory activity (Li et al., 2014). CHE is used to treat mice with acetic acid-induced ulcerative colitis, an inflammatory bowel illness, by blocking the generation of NO and TNF-α inflammatory cytokines (Xu et al., 2014; Wu et al., 2022). It has also been found that CHE can promote apoptosis and autophagy in rheumatoid arthritis by influencing the expression of genes related to autophagy and apoptosis (including Bax, Bcl-2, PARP, and ULK1) and the AMPK/mTOR/ULK-1 signaling pathway, thus inhibiting rheumatoid arthritis in vivo and in vitro (Cai et al., 2022). In addition, it regulates key signaling pathways in SARS-CoV-2 infection (including Nrf2, NF-κB, and p38 MAPK activity) to prevent excessive inflammatory immune responses (Valipour et al., 2021).
Several researchers have also isolated 6-acetonyl-5,6-dihydrosanguinarine (ADS) from C. majus and discovered that ADS can induce the production of the inflammatory cytokines TNF-α, IL-6, and IL-8 by macrophages and dendritic cells. These inflammatory cytokines are important for the inflammatory response, and their excessive production often leads to the aggravation of inflammation and the development of diseases. ADS can trigger the release of pro-inflammatory cytokines through the ROS-JNK/ERK-NF-κB signaling pathway, thus inhibiting the occurrence of the inflammatory response (Kim et al., 2013).
Furthermore, CHLD exhibits significant anti-inflammatory actions, inhibiting LPS-induced inflammatory responses in vitro and in vivo by blocking the TLR4/NF-κB signaling pathway in RAW264.7 macrophages (Liao et al., 2018). It has been experimentally demonstrated that inhibiting TNF-induced NF-κB activation and modulating NF-κB regulatory gene products display anti-inflammatory, anti-proliferative, pro-apoptotic, and anti-invasion effects. Therefore, CHLD can alleviate the inflammatory response in HCT 116 human colon cancer cells (Zhang et al., 2018). CHLD inhibits IL-1β-mediated inflammation by modulating the NF-κB pathway in vitro, thereby preventing cartilage degeneration and synovial inflammation in rats with osteoarthritis (Li et al., 2023). In particular, CHLD also suppresses the production of IL-4, IL-17, eotaxin-2, and Ovalbumin-specific IgE through the STAT6 and FOXP3 pathways and can be used to treat airway inflammation (Kim et al., 2015). CHLD can prevent inflammatory damage in porcine small intestinal epithelial cell line IPEC-J2 cells by significantly reducing pro-inflammatory factors and promoting IL-10 expression (Lin et al., 2024).
In addition to the above two alkaloids, there may be other ingredients in C. majus with anti-inflammatory activities. These components might influence the inflammatory response through different mechanisms, thereby synergistically enhancing the anti-inflammatory effects of C. majus. The anti-inflammatory actions of these alkaloids were examined in macrophage RAW264.7 cells to ascertain their inhibitory impact on the production of NO caused by LPS. CHLD and HHS exhibited strong inhibitory activities on NO production in LPS-induced macrophage RAW 264.7 cells (Park et al., 2011). The impact of five alkaloids on the secretion of IL-1β, IL-8, and TNF-α in human polymorphonuclear leukocytes (neutrophils) was determined. It was found that berberine, CHLD, and CHE notably reduced TNF-α secretion in a concentration-dependent manner, while SNG inhibited IL-1β secretion and coptisine slightly decreased TNF-α, IL-1β, and IL-8 secretion (Zielińska et al., 2020). In another study, stylopine was found to inhibit macrophage NO in a concentration-dependent manner by suppressing the expression of iNOS, COX-2, NO, and PGE2, which may be related to the anti-inflammatory activities of C. majus (Jang et al., 2004).
4.6 Anti-cancer effect
Cancer is currently the primary cause of disease-related death in humans, and both its incidence and mortality are rising globally (Chen et al., 2024). Therefore, research and treatment of cancer are particularly urgent. Researchers both domestically and overseas have carried out a significant number of in vitro and in vivo experiments on the anti-tumor effects of C. majus in recent years. Studies have indicated that alkaloids extracted and isolated from C. majus have significant biological activities in anti-tumor treatment, inhibiting the proliferation, migration, and invasion of tumor cells through various mechanisms. These alkaloids play an anti-cancer role in cervical cancer, lung cancer, liver cancer, and other cancers by promoting apoptosis, altering the cell cycle, inducing autophagy, and activating mitochondrial apoptosis. These anti-cancer effects have been summarized in Table 5.
TABLE 5.
The anti-cancer effects of alkaloids from Chelidonium majus L.
| Compounds | Models | Results | In Vivo / In Vitro | Ref. |
|---|---|---|---|---|
| Chelidonine | HepG2 cells | Inhibited the proliferation | In vitro | Noureini and Wink (2009) |
| MHCC97-H cells, LM3 cells, nude mice or BalB/c mice | Inhibited the process of EMT and enhances the antitumor effect of lenvatinib on HCC cells, IC50 = 7.72 ± 0.70 μmol/L and 6.34 ± 0.44 μmol/L, respectively | In vivo and in vitro | Hou et al. (2019) | |
| H1975 cells, nude mice | Inhibited cell growth in vitro and in vivo, IC50 = 2.58 ± 1.05 μmol/L | In vivo and in vitro | Xie et al. (2020) | |
| SGC-7901 cells | Induced mitotic slippage and apoptotic-like death, IC50 = 23.13 μmol/L | Qu et al. (2016) | ||
| BALB/c mice, Renca C | Effectively inhibited tumor proliferation | In vivo | Pan et al. (2023) | |
| KB cells | Inhibited proliferation, invasion and promoted apoptosis | In vitro | Tao and Ran (2018) | |
| BxPC-3, MIA PaCa-2 cells | Induced apoptosis | In vitro | Jang et al. (2021) | |
| MCF-7 cells | Strongly suppressed cell growth | In vitro | Noureini and Esmaili (2014) | |
| MDA-MB-231 cells | Inhibited migration and invasion of cells | In vitro | Kim et al. (2015b) | |
| T98G cells | Through multipolar spindle assembly causing G2/M arrest in T98G cells | In vitro | Lee et al. (2019) | |
| Chelerythrine chloride | SMMC-7721 cells | Inhibited tumor growth and induced apoptosis | In vitro | Zhang et al. (2011b) |
| MHCC97-H cells | Inhibited cell growth, invasion and migration | In vitro | Cheng et al. (2019) | |
| HEK-293 and SW-839 cells | Inhibited cell proliferation and induced apoptosis | In vitro | Chen et al. (2016) | |
| Chelerythrine | HepG2 cells | IC50 of 6,12,24 h was 12.98, 10.53, 11.21 μmol/L, respectively | In vitro | Han and Zhu (2016) |
| HepG2 cells | Induced apoptosis | In vitro | Lin et al. (2022) | |
| C57BL/6 mice | Inhibited tumor growth | In vivo | Jin and Lu (2024) | |
| BGC823 cells | IC50 of 24, 48, 72 h was 2.87, 0.903, 0.468 μg/mL, respectively | In vitro | Zong and Liu (2006) | |
| HeLa cells | Induced apoptosis | In vitro | Yu et al. (2000) | |
| SKOV3 cells | Inhibited the proliferation, migration, invasion, and EMT | In vitro | Zhou et al. (2024) | |
| HL-60 cells | Induced apoptosis and necrosis, IC50 = 2.6 μmol/L | In vitro | Vrba et al. (2008) | |
| B16 cells | Inhibited proliferation and induced apoptosis | In vitro | Zhang et al. (2018a) | |
| 5–8 F cells | Inhibited cell proliferation and induced apoptosis | In vitro | Chen et al. (2024) | |
| HCT-116, RKO cells | Inhibited cell growth and induced apoptosis | In vitro | Liu and Jiang (2019) | |
| Zebrafish, ACC2 cells | Inhibited cell growth and proliferation and induced apoptosis | In vivo and in vitro | Li et al. (2021a) | |
| Sanguinarine | HeLa cells | Induced apoptosis | In vitro | Alakkal et al. (2022) |
| SKOV3 cells | Inhibited cell viability, promoted cell apoptosis and suppressed cell migration and invasion | In vitro | Zhang et al. (2018b) |
4.6.1 Liver cancer
Isoquinoline alkaloids in C. majus have anti-telomerase activity, a key target of therapeutic intervention in cancer cells. Experiments have demonstrated that CHLD inhibits telomerase activity in HepG2 cells by down-regulating the expression of human telomerase reverse transcriptase and inducing apoptosis (Noureini and Wink, 2009; Noureini et al., 2017). CHLD inhibits the epithelial-mesenchymal transition (EMT) process, enhances the apoptotic effect of lenvatinib on HCC cells in nude mice, and reduces the in vivo growth of hepatocellular carcinoma tumors (Hou et al., 2019). Another study indicated that chelerythrine chloride (CHECL) significantly inhibits SMMC-7721 cell proliferation in a time-and dose-dependent manner by blocking the S-phase of SMMC-7721 cells, and activating the mitochondrial apoptosis pathway by regulating the expression of Bcl-2 family proteins, thus inducing apoptosis in SMMC-7721 cells (Zhang et al., 2011). CHECL also inhibits growth, invasion, and migration in the highly metastatic human hepatocellular carcinoma cell line MHCC97-H (Cheng et al., 2019). Additionally, CHE upregulates the relative expression of Bax and Caspase-3 proteins and mRNA, decreases the relative expression of Bcl-XL protein and mRNA, prevents proliferation in HepG2 hepatoma cells, and ultimately induces apoptosis (Han and Zhu, 2016). Experiments by Lin’s team showed that CHE exposure induces excess ROS generation and triggers oxidative stress and mitochondrial apoptosis pathways in HepG2 cells, ultimately causing apoptosis in HepG2 cells (Lin et al., 2022).
4.6.2 Lung cancer
Lung cancer (LC) is a serious health problem that can lead to significant morbidity and mortality. In the treatment of LC, CHE can reduce tumor growth in Lewis lung cancer transplanted mice by targeting the NF-κB/HIF-1α signaling pathway and down-regulating the expression levels of NF-κB and HIF-1α proteins, thereby achieving a therapeutic effect (Jin and Lu, 2024). Clinical findings indicate that the majority of non-small cell lung cancer (NSCLC) patients exhibit mutations in the epidermal growth factor receptor tyrosine kinase (EGFR), leading to increased EGFR activity and facilitating metastasis and progression of NSCLC (Jiang et al., 2022). Targeted therapy directed at mutant forms of EGFR, such as the small molecule inhibitor Gefitinib, has shown successful application in the treatment of NSCLC patients. Clinical data supports its efficacy in significantly prolonging patient survival; however, prolonged use often leads to acquired resistance in most individuals (Bai et al., 2019). Therefore, researchers found that CHLD can inhibit the growth of Gefitinib-resistant non-small cell LC cells by modulating the EGFR-AMPK signaling pathway. They also found that CHLD mediates apoptosis through the mitochondrial pathway by decreasing the expression of AKT and Bcl-2 and increasing the cleavage of Bax and Caspase-3 expression (Xie et al., 2020).
4.6.3 Gastric cancer
Gastric cancer is a malignant neoplasm originating in the upper lining of the stomach. CHE can effectively induce apoptosis in human gastric cancer (BGC 823) cells, and this apoptosis is cell cycle-dependent (Zong and Liu, 2006). In another study, CHECL was found to inhibit cell proliferation in a time - and dose-dependent manner, causing cell cycle arrest and, apoptosis in BGC-823 cells through the reduction of mitochondrial membrane potential, release of cytochrome c, activation of caspase-3, disruption of PARP, and dysregulation of BCL-2 family proteins (Zhang et al., 2012). Additionally, CHLD can induce SFC-7901 M phase arrest and mitotic slippage in human gastric cancer cells by down-regulating the expression of BubR1, Cdk1 and cyclin B1 proteins (Qu et al., 2016).
4.6.4 Renal cell carcinoma
Renal cell carcinoma is among the ten most prevalent cancers in humans. CHLD has been found to regulate the expression of proteins Smad3 and Smad7 in the TGF-β1/Smad pathway to inhibit tumor proliferation in tumor-bearing mice with renal cell carcinoma (Pan et al., 2023). Chen et al. (2016) also demonstrated that CHECL may induce apoptosis in renal cancer cells by inhibiting ERK activity.
4.6.5 Breast cancer
Breast cancer (BC) is a disease that threatens human life and health worldwide. According to TCM, BC belongs to the category of “ru yong” and “ru shi yong," mainly caused by the deficiency of zheng qi and the imbalance of yin and yang in the viscera (Liu et al., 2021). CHLD is highly cytotoxic to cancer cells and induces MCF-7 BC cell death by potently inhibiting telomerase activity and stimulating multiple mechanisms of cell death, including apoptosis, autophagy, and senescence (Noureini and Esmaili, 2014). In addition, CHLD demonstrates anti-migration and anti-invasion effects in MDA-MB-231 BC cells by preventing the formation of the integrin-linked kinase/PINCH/α-parvin complex (Kim et al., 2015). CHE can play an anti-BC role through the PI3K/AKT signaling pathway (Zhang et al., 2021). CHLD inhibits the mitosis of BC cells by inducing M-phase arrest and blocking the AKT/FOXO3/FOXM1 axis, thus exerting anti-BC effects (Li et al., 2024). Chelidonium Herba-Corydalis Rhizoma is one of the commonly used prescriptions for BC in TCM. It can suppress the expression of ERα, p-PI3K, p-Akt protein, and ESR1 mRNA, as well as inhibit the growth of MCF-7 cells (IC50 value: 693 μg/mL), suggesting that its anti-ER-positive BC effect may be connected to the modulation of ER and PI3K/Akt signaling pathways (Zou et al., 2023).
4.6.6 Cervical cancer
CHE has been proven to exert anti-tumor effects by inducing apoptosis in HeLa cells through activation of the P38/JNK signaling pathway (Yu et al., 2000). Chelerythrine hydrochloride inhibits the proliferation of cervical cancer HeLa cells by triggering mitochondrial apoptosis via the PI3K/BAD signaling pathway (Yang et al., 2020). Another isoquinoline alkaloid, CHLD, has also shown effectiveness in triggering apoptosis in HeLa cells, exerting anti-cancer effects by upregulating the expression of pro-apoptotic genes such as, p38 and p53 and downregulating the expression of anti-apoptotic genes including AKT, PI3K, JAK3, STAT3, E6, and E7 (Paul et al., 2012). Protoberberine alkaloids (coptisine, berberine, and their derivatives, like stylopine) isolated from C. majus have demonstrated anti-cervical cancer activity by interfering with reactive oxygen species production, intracellular caspase activation, and mitochondrial function (Warowicka et al., 2019). The main latex proteins in C. majus, along with berberine, 8-hydroxycheleritrine, and dihydroberberine, can reduce the in vitro activity of human cervical cancer cells (both HPV-negative and HPV-positive) and synergistically play an anti-cancer role (Nawrot et al., 2021). SNG has long been considered an anti-tumor drug; studies have shown that it induces death in HeLa cells by activating apoptosis and ferroptosis (Alakkal et al., 2022).
4.6.7 Ovarian cancer
Ovarian cancer, often termed the “silent killer” due to its high mortality rate, shows promising response to SNG in epithelial ovarian cancer cells by controlling the CASC2-EIF4A3 axis, blocking NF-κB signaling, or the PI3K/AKT/mTOR pathway (Zhang et al., 2018). Meanwhile, research on CHE indicates its effectiveness in inhibiting proliferation, migration, and invasion of human Ovarian cancer SKOV3 cells, preventing or alleviating the occurrence of SKOV3 cell epithelial-mesenchymal transition, and suppressing tumor metastasis (Zhou et al., 2024).
4.6.8 Leukemia
CHE and dihydrochelerythrine have been shown to arrest the cell cycle of HL-60 cells in the G1 phase, alter cell cycle distribution, and activate the mitochondrial apoptosis pathway, inducing apoptosis and necrosis in human leukemia HL-60 cells (Vrba et al., 2008). Studies have also indicated that CHE and SNG induce dose-dependent DNA damage and increased cytotoxicity in primary mouse spleen cells and mouse Lymphpocytic Leukemia L1210 cells, while CHLD does not exhibit significant cytotoxic or DNA damaging effects on these cells but can completely inhibit the growth of L1210 cells (Kaminskyy et al., 2008). Moreover, the Havelek R team confirmed significant cytotoxicity of CHLD and homochelidonine, effectively inducing leukemia cell death (Havelek et al., 2016). SNG, berberine, and C. majus extracts have also exhibited significant cytotoxic and pro-apoptotic activities against hematopoietic cell lines HL-60, HL-60/MX1, HL-60/MX2, CCRF/CEM and CEM/C1, J45.01, and U266B, suggesting their potential utility in treating various types of leukemia (Och et al., 2019).
4.6.9 Melanoma
Melanoma, a type of skin cancer originating from the malignant transformation of melanocytes, is concerning due to its malignancy and treatment challenges. CHE inhibits the proliferation of B16 cells in a dose- and time-dependent manner significantly increases the early and late apoptosis rates of B16 cells, and upregulates the expression levels of Caspase-3 and Bax genes, while reducing the expression levels of Bcl-2 genes. It has been demonstrated that CHE suppresses the activation of the Wnt/β-catenin signaling pathway, thereby slowing B16 cell proliferation and promoting apoptosis (Zhang et al., 2018). Experimental data have shown that uveal melanoma cells undergo necrotic cell death and apoptosis when exposed to benzophenanthine alkaloids (SNG, CHE, and CHLD) (Kemény-Beke et al., 2006). Furthermore, benzphenanthridine alkaloids, including chelilutine, CHE, and SNG, exhibit a strong antiproliferative effect on malignant melanoma cells; these alkaloids induce apoptosis by reducing levels of anti-apoptotic proteins (Bcl-xL, Mcl-1, and xIAP), leading to decreased mitochondrial membrane potential and cleavage of caspase-3 and PARP (Hammerová et al., 2011).
4.6.10 Nasopharyngeal carcinoma
SNG, in combination with 5-fluorouracil, synergistically inhibits the growth of nasopharyngeal carcinoma grafts in vivo, inducing autophagy and apoptosis related to the PI3K/AKT/mTOR signaling pathway (Peng et al., 2022). SNG inhibits the growth of human nasopharyngeal carcinoma 5–8 F cells, induces autophagy, and suppresses proliferation by activating AMPK/mTOR signaling (Su et al., 2022). Experiments confirm that both SNG and CHE inhibit nasopharyngeal carcinoma cell proliferation and induce apoptosis by regulating the PI3K/AKT and MAPK signaling pathways (Chen et al., 2024).
4.6.11 Others
CHLD increases the growth of the human oral epithelioid cancer cell line KB in a time- and dose-dependent manner, and inhibits KB cells invasion in a dose-dependent manner. It upregulates Bax expression and decreases Bcl-2 expression, activating Caspase-3 and inducing KB cell apoptosis. This effect may be mediated through dual inhibition of Akt and MAPK signaling pathways, inhibiting Bcl-2 expression and promoting Caspase-3 expression, thereby suppressing cancer cell proliferation, fostering apoptosis, and impeding tumor growth and metastasis (Tao and Ran, 2018). Moreover, CHLD induces G2/M phase block in BxPC-3 and MIA PaCa-2 cells by downregulating CDK 1, and increases S-phase block induced by GADD 45a by upregulating p21 and p53, culminating in pancreatic cancer cell apoptosis through Caspase-3 cleavage (Jang et al., 2021). Dihydrosanguinarine exhibits inhibitory effects on K-Ras and TP53 mutant pancreatic cancer cell lines by bidirectionally modulating mut-p53/-Ras and WT-p53/-Ras proteins (Wu et al., 2019). CHLD induces apoptosis in human glioblastoma cells through G2/M phase arrest and Mcl-1 degradation (Lee et al., 2019). CHE triggers apoptosis by activating ROS-mediated mitochondrial dysfunction in colorectal cancer cells (Liu and Jiang, 2019). CHE effectively inhibits the growth and proliferation of adenoid cystic carcinoma cells and induces apoptosis by increasing ROS levels and upregulating NF-κB, p-JNK and p-p38 expression in cells (Li et al., 2021).
4.7 Anti-hepatic fibrosis effect
The mRNA expression of TGF-1, Smad3, Smad4, and the negative regulator Smad7 varied significantly following different doses of CHE in the mouse model of carbon tetrachloride-induced hepatic fibrosis, suggesting that CHE could inhibit the signaling of the TGF-β receptor complex from the cytoplasm to the nucleus. Additionally, CHE interfered with the expression of TGF-β1, Smad4, and Smad7 proteins, further confirming its inhibitory effect on hepatic fibrosis in mice, associated with the TGF-β/Smads signaling pathway (Li et al., 2018). After administering different doses of CHLD to rats with carbon tetrachloride-induced hepatic fibrosis, the protein phosphorylation levels of PI3K, Akt, mTOR, and mRNA expression levels of corresponding genes in their tissues were increased to varying degrees. This indicates that CHLD can regulate the mRNA and protein expression of genes related to the PI3K/Akt/mTOR pathway, affecting the expression of autophagy marker proteins LC3 and p62, preventing the activation of hepatic stellate cells, and inhibiting liver fibrosis (Li et al., 2021). Using TGF-β1-activated rat hepatic stellate cells CFSC-8B as a model of hepatic fibrosis, it was observed that CHLD inhibited the proliferation of TGF-β1-activated hepatic stellate cells, further demonstrating its potential to reverse liver fibrosis (Li et al., 2019).
4.8 Anti-alzheimer effect
Alzheimer’s disease (AD) is a progressive neurodegenerative disease with an insidious onset that causes neuronal damage in the brain, leading to memory loss, cognitive decline, and behavioral changes. Acetylcholinesterase plays a critical role in nerve function, as it is a serine protease produced by motor neurons and muscle junctions that hydrolyzes the neurotransmitter acetylcholine into acetic acid and choline, thereby terminating nerve impulses. Decreased levels of acetylcholine are a key factor in the onset of AD. Two compounds, HHC and HHS, isolated from C. majus, exhibit strong inhibitory activity against acetylcholinesterase. They slow down the breakdown of acetylcholine and increase its levels in the brain. Therefore, these compounds have been suggested as potential alternatives to anti-dementia drugs (Cho et al., 2006).
4.9 Anthelmintic effect
Chelidonium majus not only holds medicinal value, but also serves as a pesticide. Plant-derived alkaloid insecticides are crucial components of plant pesticides, showing significant repellent and insecticidal activities against numerous pests. CHLD has been found to possess substantial anthelmintic activity against Dactylogyrus intermedius, achieving a 100% anthelmintic effect at 0.9 mg L−1. The EC50 value after 48 h of exposure (the concentration required to achieve a 50% deworming effect) was 0.48 mg L−1 (Yao et al., 2011). The insecticidal mechanisms of alkaloids in C. majus are diverse, contributing to larvicidal effects by disrupting enzyme activity, reducing food intake, affecting nutritional indexes, and downregulating mRNA expression of enzyme genes (Zou et al., 2017). In another study, alkaloids in C. majus induced resistance to dietary intake and larval mortality in Lymantria dispar by inhibiting food intake and digestive enzymes (Zou et al., 2019).
4.10 Other effects
In the isolated ileal spasmolytic test model of guinea pigs, CHLD and protopine, when their concentration reaches 1 × 10−5 g/mL, can significantly induce ileal relaxation in response to barium chloride stimulation, with relaxation rates reaching 68.8% and 54.8%, respectively (Hiller et al., 1998). The DPPH free radical scavenging rate was determined using the DPPH method, with ascorbic acid as a positive control to evaluate the antioxidative activity of CHE solid dispersion (SD). The results showed that different mass concentrations of CHE-PEG-SD exhibited certain DPPH radical scavenging ability (IC50 = 0.124 mg/mL), albeit weaker than ascorbic acid (IC50 = 0.041 mg/mL) (Wang et al., 2020). Traditional medicine traditions utilize natural small-molecule compounds from C. majus to treat various gynecological disorders, including coptisine, protopine, berberine, and dihydroberberine (Lans et al., 2018). Supplementation with the protoberberine-rich fraction from C. majus in the diet of rats with experimentally induced endometriosis has been shown to inhibit the recurrence of endometriosis (Warowicka et al., 2021).
5 Toxicology
The utilization of plants and herbs in traditional medicine has a long history, with many herbs used to treat various ailments. However, it’s crucial to remember that not all herbs are harmless. Chelidonium majus was recorded as a toxic Chinese medicine in the Chinese Pharmacopoeia of the People’s Republic of China (2020 edition). The alkaloids found in C. majus, including CHLD, SNG, berberine, coptisine, and CHE, either alone or in combination, possess potential toxicity (Benninger et al., 1999). Following evaluation using liver-targeted causality assessment methods, several cases of hepatotoxicity were found to be likely or highly likely related to C. majus. Therefore, the toxicity of C. majus to liver function has always been a topic of concern, with some components potentially causing liver damage through different mechanisms. The potential hepatotoxicity of C. majus has also been confirmed in several reports from European countries, defining this hepatotoxicity as a unique form of herbal-induced liver injury (HILI) due to idiosyncratic metabolic reactions (Pantano et al., 2017). Ciornolutchii et al. reported two case studies in which patients exhibited liver damage after using pharmaceutical preparations containing C. majus, characterized by severe hypertransaminasemia. Their literature review also identified multiple HILI cases related to C. majus preparations (Ciornolutchii et al., 2024). The metabolism of CHLD was studied in a human liver microsomal model, revealing two demethylated metabolites containing phenolic hydroxyl groups after incubation with liver microsomes. These hydroxyl groups are easily oxidized into quinone compounds, which can combine with glutathione to form quinone-sulfide, depleting glutathione in the liver and causing hepatotoxicity (Zhang et al., 2018).
Alkaloids, in C. majus, such as CHE, SNG, berberine and coptisine, have significant inhibitory effects on mitochondrial respiration in mice, suppressing liver respiration by inhibiting mitochondrial enzymes like NADH dehydrogenase or succinic acid (Barreto et al., 2003). Furthermore, SNG induces chromosome breaks and DNA damage in mouse bone marrow cells (Das et al., 2004). Additionally, CHLD, berberine, and SNG from C. majus distinctly block hERG potassium channels, delaying heart repolarization and prolonging QT intervals, potentially increasing the risk of death (Orvos et al., 2015). Chronic exposure to C. majus can also cause toxic effects in specific organs; CHE has been observed to induce a dose-dependent long-term toxicity in rat lung tissue, resulting in symptoms of pulmonary congestion and bloody ascites (Liu et al., 2019). Despite C. majus having abundant pharmacological effects, caution should be exercised to avoid excessive or prolonged use to prevent toxic reactions.
Chinese herbal medicines can be processed to reduce or eliminate drug toxicity and side effects, while also modifying drug function and flavor, thereby improving therapeutic efficacy. Researchers have processed C. majus and discovered that the content of SNG and CHE in licorice products derived from C. majus is significantly lower than in raw products. The optimal processing method involves using 15% licorice, moistening for 3 h, and drying at 60°C for 12 h. This suggests that licorice processing technology has a certain detoxification effect (Xiao et al., 2021). In conclusion, although C. majus possesses numerous pharmacological effects and holds certain application value in the pharmaceutical field, its potential toxicity risks must also be given sufficient attention. A deeper understanding of its efficacy and safety through scientific research is essential, and it should be used cautiously, with a full understanding of its potential risks to prevent toxic reactions from excessive or long-term use.
6 Discussion
This plant has been revered for its antibacterial, antiviral, antitumor, anti-inflammatory, and other pharmacological effects for centuries. Alkaloids from C. majus, particularly benzophenanthridine and protoberberine alkaloids, show promising potential for application in anti-tumor therapy (Yang et al., 2024). Compounds such as CHE, CHLD, and SNG, known for their high content and notable anti-tumor activity, have been extensively studied. The alkaloids exert anti-tumor effects by promoting apoptosis, altering the cell cycle, inducing autophagy, and activating mitochondrial apoptosis. Even alkaloids present in small amounts, such as berberine (Mohammadlou et al., 2021), dihydrochelerythrine (Silva et al., 2018), 6-methoxydihydrosanguinarine (Wang et al., 2023), and berberrubine (He et al., 2023), demonstrate significant potential in tumor treatment. UkrainTM, a derivative of C. majus alkaloids comprising components like CHLD, CHE, SNG, protopine, and allocryptopine, induces apoptosis in cells and exerts toxic effects on cancer cells (Habermehl et al., 2006). However, the exact anti-tumor mechanisms of C. majus alkaloids remain unclear, necessitating further in-depth studies to elucidate their mechanisms of action.
Chelidonium majus alkaloids not only exhibit anti-tumor effects but also demonstrate significant antimicrobial effects. These alkaloid components are applied to human diseases and also play a role in preventing and controlling on plant diseases as well. Simultaneously, these alkaloids exert anti-inflammatory effects and treat various inflammations by regulating the immune system and various inflammation-related signaling pathways. As research on the pharmacological effects of C. majus alkaloids progresses, there is potential for further expanding their clinical applications. Analysis of other alkaloids with lower content has revealed that aporphine alkaloids, such as magnoflorine, may reduce blood glucose levels by promoting insulin release and stimulating insulin activity mechanisms, potentially improving postprandial hyperglycemia and demonstrating anti-diabetic effects (Patel and Mishra, 2011). Vennerstrom et al. discovered that protoberberine alkaloids showed potential antimalarial activity in vitro experiments (Vennerstrom and Klayman, 1988), while Xiang et al. explored the potential uses of these alkaloids in treating stomach diseases and providing gastric protective effects (Xiang et al., 2024). Berberine has also been noted to prevent and delay AD (Wang et al., 2024), while protopine may hold a potential role in asthma treatment (Yang et al., 2024). However, studies on these effects are limited and mostly at a preliminary stage. The results of pharmacological activity studies are mostly based on animal experiments, and the results of clinical studies may differ; further research is needed to verify their effects and mechanisms. Additionally, the synergistic effects between C. majus alkaloids and other drugs or ingredients cannot be overlooked and warrant further exploration.
The rich chemical composition and extensive biological activity of C. majus alkaloids provide valuable resources for the development of pharmaceutical drugs. However, there still exist limitations in the extraction process, content determination, and the relationship between structure and activity. Future research efforts should focus on optimizing extraction processes, improving the purity of bioactive alkaloids, delving into the relationship between structure and activity, elucidating the underlying mechanisms of action, and facilitating their practical application in the field of medicine.
7 Conclusions and perspectives
Chelidonium majus, a medicinal plant with a long history of application, has been extensively used in European countries as well as in China’s TCM, highlighting its extremely high medicinal value. Alkaloids are the main active components of the plant, which have garnered attention from many scholars. These alkaloids are abundant in content and variety, but each alkaloid exhibits unique chemical structures and can act on multiple biological targets to play a therapeutic role in diseases. This makes C. majus a vast potential area for research and development in drug research.
To date, researchers both domestically and internationally have isolated and identified 94 alkaloids from C. majus. This review therefore summarizes the research findings on the phytochemistry, pharmacology, and toxicology of these alkaloids. Additionally, it systematically classifies these alkaloids according to their structural properties, providing a foundational basis for the phytochemical classification of C. majus.
Through further in-depth study of these alkaloids, more candidate compounds can be provided for drug research and development, expanding their potential applications in the pharmaceutical field. However, it is worth noting that these alkaloids also have some toxicity. Therefore, in the application of C. majus for drug development or treatment of diseases, it is necessary to further explore the toxic mechanisms of these alkaloids to optimize the extraction process, reduce toxicity, and improve efficacy, thereby laying a solid foundation for their broad application in the pharmaceutical field. Simultaneously, strengthening the quality control and safety evaluation of C. majus is also crucial for future research directions, to effectively manage potential risks during drug development and ensure the safety and effectiveness of drugs. In summary, this review provides a basis for understanding the current research status of alkaloid components in C. majus. As science and technology advance and research methods improve, more comprehensive studies on C. majus alkaloids are expected, aiming to develop clinically valuable drugs and provide a solid theoretical framework for the in-depth research and application of C. majus.
Acknowledgments
We would like to thank Editage (http://www.editage.cn) for English language editing.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by Heilongjiang Provincial Key Research Plan (Grant No. GA22B012), the Excellent Innovative Talents Support Program of Heilongjiang University of Chinese Medicine (No. 2018RCD03), the Heilongjiang Province Education Department project (No. 12511512), the Heilongjiang Postdoctoral Foundation (No LBH-Z10021), the Heilongjiang Touyan Innovation Team Program (Grant No: [2019] No. 5), the National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Construction Program of National Administration of TCM (Grant Number: (2022) No. 75), the Seventh Batch of National Famous Old Traditional Chinese Medicine Experts Experience Heritage Construction Program of National Administration of TCM (Grant Number: (2022) No. 76).
Author contributions
X-LL: Writing–original draft. Y-PS: Writing–original draft. MW: Writing–review and editing. Z-BW: Writing–review and editing. H-XK: Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
C. majus, Chelidonium majus L.; TCM, Traditional Chinese medicine; CHE, Chelerythrine; SNG, Sanguinarine; CHLD, Chelidonine; CHECL, Chelerythrine chloride; HHS, 8-hydroxydihydrosanguinarine; HHC, 8-hydroxydihydrochelerythrine.
References
- Alakkal A., Thayyullathil F., Pallichankandy S., Subburayan K., Cheratta A. R., Galadari S. (2022). Sanguinarine induces H2O2-dependent apoptosis and ferroptosis in human cervical cancer. Biomedicines 10, 1795. 10.3390/biomedicines10081795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B., Zhang W. W. (2009). The latest research progress of Chelidonium majus L. Heilongjiang Med. J. 22, 794–796. 10.3969/j.issn.1006-2882.2009.06.015 [DOI] [Google Scholar]
- Bai X. J., Zhu M. L., Li B. H., Li H. M., Huo Q., Wu C. Z. (2019). Effects of parthenolide on apoptosis, invasion and migration of human non-small cell lung cancer H1975 cells. Chin. Pharmacol. Bull. 35, 673–679. 10.3969/j.issn.1001-1978.2019.05.017 [DOI] [Google Scholar]
- Barreto M. C., Pinto R. E., Arrabaça J. D., Pavão M. L. (2003). Inhibition of mouse liver respiration by Chelidonium majus isoquinoline alkaloids. Toxicol. Lett. 146, 37–47. 10.1016/j.toxlet.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Benninger J., Schneider H. T., Schuppan D., Kirchner T., Hahn E. G. (1999). Acute hepatitis induced by greater celandine (Chelidonium majus). Gastroenterology 117 (5), 1234–1237. 10.1016/s0016-5085(99)70410-5 [DOI] [PubMed] [Google Scholar]
- Bisai V., Saina Shaheeda M. K., Gupta A., Bisai A. (2019). Biosynthetic relationships and total syntheses of naturally occurring benzo [c] phenanthridine alkaloids. Asian J. Org. Chem. 8, 946–969. 10.1002/ajoc.201900244 [DOI] [Google Scholar]
- Bozhadze A. D., Vachnadze V. I., Dzhokhadze M. S., Berashvili D. T., Bakuridze A. D. (2013). Study on the separation process of pharmacological active total alkaloids from Chelidonium majus L. growing in Georgia. Georgian Med. News, 61–65. [PubMed] [Google Scholar]
- Bugatti C., Colombo M. L., Tomè F. (1987). High-performance liquid chromatographic separation of quaternary alkaloids of Chelidonium majus L. roots. J. Chromatogr. 393, 312–316. 10.1016/s0021-9673(01)94228-1 [DOI] [PubMed] [Google Scholar]
- Cai J., Zhang L.-C., Zhao R.-J., Pu L.-M., Chen K.-Y., Nasim A. A., et al. (2022). Chelerythrine ameliorates rheumatoid arthritis by modulating the AMPK/mTOR/ULK-1 signaling pathway. Phytomedicine 104, 154140. 10.1016/j.phymed.2022.154140 [DOI] [PubMed] [Google Scholar]
- Chang Y.-C., Chang F.-R., Khalil A. T., Hsieh P.-W., Wu Y.-C. (2003). Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z. Naturforsch. C J. Biosci. 58, 521–526. 10.1515/znc-2003-7-813 [DOI] [PubMed] [Google Scholar]
- Chen S. R., Wu T. H., Liu J., Zhang W. Q., Yao J. X., He Y. C., et al. (2024). Study on the material basis and potential mechanism of Chelidonium majus in the treatment of nasopharyngeal carcinoma based on network pharmacology, molecular docking and experimental study. J. Hunan Univ. Chin. Med. 44, 278–287. [Google Scholar]
- Chen X., Xu D. X., Cheng R. B. (2011). Scanning Electron Microscopic Observation of Inhibitory Effect of Chelerythrine on the Adherence of Streptococcus Mutans . J. China. Med. Univ. 40, 413–416. 10.3969/j.issn.1671-7651.2007.06.039 [DOI] [Google Scholar]
- Chen X.-M., Zhang M., Fan P.-L., Qin Y.-H., Zhao H.-W. (2016). Chelerythrine chloride induces apoptosis in renal cancer HEK-293 and SW-839 cell lines. Oncol. Lett. 11, 3917–3924. 10.3892/ol.2016.4520 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen Y., Liang Y. P., Guo L., Sun J., Zhou Z. D., Hu Y. H., et al. (2014). Effect of chelerythrine pre-administration on pain behavior and activation of spinal cord astrocytes in neuropathic pain rats. J. Clin. Anesth. 30, 77–80. [Google Scholar]
- Cheng J. Y., Zhao L. Z., Li W., Xie G. Y. (2019). The regulatory effect of chelerythrine chloride on the growth, invasion and migration of human HCC cell line MHCC97-H. Chin. J. Integr.Tradit. Wes. Med. Liver Dis. 29, 149–151+166. 10.3969/j.issn.1005-0264.2019.02.017 [DOI] [Google Scholar]
- Cho K.-M., Yoo I.-D., Kim W.-G. (2006). 8-hydroxydihydrochelerythrine and 8-hydroxydihydrosanguinarine with a potent acetylcholinesterase inhibitory activity from Chelidonium majus L. Biol. Pharm. Bull. 29, 2317–2320. 10.1248/bpb.29.2317 [DOI] [PubMed] [Google Scholar]
- Ciornolutchii V., Ismaiel A., Sabo C. M., Al Hajjar N., Seicean A., Dumitrascu D. L. (2024). A hidden cause of hypertransaminasemia: liver toxicity caused by Chelidonium majus L.: report of two cases of herb-induced liver injury and literature review. Am. J. Ther. 31, e382–e387. 10.1097/MJT.0000000000001708 [DOI] [PubMed] [Google Scholar]
- Colombo M. L., Bosisio E. (1996). Pharmacological activities of Chelidonium majus L. (Papaveraceae). Pharmacol. Res. 33, 127–134. 10.1006/phrs.1996.0019 [DOI] [PubMed] [Google Scholar]
- Das A., Mukherjee A., Chakrabarti J. (2004). Sanguinarine: an evaluation of in vivo cytogenetic activity. Mutat. Res. 563, 81–87. 10.1016/j.mrgentox.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Deng A.-J., Zhang H.-J., Li Q., Li Z.-H., Zhang Z.-H., Wu L.-Q., et al. (2017). Six scalemic mixtures of 6-monosubstituted dihydrobenzophenanthridine alkaloids from Chelidonium majus and optically active structures of enantiomers. Phytochemistry 144, 159–170. 10.1016/j.phytochem.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Deng A.-J., Zhang Z.-H., Li Q., Ma L., Qin H.-L. (2016). Two new hopane-type triterpenes from the aerial part of Chelidonium majus . Phytochem. Lett. 17, 75–78. 10.1016/j.phytol.2016.07.009 [DOI] [Google Scholar]
- Fan L., Fan Y., Liu L., Tao W., Shan X., Dong Y., et al. (2018). Chelerythrine attenuates the inflammation of lipopolysaccharide-induced acute lung inflammation through NF-κB signaling pathway mediated by Nrf2. Front. Pharmacol. 9, 1047. 10.3389/fphar.2018.01047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerenčer M., Turecek P. L., Kistner O., Mitterer A., Savidis-Dacho H., Barrett N. P. (2006). In vitro and in vivo anti-retroviral activity of the substance purified from the aqueous extract of Chelidonium majus L. Antivir. Res. 72, 153–156. 10.1016/j.antiviral.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Gilca M., Gaman L., Panait E., Stoian I., Atanasiu V. (2010). Chelidonium majus--an integrative review: traditional knowledge versus modern findings. Forsch. Komplementarmedizin. 17, 241–248. 10.1159/000321397 [DOI] [PubMed] [Google Scholar]
- Golkiewicz W., Gadzikowska M. (1999). Isolation of some quaternary alkaloids from the extract of roots of Chelidonium majus L. by column and thin-layer chromatography. Chromatographia 50, 52–60. 10.1007/bf02493617 [DOI] [Google Scholar]
- Gong Y., Li S., Wang W., Li Y., Ma W., Sun S. (2019). In vitro and in vivo activity of chelerythrine against Candida albicans and underlying mechanisms. Future Microbiol. 14, 1545–1557. 10.2217/fmb-2019-0178 [DOI] [PubMed] [Google Scholar]
- Guo W., Lu X., Liu B., Yan H., Feng J. (2021). Anti-TMV activity and mode of action of three alkaloids isolated from Chelidonium majus . Pest Manag. Sci. 77, 510–517. 10.1002/ps.6049 [DOI] [PubMed] [Google Scholar]
- Habermehl D., Kammerer B., Handrick R., Eldh T., Gruber C., Cordes N., et al. (2006). Proapoptotic activity of Ukrain is based on Chelidonium majus L. alkaloids and mediated via a mitochondrial death pathway. BMC Cancer 6, 14. 10.1186/1471-2407-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerová J., Uldrijan S., Táborská E., Slaninová I. (2011). Benzo [c] phenanthridine alkaloids exhibit strong anti-proliferative activity in malignant melanoma cells regardless of their p53 status. J. Dermatol. Sci. 62, 22–35. 10.1016/j.jdermsci.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Han C., Zhu G. F. (2016). Chelerythrine induces apoptosis of human hepatocellular carcinoma cells HepG2 and its mechanism. Chin. J. Exp. Tradit. Med. Formulae. 22, 127–130. 10.13422/j.cnki.syfjx.2016110127 [DOI] [Google Scholar]
- Han N., Yang Z., Liu Z., Liu H., Yin J. (2016). Research progress on natural benzophenanthridine alkaloids and their pharmacological functions: a review. Nat. Prod. Commun. 11, 1934578X1601100–1188. 10.1177/1934578x1601100838 [DOI] [PubMed] [Google Scholar]
- Hanaoka M., Chc W. J., Yoshida S., Mukai C. (1991). Chemical transformation of protoberberines. XVII. Biomimetic introduction of an oxy functionality at the C-10 position in the benzo(c)phenanthridine skeleton: synthesis of 2,3,7,8,10-pentaoxygenated benzo(c)phenanthridine alkaloids, chelilutine and sanguilutine. Chem. Pharm. Bul. 39, 1163–1166. 10.1248/cpb.39.1163 [DOI] [Google Scholar]
- Havelek R., Seifrtova M., Kralovec K., Krocova E., Tejkalova V., Novotny I., et al. (2016). Comparative cytotoxicity of chelidonine and homochelidonine, the dimethoxy analogues isolated from Chelidonium majus L. (Papaveraceae), against human leukemic and lung carcinoma cells. Phytomedicine 23, 253–266. 10.1016/j.phymed.2016.01.001 [DOI] [PubMed] [Google Scholar]
- He X., Cui J., Ma H., Abuduaini N., Huang Y., Tang L., et al. (2023). Berberrubine is a novel and selective IMPDH2 inhibitor that impairs the growth of colorectal cancer. Biochem. Pharmacol. 218, 115868. 10.1016/j.bcp.2023.115868 [DOI] [PubMed] [Google Scholar]
- Hiller K. O., Ghorbani M., Schilcher H. (1998). Antispasmodic and relaxant activity of chelidonine, protopine, coptisine, and Chelidonium majus extracts on isolated guinea-pig ileum. Planta Med. 64, 758–760. 10.1055/s-2006-957576 [DOI] [PubMed] [Google Scholar]
- Hong B., Meng Q., Jiang J. M., Zhu Y. J., Xu Y. H., Zhao L. (2022). Research progress on alkaloids of Chelidonium majus L. Ginseng Res. 34, 58–62. 10.19403/j.cnki.1671-1521.2022.02.016 [DOI] [Google Scholar]
- Hou F.-J., Guo L.-X., Zheng K.-Y., Song J.-N., Wang Q., Zheng Y.-G. (2019). Chelidonine enhances the antitumor effect of lenvatinib on hepatocellular carcinoma cells. Onco Targets Ther. 12, 6685–6697. 10.2147/OTT.S215103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Zhang C., Zhong J., Hu H., Li C., Zhao Y., et al. (2023). Chelerythrine inhibits HSV-1 infection by suppressing virus binding to the cells. Pharmacol. Res. - Mod. Chin. Med. 6, 100223. 10.1016/j.prmcm.2023.100223 [DOI] [Google Scholar]
- Huang X.-Y., Shao Z.-X., An L.-J., Xue J.-J., Li D.-H., Li Z.-L., et al. (2019). New lignanamides and alkaloids from Chelidonium majus and their anti-inflammation activity. Fitoterapia 139, 104359. 10.1016/j.fitote.2019.104359 [DOI] [PubMed] [Google Scholar]
- Jang H.-J., Yang J. H., Hong E., Jo E., Lee S., Lee S., et al. (2021). Chelidonine induces apoptosis via GADD45a-p53 regulation in human pancreatic cancer cells. Integr. Cancer Ther. 20, 15347354211006191. 10.1177/15347354211006191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. I., Kim B. H., Lee W. Y., An S. J., Choi H. G., Jeon B. H., et al. (2004). Stylopine from Chelidonium majus inhibits LPS-induced inflammatory mediators in RAW 264.7 cells. Arch. Pharm. Res. 27, 923–929. 10.1007/BF02975845 [DOI] [PubMed] [Google Scholar]
- Jeong W. T., Lim H. B. (2017). Determination of isoquinoline alkaloids by UPLC-ESI-Q-TOF MS: application to Chelidonium majus L. Anal. Sci. Technol. 30, 379–389. 10.5806/AST.2017.30.6.379 [DOI] [Google Scholar]
- Jiang J., Wang Y., Gao Y., Sugimura H., Minervini F., Uchino J., et al. (2022). Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. Transl. Lung Cancer Res. 11, 277–294. 10.21037/tlcr-22-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X. J., Lu C. Y. (2024). Inhibitory effect of leucovorin on growth and angiogenesis of subcutaneous transplanted tumors in mouse lung cancer cells and its mechanism. J. Jilin Univ. Ed. 50, 612–619. 10.13481/j.1671-587X.20240304 [DOI] [Google Scholar]
- Kaczmarek F., Malek B. (1959). Zur papierchromatographie der alkaloide von chelidonium majus l. Planta Med. 7, 171–173. 10.1055/s-0028-1101598 [DOI] [Google Scholar]
- Kadan G., Gözler T., Hesse M. (1992). (+)-Norchelidonine from Chelidonium majus . Planta Med. 58, 477. 10.1055/s-2006-961523 [DOI] [PubMed] [Google Scholar]
- Kadan G., Gözler T., Shamma M. (1990). (-)-Turkiyenine, a new alkaloid from Chelidonium majus . J. Nat. Prod. 53, 531–532. 10.1021/np50068a046 [DOI] [Google Scholar]
- Kaminskyy V., Lin K.-W., Filyak Y., Stoika R. (2008). Differential effect of sanguinarine, chelerythrine and chelidonine on DNA damage and cell viability in primary mouse spleen cells and mouse leukemic cells. Cell Biol. Int. 32, 271–277. 10.1016/j.cellbi.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Kemény-Beke A., Aradi J., Damjanovich J., Beck Z., Facskó A., Berta A., et al. (2006). Apoptotic response of uveal melanoma cells upon treatment with chelidonine, sanguinarine and chelerythrine. Cancer Lett. 237, 67–75. 10.1016/j.canlet.2005.05.037 [DOI] [PubMed] [Google Scholar]
- Kim D. H., Lee J. H., Park S., Oh S. S., Kim S., Kim D. W., et al. (2013). 6-Acetonyl-5,6-dihydrosanguinarine (ADS) from Chelidonium majus L. triggers proinflammatory cytokine production via ROS-JNK/ERK-NFκB signaling pathway. Food Chem. Toxicol. 58, 273–279. 10.1016/j.fct.2013.04.051 [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Lee J. H., Song Y. H., Jeong W. M., Tan X., Uddin Z., et al. (2015a). Human neutrophil elastase inhibitory alkaloids from Chelidonium majus L. J . Appl. Biol. Chem. 58, 281–285. 10.3839/jabc.2015.044 [DOI] [Google Scholar]
- Kim O., Hwangbo C., Kim J., Li D.-H., Min B.-S., Lee J.-H. (2015b). Chelidonine suppresses migration and invasion of MDA-MB-231 cells by inhibiting formation of the integrin-linked kinase/PINCH/α-parvin complex. Mol. Med. Rep. 12, 2161–2168. 10.3892/mmr.2015.3621 [DOI] [PubMed] [Google Scholar]
- Kim S.-H., Hong J.-H., Lee Y.-C. (2015c). Chelidonine, a principal isoquinoline alkaloid of Chelidonium majus, attenuates eosinophilic airway inflammation by suppressing IL-4 and eotaxin-2 expression in asthmatic mice. Pharmacol. Rep. PR. 67, 1168–1177. 10.1016/j.pharep.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Kim S. R., Hwang S. Y., Jang Y. P., Park M. J., Markelonis G. J., Oh T. H., et al. (1999). Protopine from Corydalis ternata has anticholinesterase and antiamnesic activities. Planta Med. 65, 218–221. 10.1055/s-1999-13983 [DOI] [PubMed] [Google Scholar]
- Kopyt’ko Y. F., Dargaeva T. D., Sokol’Skaya T. A., Grodnitskaya E. I., Kopnin A. (2005). New methods for the quality control of a homeopathic matrix tincture of greater celandine. Pharm. Chem. J. 39, 603–609. 10.1007/s11094-006-0028-4 [DOI] [Google Scholar]
- Kwasniewski V. (1958). Discovery of saponins in celandine, Chelidonium majus . Arch. Pharm. Ber. Dtsch. Pharm. Ges. 291/63, 209–211. 10.1002/ardp.19582910410 [DOI] [PubMed] [Google Scholar]
- Laines-Hidalgo J. I., Muñoz-Sánchez J. A., Loza-Müller L., Vázquez-Flota F. (2022). An update of the sanguinarine and benzophenanthridine alkaloids’ biosynthesis and their applications. Mol. Basel Switz. 27, 1378. 10.3390/molecules27041378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans C., Taylor-Swanson L., Westfall R. (2018). Herbal fertility treatments used in North America from colonial times to 1900, and their potential for improving the success rate of assisted reproductive technology. Reprod. Biomed. Soc. Online 5, 60–81. 10.1016/j.rbms.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T. P. L., Lee J. W., Kim J. G., Han J. S., Kwon H., Lee D., et al. (2021). Tetrahydroprotoberberine N-oxides from Chelidonium majus and their inhibitory effects on NO production in RAW 264.7 cells. Phytochem. Lett. 41, 38–42. 10.1016/j.phytol.2020.10.014 [DOI] [Google Scholar]
- Lee Y.-K., Lee K. W., Kim M., Lee Y., Yoo J., Hwangbo C., et al. (2019). Chelidonine induces caspase-dependent and caspase-independent cell death through G2/M arrest in the T98G human glioblastoma cell line. Evid. Based Complement. Altern. Med. 2019, 6318179. 10.1155/2019/6318179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Tang X., Sun Z., Qu Z., Zou X. (2024). Integrating bioinformatics and experimental models to investigate the mechanism of the chelidonine-induced mitotic catastrophe via the AKT/FOXO3/FOXM1 axis in breast cancer cells. Biomol. Biomed. 24, 560–574. 10.17305/bb.2023.9665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Tian F., Li M. Y., Liu M., Zhao X. M. (2013). Analgesic and Anti-inflammatory Effect of the Alkaloid in Chelidonium majus Extract. Chin. J. Exp. Tradit. Med. Formulae. 19, 262–265. 10.11653/syfj2013080262 [DOI] [Google Scholar]
- Li L., Yang X. L., Zhang Y., Liu K. C., Wang X., Wang R. C. (2021a). Inhibitory effect of chelerythrine on growth of adenoid cystic carcinoma and its mechanism. Drug Eval. Res. 44, 1361–1367. 10.7501/j.issn.16746376.2021.07.001 [DOI] [Google Scholar]
- Li M., Zhu Y., Shao J., Wang C., Dong B., Cui H., et al. (2023). Chelidonine reduces IL-1β-induced inflammation and matrix catabolism in chondrocytes and attenuates cartilage degeneration and synovial inflammation in rats. Braz. J. Med. Biol. Res. 56, e12604. 10.1590/1414-431X2023e12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Fan T., Zhang Y., Niu X., Xing W. (2012). Effect of chelerythrine against endotoxic shock in mice and its modulation of inflammatory mediators in peritoneal macrophages through the modulation of mitogen-activated protein kinase (MAPK) pathway. Inflammation 35, 1814–1824. 10.1007/s10753-012-9502-1 [DOI] [PubMed] [Google Scholar]
- Li W.-F., Hao D.-J., Fan T., Huang H.-M., Yao H., Niu X.-F. (2014). Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem. Biol. Interact. 208, 18–27. 10.1016/j.cbi.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Li X. M., Lin P. F., Dong M. X., Xu T. J., Yu C. L., Rong H., et al. (2019). Effects of chelidonine on proliferation, collagen synthesis and TGF-β1 receptor of activated rat hepatic stellate cells CFSC-8B in rats. China Pharm. 30, 1759–1763. 10.6039/j.issn.1001-0408.2019.13.07 [DOI] [Google Scholar]
- Li X. M., Ouyang T. T., Dong M. X., Cui T., Guo L. N., Dong W., et al. (2018). Effect of chelidonine on TGF-β/Smads signaling pathway in mice with hepatic fibrosis. Chin. J. Pathophysiol. 34, 1323–1328. 10.3969/j.issn.1000-4718.2018.07.028 [DOI] [Google Scholar]
- Li X. M., Wang W. B., Guo L. N., Song B., Dong W., Xu T. J., et al. (2021b). Improvement effects of chelidonine on CCl4-induced hepatic fibrosis model rats and its mechanism. China Pharm. 32, 2868–2874. 10.6039/j.issn.1001-0408.2021.23.09 [DOI] [Google Scholar]
- Liao W., He X., Yi Z., Xiang W., Ding Y. (2018). Chelidonine suppresses LPS-Induced production of inflammatory mediators through the inhibitory of the TLR4/NF-κB signaling pathway in RAW264.7 macrophages. Biomed. Pharmacother. 107, 1151–1159. 10.1016/j.biopha.2018.08.094 [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Yang L. Y., Li J. W., Lin F., Li J. (2024). Preventive effect of chelidonine on IPEC-J2 cells induced by H2O2. Chin. J. Vet. Med. 44, 128–134. 10.16303/j.cnki.1005-4545.2024.01.18 [DOI] [Google Scholar]
- Lin X. W., Wang R. Q., Tan Y. F., Zhang X. P. (2020). Advances on pharmacokinetic researches on aporphine alkaloids. Prog. Pharm. Sci. 44, 451–458. [Google Scholar]
- Lin Y., Zhang Q., Xie B., Jiang H., Shen J., Tang S., et al. (2022). Chelerythrine-induced apoptotic cell death in HepG2 cells involves the inhibition of Akt pathway and the activation of oxidative stress and mitochondrial apoptotic pathway. Antioxid. Basel Switz. 11, 1837. 10.3390/antiox11091837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Jiang X. M. (2019). Effect of chelerythrine on apoptosis of human colorectal cancer cells and its mechanism. Chin. J. Mod. Appl. Pharm. 36, 3034–3039. 10.13748/j.cnki.issn1007-7693.2019.24.0062019 [DOI] [Google Scholar]
- Liu J. M., Liu C. C., Liu X. M., Zeng M., Jiang Q. (2019). Long-term toxic effect of chelerythrine on lung tissue and its effect on NF-κB expression in lung tissue. J. Jilin Univ. 45, 518–523. 10.13481/j.1671-587x.20190309 [DOI] [Google Scholar]
- Liu X. Y., Li J. W., Liu X. F., Sun Y. A. (2021). Research progress of Chinese medicine joined with neoadjuvant chemotherapy in treating breast cancer. Glob. Tradit. Chin. Med. 14, 357–362. 10.3969/j.issn.1674-1749.2021.02.044 [DOI] [Google Scholar]
- Liu X. Z., Yu M. J., Liang J. H. (2023). Research progress on the synthesis of protoberberine skeleton and its anti-inflammatory activity. Chin. J. Org. Chem. 43, 1325–1340. 10.6023/cjoc202209037 [DOI] [Google Scholar]
- MacLean D. B., Gracey D. E. F., Saunders J. K., Rodrigo R., Manske R. H. F. (1969). Some benzophenanthridine alkaloids from Bocconia arborea. Can. J. Chem. 47, 1951–1956. 10.1139/v69-317 [DOI] [Google Scholar]
- Maji A. K., Banerji P. (2015). Chelidonium majus L. (Greater celandine)–A review on its phytochemical and therapeutic perspectives. Int. J. Herb. Med. 3, 10–27. 10.22271/flora.2015.v3.i1.03 [DOI] [Google Scholar]
- Marek J., Dostál J., Slavík J. (1998). Crystal structures of α-and β-allocryptopine. Collect. Czechoslov. Chem. Commun. 63, 416–424. 10.1135/cccc19980416 [DOI] [Google Scholar]
- Miao F., Yang X. J., Zhou L., Hu H. J., Zheng F., Ding X. D., et al. (2011). Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 25, 863–875. 10.1080/14786419.2010.482055 [DOI] [PubMed] [Google Scholar]
- Mohammadlou M., Abdollahi M., Hemati M., Baharlou R., Doulabi E. M., Pashaei M., et al. (2021). Apoptotic effect of berberine via Bcl-2, ROR1, and mir-21 in patients with B-chronic lymphocytic leukemia. Phytother. Res. PTR 35, 2025–2033. 10.1002/ptr.6945 [DOI] [PubMed] [Google Scholar]
- Móricz Á. M., Fornal E., Jesionek W., Majer-Dziedzic B., Choma I. M. (2015). Effect-directed isolation and identification of antibacterial Chelidonium majus L. alkaloids. Chromatographia 78, 707–716. 10.1007/s10337-015-2870-6 [DOI] [Google Scholar]
- Musidlak O., Warowicka A., Broniarczyk J., Adamczyk D., Goździcka-Józefiak A., Nawrot R. (2022). The activity of Chelidonium majus L. Latex and its components on HPV reveal insights into the antiviral molecular mechanism. Int. J. Mol. Sci. 23, 9241. 10.3390/ijms23169241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot R., Warowicka A., Rudzki P. J., Musidlak O., Dolata K. M., Musijowski J., et al. (2021). Combined protein and alkaloid research of Chelidonium majus latex reveals CmMLP1 accompanied by alkaloids kwith cytotoxic potential to human cervical carcinoma cells. Int. J. Mol. Sci. 22, 11838. 10.3390/ijms222111838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X.-F., Zhou P., Li W.-F., Xu H.-B. (2011). Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia 82, 620–625. 10.1016/j.fitote.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Noureini S. K., Esmaeili H., Abachi F., Khiali S., Islam B., Kuta M., et al. (2017). Selectivity of major isoquinoline alkaloids from Chelidonium majus towards telomeric G-quadruplex: a study using a transition-FRET (t-FRET) assay. Biochim. Biophys. Acta Gen. Subj. 1861, 2020–2030. 10.1016/j.bbagen.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Noureini S. K., Esmaili H. (2014). Multiple mechanisms of cell death induced by chelidonine in MCF-7 breast cancer cell line. Chem. Biol. Interact. 223, 141–149. 10.1016/j.cbi.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Noureini S. K., Wink M. (2009). Transcriptional down regulation of hTERT and senescence induction in HepG2 cells by chelidonine. World J. Gastroenterol. 15, 3603–3610. 10.3748/wjg.15.3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiang-Obounou B. W., Kang O. H., Choi J. G., Keum J. H., Kim S. B., Mun S. H., et al. (2011). The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus . J. Toxicol. Sci. 36, 277–283. 10.2131/jts.36.277 [DOI] [PubMed] [Google Scholar]
- Och A., Zalewski D., Komsta Ł., Kołodziej P., Kocki J., Bogucka-Kocka A. (2019). Cytotoxic and proapoptotic activity of sanguinarine, berberine, and extracts of Chelidonium majus L. And berberis thunbergii DC. Toward hematopoietic cancer cell lines. Toxins 11, 485. 10.3390/toxins11090485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin S. M., König G. M., Oechslin-Merkel K., Wright A. D., Kinghorn A. D., Sticher O., et al. (1991). An NMR study of four benzophenanthridine alkaloids. J. Nat. Prod. 54, 519–524. 10.1021/np50074a026 [DOI] [Google Scholar]
- Orvos P., Virág L., Tálosi L., Hajdú Z., Csupor D., Jedlinszki N., et al. (2015). Effects of Chelidonium majus extracts and major alkaloids on hERG potassium channels and on dog cardiac action potential - a safety approach. Fitoterapia 100, 156–165. 10.1016/j.fitote.2014.11.023 [DOI] [PubMed] [Google Scholar]
- Pan S. J., Jiang J. L., Zhao J. F., Wang J. T. (2023). Effect of chelidonine on TGF-β1/Smad signaling pathway on proliferation mechanism of renal carcinoma. Chin. J. Gerontol. 43, 4012–4015. 10.3969/j.issn.1005-9202.2023.16.046 [DOI] [Google Scholar]
- Pantano F., Mannocchi G., Marinelli E., Gentili S., Graziano S., Busardò F. P., et al. (2017). Hepatotoxicity induced by greater celandine (Chelidonium majus L.): a review of the literature. Eur. Rev. Med. Pharmacol. Sci. 21, 46–52. [PubMed] [Google Scholar]
- Park J. E., Cuong T. D., Hung T. M., Lee I., Na M., Kim J. C., et al. (2011). Alkaloids from Chelidonium majus and their inhibitory effects on LPS-induced NO production in RAW264.7 cells. Bioorg. Med. Chem. Lett. 21, 6960–6963. 10.1016/j.bmcl.2011.09.128 [DOI] [PubMed] [Google Scholar]
- Patel M. B., Mishra S. (2011). Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine 18, 1045–1052. 10.1016/j.phymed.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Paul A., Bishayee K., Ghosh S., Mukherjee A., Sikdar S., Chakraborty D., et al. (2012). Chelidonine isolated from ethanolic extract of Chelidonium majus promotes apoptosis in HeLa cells through p38-p53 and PI3K/AKT signalling pathways. Zhong Xi Yi Jie He Xue Bao 10, 1025–1038. 10.3736/jcim20120912 [DOI] [PubMed] [Google Scholar]
- Paul L. D., Maurer H. H. (2003). Studies on the metabolism and toxicological detection of the Eschscholtzia californica alkaloids californine and protopine in urine using gas chromatography-mass spectrometry. J. Chromatogr. 789, 43–57. 10.1016/s1570-0232(03)00124-7 [DOI] [PubMed] [Google Scholar]
- Paulsen J., Yahyazadeh M., Hänsel S., Kleinwächter M., Ibrom K., Selmar D. (2015). 13,14-dihydrocoptisine--the genuine alkaloid from Chelidonium majus . Phytochemistry 111, 149–153. 10.1016/j.phytochem.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Peng J. X., Tao Y. Y., Peng J. M., Yuan J., Liu L. Z., He Y. C., et al. (2022). Effects of sanguinarine combined with 5-fluorouracil on autophagy and apoptosis of nasopharyngeal carcinoma transplanted tumor cells in nude mice. Chin. Pharm. J. 57, 2092–2098. 10.11669/cpj.2022.24.007 [DOI] [Google Scholar]
- Pfeifer S., Banerjee S. K., Dolejs L., Hanus V. (1965). On the structure of papaverrubins. Mass spectrometry of rhoeadin alkaloids. Pharma 20, 45–46. [PubMed] [Google Scholar]
- Qu Z., Zou X., Zhang X., Sheng J., Wang Y., Wang J., et al. (2016). Chelidonine induces mitotic slippage and apoptotic-like death in SGC-7901 human gastric carcinoma cells. Mol. Med. Rep. 3, 1336–1344. 10.3892/mmr.2015.4683 [DOI] [PubMed] [Google Scholar]
- Rosa S. D., Vincenzo G. D. (1992). Isochelidonine, a benzophenanthridine alkaloid from chelidonium majus . Phytochemistry 31, 1085–1086. 10.1016/0031-9422(92)80235-7 [DOI] [Google Scholar]
- Rui-Zhi F., Wen-Yan L., Gui-Xiang F., Pei-Gen X. (1985). Chemotaxonomy and resource utilization of the tribe Chelidonieae (Papaveraceae). J. Syst. Evol. 23, 36. [Google Scholar]
- Sárközi Á., Janicsák G., Kursinszki L., Kéry Á. (2006). Alkaloid composition of Chelidonium majus L. Studied by different chromatographic techniques. Chromatographia 63, S81–S86. 10.1365/s10337-006-0728-7 [DOI] [Google Scholar]
- Schrittwieser J. H., Resch V., Wallner S., Lienhart W. D., Sattler J. H., Resch J., et al. (2011). Biocatalytic organic synthesis of optically pure (S)-scoulerine and berbine and benzylisoquinoline alkaloids. J. Org. Chem. 76, 6703–6714. 10.1021/jo201056f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger C., Sturm S., Strasser E. M., Ellmerer E., Stuppner H. (2004). 1H and 13C NMR signal assignment of benzylisoquinoline alkaloids from Fumaria officinalis L. (Papaveraceae). Magn. Reson. Chem. MRC 42, 882–886. 10.1002/mrc.1417 [DOI] [PubMed] [Google Scholar]
- Shafiee A., Jafarabadi A. H. (1998). Corydine and norcorydine from the roots of Chelidonium majus . Planta Med. 64, 489. 10.1055/s-2006-957498 [DOI] [PubMed] [Google Scholar]
- Silva T. C. C., de Faria Lopes G. P., de J Menezes-Filho N., de Oliveira D. M., Pereira E., Pitanga B. P. S., et al. (2018). Specific cytostatic and cytotoxic effect of dihydrochelerythrine in glioblastoma cells: role of NF-κB/β-catenin and STAT3/IL-6 pathways. Anticancer Agents Med. Chem. 18, 1386–1393. 10.2174/1871520618666180412122101 [DOI] [PubMed] [Google Scholar]
- Slavik J., Slavikova L. (1977). Minor alkaloids from Chelidonium majus L. Collect. Czechoslov. Chem. Commun. 42, 2686–2693. 10.1135/cccc19772686 [DOI] [Google Scholar]
- Su L. D., He Y. C., Huang L. Z., Liu J. (2022). Role of autophagy in sanguinarine inhibiting nasopharyngeal carcinoma cells proliferation via AMPK/mTOR signaling pathway. Chin. J. Immunol. 38, 2870–2875. 10.3969/j.issn.1000-484X.2022.23.010 [DOI] [Google Scholar]
- Táborská E., Bochoráková H., Paulová H., Dostál J. (1994). Separation of alkaloids in Chelidonium majus by reversed phase HPLC. Planta. Med. 60, 380–381. 10.1055/s-2006-959508 [DOI] [PubMed] [Google Scholar]
- Tanahashi T., Zenk M. H. (1990). New hydroxylated benzo[c]phenanthridine alkaloids from Eschscholtzia californica cell suspension cultures. J. Nat. Prod. 53, 579–586. 10.1021/np50069a007 [DOI] [PubMed] [Google Scholar]
- Tao R. T., Ran L. T. (2018). Anti- tumor mechanisms of chelidonine on KB cells. J. Mod. Oncol. 26, 1498–1502. 10.3969/j.issn.1672-4992.2018.10.005 [DOI] [Google Scholar]
- Tin‐wa M., Fong H. H. S., Abraham D. J., Trojanek J., Farnsworth N. R. (1972a). Structure of sanguidimerine, a new major alkaloid from Sanguinaria canadensis (Papaveraceae). J. Pharm. Sci. 61, 1846–1847. 10.1002/jps.2600611140 [DOI] [Google Scholar]
- Tin-wa M., Kim H. K., Fong H. H., Farnsworth N. R. (1972b). The structure of chelidimerine, a new alkaloid from Chelidonium majus. Chelidonium majus. Lloydia 35 (1), 87–89. [PubMed] [Google Scholar]
- Tomè F., Colombo M. L. (1995). Distribution of alkaloids in Chelidonium majus and factors affecting their accumulation. Phytochemistry 40, 37–39. 10.1016/0031-9422(95)00055-C [DOI] [Google Scholar]
- Tomita M., Kikuchi T. (1955). Studies on the alkaloids of menispermaceous plants. CXXIV. Alkaloids of menispermum dauricum DC. (Suppl. 1). Pharm. Bull. 3, 100–104. 10.1248/cpb1953.3.100 [DOI] [PubMed] [Google Scholar]
- Tuzimski T., Petruczynik A. (2023). New trends in the practical use of isoquinoline alkaloids as potential drugs applicated in infectious and non-infectious diseases. Biomed. Pharmacother. 168, 115704. 10.1016/j.biopha.2023.115704 [DOI] [PubMed] [Google Scholar]
- Valipour M., Zarghi A., Ebrahimzadeh M. A., Irannejad H. (2021). Therapeutic potential of chelerythrine as a multi-purpose adjuvant for the treatment of COVID-19. Georget. Tex 20, 2321–2336. 10.1080/15384101.2021.1982509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennerstrom J. L., Klayman D. L. (1988). Protoberberine alkaloids as antimalarials. J. Med. Chem. 31, 1084–1087. 10.1021/jm00401a006 [DOI] [PubMed] [Google Scholar]
- Vrba J., Dolezel P., Vicar J., Modrianský M., Ulrichová J. (2008). Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 22, 1008–1017. 10.1016/j.tiv.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Wang L.-L., Li R.-T., Zang Z.-H., Song Y.-X., Zhang Y.-Z., Zhang T.-F., et al. (2023). 6-Methoxydihydrosanguinarine exhibits cytotoxicity and sensitizes TRAIL-induced apoptosis of hepatocellular carcinoma cells through ROS-mediated upregulation of DR5. Med. Oncol. N. Lond. Engl. 40, 266. 10.1007/s12032-023-02129-z [DOI] [PubMed] [Google Scholar]
- Wang X. J., Zhong G. C., Li S. T., Zhang Q., Luo B. J., Wang Q. (2024). Research progress in pharmacological effect of berberine for treatment of Alzheimer's disease. Chin. J. Exp. Tradit. Med. Formulae., 1–15. 10.13422/j.cnki.syfjx.20240741 [DOI] [Google Scholar]
- Wang Y., Li P. P., Gao Z. S., Zhang X. P., Wang Z., Zhou H., et al. (2020). Preparation of chelerythrine solid dispersion and study on its physicochemical properties and antioxidant activity. China Pharm. 31, 1054–1061. 10.6039/j.issn.1001-0408.2020.09.07 [DOI] [Google Scholar]
- Warowicka A., Popenda Ł., Bartkowiak G., Musidlak O., Litowczenko-Cybulska J., Kuźma D., et al. (2019). Protoberberine compounds extracted from Chelidonium majus L. as novel natural photosensitizers for cancer therapy. Phytomedicine 64, 152919. 10.1016/j.phymed.2019.152919 [DOI] [PubMed] [Google Scholar]
- Warowicka A., Qasem B., Dera-Szymanowska A., Wołuń-Cholewa M., Florczak P., Horst N., et al. (2021). Effect of protoberberine-rich fraction of Chelidonium majus L. On endometriosis regression. Pharmaceutics 13, 931. 10.3390/pharmaceutics13070931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q. H., Cui D. Z., Liu X. F., Chai Y. Y., Zhao N., Wang J. Y., et al. (2020). In vitro antifungal activity and possible mechanisms of action of chelerythrine. Pestic. Biochem. Physiol. 164, 140–148. 10.1016/j.pestbp.2020.01.007 [DOI] [PubMed] [Google Scholar]
- Wei W.-J., Chen X.-H., Guo T., Liu X.-Q., Zhao Y., Wang L.-L., et al. (2021). A review on classification and biological activities of alkaloids from the genus zanthoxylum species. Mini Rev. Med. Chem. 21, 336–361. 10.2174/1389557520666200910091905 [DOI] [PubMed] [Google Scholar]
- Wei Z. Q., Zou X., Qu Z. Y., Qiao Y. B. (2009). Research progress on chemical constituents and pharmacological effects of Chelidonium majus . Chin. Tradit. Herb. Drugs. 40, 38–40. [Google Scholar]
- Wu J.-S., Liu H.-J., Han S.-J., Mao N.-F., Liu X.-F. (2022). Chelerythrine ameliorates acetic acid-induced ulcerative colitis via suppression of inflammation and oxidation. Nat. Prod. Commun. 17, 1934578X2211324. 10.1177/1934578X221132417 [DOI] [Google Scholar]
- Wu S.-Z., Xu H.-C., Wu X.-L., Liu P., Shi Y.-C., Pang P., et al. (2019). Dihydrosanguinarine suppresses pancreatic cancer cells via regulation of mut-p53/WT-p53 and the Ras/Raf/Mek/Erk pathway. Phytomedicine 59, 152895. 10.1016/j.phymed.2019.152895 [DOI] [PubMed] [Google Scholar]
- Xiang Z.-D., Guan H.-D., Zhao X., Xie Q., Cai F.-J., Xie Z.-J., et al. (2024). Protoberberine alkaloids: a review of the gastroprotective effects, pharmacokinetics, and toxicity. Phytomedicine Int. J. Phytother. Phytopharm. 126, 155444. 10.1016/j.phymed.2024.155444 [DOI] [PubMed] [Google Scholar]
- Xiao Z. F., Li R. H., Jia T. Z. (2021). Study on the toxicity-attenuating processing technology of atractylodes Chelidonium majus with licorice. China Pharm. 24, 1923–1927. 10.19962/j.cnki.issn1008-049X.2021.10.031 [DOI] [Google Scholar]
- Xie Y.-J., Gao W.-N., Wu Q.-B., Yao X.-J., Jiang Z.-B., Wang Y.-W., et al. (2020). Chelidonine selectively inhibits the growth of gefitinib-resistant non-small cell lung cancer cells through the EGFR-AMPK pathway. Pharmacol. Res. 159, 104934. 10.1016/j.phrs.2020.104934 [DOI] [PubMed] [Google Scholar]
- Xu X. Q., Huang S., Du X. H., Zhou J. L., Huang H. D. (2014). Effect of chelerythrine on ulcerative colitis in mice. Chin. J. Exp. Tradit. Med. Formulae. 20, 171–174. 10.13422/j.cnki.syfjx.2014210171 [DOI] [Google Scholar]
- Yahyazadeh M., Ratmoyo P., Bittner F., Sato F., Selmar D. (2017). Cloning and characterization of cheilanthifoline and stylopine synthase genes from Chelidonium majus . Plant Cell Physiol. 58, 1421–1430. 10.1093/pcp/pcx077 [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang M., Luo Y., Xu F., Gao F., Sun Y., et al. (2024a). Protopine ameliorates OVA-induced asthma through modulatingTLR4/MyD88/NF-κB pathway and NLRP3 inflammasome-mediated pyroptosis. Phytomedicine 126, 155410. 10.1016/j.phymed.2024.155410 [DOI] [PubMed] [Google Scholar]
- Yang L. Z., Li X. Z., Shi J. R., Huang W. L., Jiang Y., Song X. M., et al. (2024b). Research progress on the chemical constituents and pharmacological effects of Rhizoma of Chelidonium majus L. J. Shaanxi Univ. Chin. Med., 1–9. [Google Scholar]
- Yang P., Qing Z. X., Zuo Z., Yu K., Zeng J. G. (2017). Identification of isoquinoline alkaloids in Chelidonium majus by HPLC-Q-TOF/MS. Mod. Chin. Med. 19, 174–182. 10.13313/j.issn.1673-4890.2017.2.003 [DOI] [Google Scholar]
- Yang T., Xu R., Su Q., Wang H., Liu F., Dai B., et al. (2020). Chelerythrine hydrochloride inhibits proliferation and induces mitochondrial apoptosis in cervical cancer cells via PI3K/BAD signaling pathway. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 68, 104965. 10.1016/j.tiv.2020.104965 [DOI] [PubMed] [Google Scholar]
- Yao J.-Y., Zhou Z.-M., Pan X., Hao G., Li X.-L., Xu Y., et al. (2011). In vivo anthelmintic activity of chelidonine from Chelidonium majus L. against Dactylogyrus intermedius in Carassius auratus . Parasitol. Res. 109, 1465–1469. 10.1007/s00436-011-2416-2 [DOI] [PubMed] [Google Scholar]
- Yu R., Mandlekar S., Tan T. H., Kong A. N. (2000). Activation of p38 and c-Jun N-terminal kinase pathways and induction of apoptosis by chelerythrine do not require inhibition of protein kinase C. J. Biol. Chem. 275, 9612–9619. 10.1074/jbc.275.13.9612 [DOI] [PubMed] [Google Scholar]
- Yuan Y. Z., Huang X. Y., Zhang X., Li D. H., Bai J., Li Z. L., et al. (2022). Separation and structure identification of chemical constituents from Chelidonium majus . Chin. J. Med. Chem. 32, 454–461. 10.14142/j.cnki.cn21-1313/r.2022.06.004 [DOI] [Google Scholar]
- Zhang B. X., Zhao X. M., Cheng Q., Li X. Q. (2018a). Effect of chelerythrine on proliferation inhibition and apoptosis induction of melanoma B16 cells. Lishizhen Med. Mat. Medica Res. 29, 793–795. 10.3969/j.issn.1008-0805.2018.04.008 [DOI] [Google Scholar]
- Zhang G. Z., Wan T. H., Cao Z., Tao Z. J., Zhang T., Wei W., et al. (2023). Study on the mechanism of Chelidonium alkaloids in the treatment of functional abdominal pain based on network pharmacology and molecular docking. Liaoning J. Tradit. Chin. Med. 50, 5–10+253. 10.13192/j.issn.1000-1719.2023.09.002 [DOI] [Google Scholar]
- Zhang L., Wang M., Zhang X. X., Zhang X. Y., Zhang F. X., Cao Z., et al. (2021). The mechanism of chelerythrine against breast cancer by network pharmacology and molecular docking. J. Xian Jiaot. Univ. Med. Sci. 42, 554–561+573. 10.7652/jdyxb202104011 [DOI] [Google Scholar]
- Zhang S., Leng T., Zhang Q., Zhao Q., Nie X., Yang L. (2018b). Sanguinarine inhibits epithelial ovarian cancer development via regulating long non-coding RNA CASC2-EIF4A3 axis and/or inhibiting NF-κB signaling or PI3K/AKT/mTOR pathway. Biomed. Pharmacother. Biomedecine Pharmacother. 102, 302–308. 10.1016/j.biopha.2018.03.071 [DOI] [PubMed] [Google Scholar]
- Zhang S., Wang M., Wang C. (2011a). Preparative separation and purification of alkaloids from Rhizoma coptidis by high-speed counter-current chromatography. Sep. Purif. Technol. 76, 428–431. 10.1016/j.seppur.2010.10.019 [DOI] [Google Scholar]
- Zhang W.-J., You C.-X., Wang C.-F., Fan L., Wang Y., Su Y., et al. (2014). One new alkaloid from Chelidonium majus L. Nat. Prod. Res. 28, 1873–1878. 10.1080/14786419.2014.953497 [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y., Dong W. H., Xu J. J., Zhu H., Li L. D., Sun L. (2018c). Identification of chelidonine metabolites in human liver microsomes. J. Shenyang Pharm. Univ. 35, 477–483. 10.14066/j.cnki.cn21-1349/r.2018.06.008 [DOI] [Google Scholar]
- Zhang Z., Guo Y., Zhang L., Zhang J., Wei X. (2012). Chelerythrine chloride from Macleaya cordata induces growth inhibition and apoptosis in human gastric cancer BGC-823 cells. Acta. Pharm. Sin. B 2, 464–471. 10.1016/j.apsb.2011.12.013 [DOI] [Google Scholar]
- Zhang Z.-F., Guo Y., Zhang J.-B., Wei X.-H. (2011b). Induction of apoptosis by chelerythrine chloride through mitochondrial pathway and Bcl-2 family proteins in human hepatoma SMMC-7721 cell. Arch. Pharm. Res. 34, 791–800. 10.1007/s12272-011-0513-5 [DOI] [PubMed] [Google Scholar]
- Zhang Z. H., Mi C., Wang K. S., Wang Z., Li M. Y., Zuo H. X., et al. (2018d). Chelidonine inhibits TNF-α-induced inflammation by suppressing the NF-κB pathways in HCT116 cells. Phytother. Res. PTR. 32, 65–75. 10.1002/ptr.5948 [DOI] [PubMed] [Google Scholar]
- Zhao T. Y., Wang W., Qi H. W., Jin H. L., Yan S. (2020). Rapid identification of chemical composition of alkaloids from Chelidonium majus based on HPLC-Q-TOF/MS. Acta Pharm. Sin. 55, 1273–1281. 10.16438/j.0513-4870.2020-0009 [DOI] [Google Scholar]
- Zhou J., Qiu Z. D., Lin Z., Lv G. F., Xu J. M., Lin H., et al. (2024). Effect of chelerythrine on migration, invasion and epithelial-mesenchymal transformation of SKOV3 cells in human ovarian cancer. J. Jilin Univ. 50, 25–32. 10.13481/j.1671-587X.20240104 [DOI] [Google Scholar]
- Zhou J. Y., Chen B. Z., Tong X. J., Lian W. Y., Fang Q. C. (1989). Chemical study of alkaloids of Chelidonium majus . Chin. Tradit. Herb. Drugs 20, 2–4. 10.7501/j.issn.0253-2670.1989.4.057 [DOI] [Google Scholar]
- Zielińska S., Czerwińska M. E., Dziągwa-Becker M., Dryś A., Kucharski M., Jezierska-Domaradzka A., et al. (2020). Modulatory effect of Chelidonium majus extract and its alkaloids on LPS-stimulated cytokine secretion in human neutrophils. Molecules 25, 842. 10.3390/molecules25040842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska S., Jezierska-Domaradzka A., Wójciak-Kosior M., Sowa I., Junka A., Matkowski A. M. (2018). Greater celandine’s ups and downs-21 centuries of medicinal uses of Chelidonium majus from the viewpoint of today’s pharmacology. Front. Pharmacol. 9, 299. 10.3389/fphar.2018.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska S., Wójciak-Kosior M., Dziągwa-Becker M., Gleńsk M., Sowa I., Fijałkowski K., et al. (2019). The activity of isoquinoline alkaloids and extracts from Chelidonium majus against pathogenic bacteria and Candida sp . Toxins 11 (7), 406. 10.3390/toxins11070406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y. L., Liu Y. P. (2006). Proliferation inhibition and apoptosis induction of chelerythrine in human gastric carcinoma BGC823cells. Chin. Tradit. Herb. Drugs 37, 1054–1056. 10.7501/j.issn.0253-2670.2006.7.454 [DOI] [Google Scholar]
- Zou C., Lv C., Wang Y., Cao C., Zhang G. (2017). Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar . Pestic. Biochem. Physiol. 142, 123–132. 10.1016/j.pestbp.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Zou C., Wang Y., Zou H., Ding N., Geng N., Cao C., et al. (2019). Sanguinarine in Chelidonium majus induced antifeeding and larval lethality by suppressing food intake and digestive enzymes in Lymantria dispar . Pestic. Biochem. Physiol. 153, 9–16. 10.1016/j.pestbp.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Zou X., Shu Q., Wu S., Yu J. H., Zhang X. R., Suan Y. H., et al. (2023). Pharmacodynamic substances and mechanism of Chelidonii Herba-Corydalis Rhizoma against estrogen receptor-positive breast cancer. China Pharm. 34, 935–940. 10.6039/j.issn.1001-0408.2023.08.08 [DOI] [Google Scholar]
- Zuo G. Y., Meng F. Y., Hao X. Y., Zhang Y. L., Wang G. C., Xu G. L. (2008). Antibacterial alkaloids from chelidonium majus linn (papaveraceae) against clinical isolates of methicillin-resistant Staphylococcus aureus . J. Pharm. Pharm. Sci. 11, 90–94. 10.18433/j3d30q [DOI] [PubMed] [Google Scholar]
- Zwerger M., Boeck L., Manzl J., Schwaiger S., Ganzera M. (2024). Novel approaches for the analysis and isolation of benzylisoquinoline alkaloids in Chelidonium majus . Planta Med. 90, 523–533. 10.1055/a-2204-5686 [DOI] [PMC free article] [PubMed] [Google Scholar]