Abstract
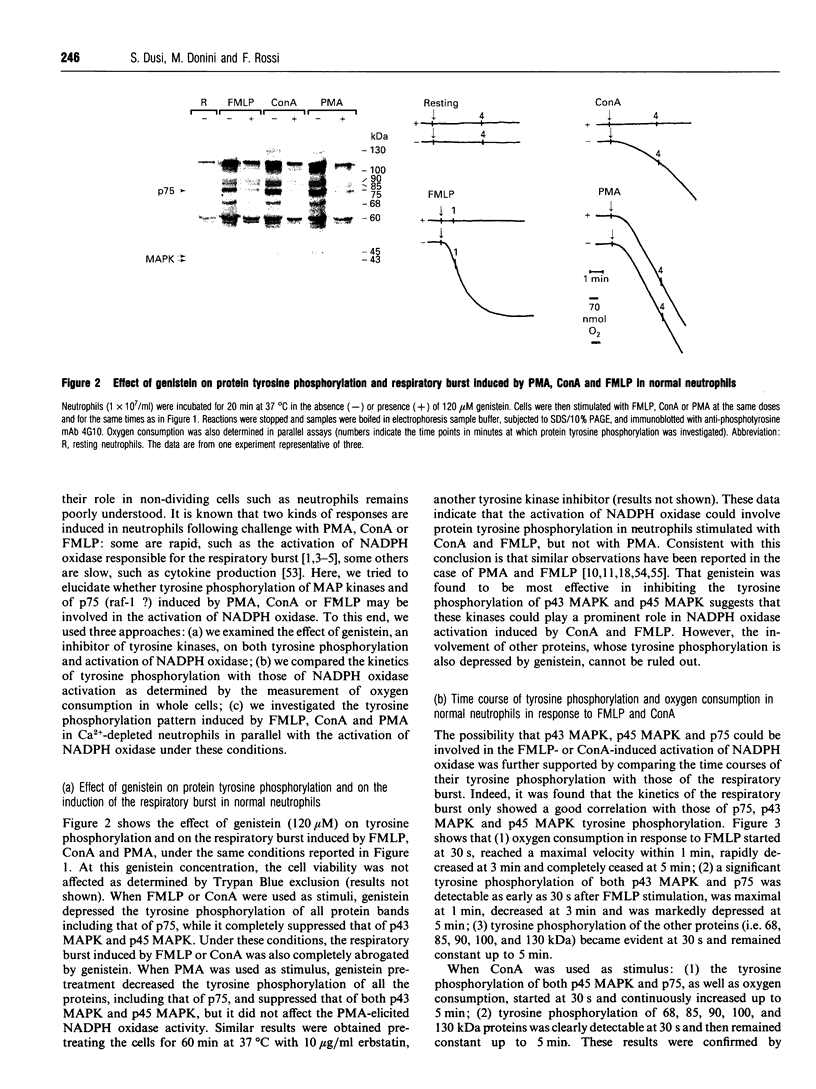
Challenge of neutrophils with concanavalin A (ConA), formyl-methionyl-leucyl-phenylalanine (FMLP), and phorbol 12-myristate 13-acetate (PMA) induced the tyrosine phosphorylation of several proteins. Among these proteins we have identified two mitogen-activated protein kinase (MAPK) isoforms of 43 kDa (p43 MAPK) and 45 kDa (p45 MAPK) molecular mass. Moreover here we show that: (1) FMLP induced the tyrosine phosphorylation of the p43 MAPK, and ConA that of p45 MAPK, while PMA induced the tyrosine phosphorylation of both p43 and p45 MAPK; all these agonists induced the tyrosine phosphorylation of a 75 kDa protein (p75). (2) With FMLP or ConA as agonists, tyrosine phosphorylations of MAPK and p75 can be involved in the process of NADPH oxidase activation. On the contrary, PMA can activate the respiratory burst independently of these phosphorylations. (3) In Ca(2+)-depleted neutrophils, where phospholipid hydrolysis did not take place, ConA or FMLP did not activate the respiratory burst, but while ConA induced the tyrosine phosphorylation of p45 MAPK and p75, FMLP was not able to phosphorylate p43 MAPK and p75. (4) As previously observed in our laboratory, a double stimulation of Ca(2+)-depleted neutrophils with ConA plus FMLP induced a respiratory burst in the absence of activation of second messengers derived from phospholipase C, D and A2 activity. This respiratory burst was accompanied by tyrosine phosphorylation of both p43 and p45 MAPKs. These results indicate that when FMLP is the agonist, both the tyrosine phosphorylation of p43 MAPK and p75, and the activation of NADPH oxidase, are coupled to Ca(2+)-dependent mechanisms. On the contrary, ConA can induce the tyrosine phosphorylation of p45 MAPK and p75 independently of calcium, but an unknown Ca(2+)-dependent mechanism is necessary for the activation of NADPH oxidase by this agonist. This mechanism could be substituted by the induction of tyrosine phosphorylation of both p43 MAPK and p45 MAPK when Ca(2+)-depleted neutrophils are stimulated with ConA plus FMLP.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkow R. L., Dodson R. W., Kraft A. S. Human neutrophils contain distinct cytosolic and particulate tyrosine kinase activities: possible role in neutrophil activation. Biochim Biophys Acta. 1989 Aug 31;997(3):292–301. doi: 10.1016/0167-4838(89)90200-8. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Dodson R. W. Tyrosine-specific protein phosphorylation during activation of human neutrophils. Blood. 1990 Jun 15;75(12):2445–2452. [PubMed] [Google Scholar]
- Bianchini L., Todderud G., Grinstein S. Cytosolic [Ca2+] homeostasis and tyrosine phosphorylation of phospholipase C gamma 2 in HL60 granulocytes. J Biol Chem. 1993 Feb 15;268(5):3357–3363. [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Bourgoin S., Grinstein S. Peroxides of vanadate induce activation of phospholipase D in HL-60 cells. Role of tyrosine phosphorylation. J Biol Chem. 1992 Jun 15;267(17):11908–11916. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai H., Erhardt P., Troppmair J., Diaz-Meco M. T., Sithanandam G., Rapp U. R., Moscat J., Cooper G. M. Hydrolysis of phosphatidylcholine couples Ras to activation of Raf protein kinase during mitogenic signal transduction. Mol Cell Biol. 1993 Dec;13(12):7645–7651. doi: 10.1128/mcb.13.12.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Bazzoni F., Ceska M., Ferro I., Baggiolini M., Berton G. IL-8 production by human polymorphonuclear leukocytes. The chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol. 1992 May 15;148(10):3216–3220. [PubMed] [Google Scholar]
- Connelly P. A., Farrell C. A., Merenda J. M., Conklyn M. J., Showell H. J. Tyrosine phosphorylation is an early signaling event common to Fc receptor crosslinking in human neutrophils and rat basophilic leukemia cells (RBL-2H3). Biochem Biophys Res Commun. 1991 May 31;177(1):192–201. doi: 10.1016/0006-291x(91)91967-h. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Erikson R. L. Extracellular signals and reversible protein phosphorylation: what to Mek of it all. Cell. 1993 Jul 30;74(2):215–217. doi: 10.1016/0092-8674(93)90411-i. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Schomisch S. J., Kusner D. J., Xie M. Phospholipase D activity in phagocytic leucocytes is synergistically regulated by G-protein- and tyrosine kinase-based mechanisms. Biochem J. 1993 May 15;292(Pt 1):121–128. doi: 10.1042/bj2920121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusi S., Della Bianca V., Grzeskowiak M., Rossi F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca(2+)-depleted human neutrophils. Biochem J. 1993 Feb 15;290(Pt 1):173–178. doi: 10.1042/bj2900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusi S., Rossi F. Activation of NADPH oxidase of human neutrophils involves the phosphorylation and the translocation of cytosolic p67phox. Biochem J. 1993 Dec 1;296(Pt 2):367–371. doi: 10.1042/bj2960367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian J. R., Daar I. O., Morrison D. K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993 Nov;13(11):7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry M., Caon A. C., Naccache P. H. Modulation of the activity and subcellular distribution of protein tyrosine kinases in human neutrophils by phorbol esters. FASEB J. 1993 May;7(8):687–693. doi: 10.1096/fasebj.7.8.7684713. [DOI] [PubMed] [Google Scholar]
- Gaudry M., Roberge C. J., de Médicis R., Lussier A., Poubelle P. E., Naccache P. H. Crystal-induced neutrophil activation. III. Inflammatory microcrystals induce a distinct pattern of tyrosine phosphorylation in human neutrophils. J Clin Invest. 1993 Apr;91(4):1649–1655. doi: 10.1172/JCI116373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser K. B., Asmis R., Dennis E. A. Bacterial lipopolysaccharide priming of P388D1 macrophage-like cells for enhanced arachidonic acid metabolism. Platelet-activating factor receptor activation and regulation of phospholipase A2. J Biol Chem. 1990 May 25;265(15):8658–8664. [PubMed] [Google Scholar]
- Gomez-Cambronero J., Colasanto J. M., Huang C. K., Sha'afi R. I. Direct stimulation by tyrosine phosphorylation of microtubule-associated protein (MAP) kinase activity by granulocyte-macrophage colony-stimulating factor in human neutrophils. Biochem J. 1993 Apr 1;291(Pt 1):211–217. doi: 10.1042/bj2910211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Bonak V. A., Wang E., Casnellie J. E., Shiraishi T., Sha'afi R. I. Tyrosine phosphorylation in human neutrophil. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1478–1485. doi: 10.1016/0006-291x(89)90841-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Gomez-Cambronero T. M., Waterman W. H., Becker E. L., Sha'afi R. I. Granulocyte-macrophage colony-stimulating factor-induced protein tyrosine phosphorylation of microtubule-associated protein kinase in human neutrophils. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7551–7555. doi: 10.1073/pnas.89.16.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Wang E., Johnson G., Huang C. K., Sha'afi R. I. Platelet-activating factor induces tyrosine phosphorylation in human neutrophils. J Biol Chem. 1991 Apr 5;266(10):6240–6245. [PubMed] [Google Scholar]
- Gomez-Cambronero J., Yamazaki M., Metwally F., Molski T. F., Bonak V. A., Huang C. K., Becker E. L., Sha'afi R. I. Granulocyte-macrophage colony-stimulating factor and human neutrophils: role of guanine nucleotide regulatory proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3569–3573. doi: 10.1073/pnas.86.10.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Butler J. R., Tseng J. Receptor-mediated activation of multiple serine/threonine kinases in human leukocytes. J Biol Chem. 1993 Sep 25;268(27):20223–20231. [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. J Biol Chem. 1992 Sep 5;267(25):18122–18125. [PubMed] [Google Scholar]
- Grzeskowiak M., Della Bianca V., Cassatella M. A., Rossi F. Complete dissociation between the activation of phosphoinositide turnover and of NADPH oxidase by formyl-methionyl-leucyl-phenylalanine in human neutrophils depleted of Ca2+ and primed by subthreshold doses of phorbol 12,myristate 13,acetate. Biochem Biophys Res Commun. 1986 Mar 28;135(3):785–794. doi: 10.1016/0006-291x(86)90997-6. [DOI] [PubMed] [Google Scholar]
- Grzeskowiak M., Della Bianca V., De Togni P., Papini E., Rossi F. Independence with respect to Ca2+ changes of the neutrophil respiratory and secretory response to exogenous phospholipase C and possible involvement of diacylglycerol and protein kinase C. Biochim Biophys Acta. 1985 Jan 18;844(1):81–90. doi: 10.1016/0167-4889(85)90237-x. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Kölch W., Morrison D. K., Rapp U. R. The role of Raf-1 phosphorylation in signal transduction. Adv Cancer Res. 1992;58:53–73. doi: 10.1016/s0065-230x(08)60290-0. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Badwey J. A. Protein phosphorylation associated with the stimulation of neutrophils. Modulation of superoxide production by protein kinase C and calcium. J Bioenerg Biomembr. 1990 Feb;22(1):1–26. doi: 10.1007/BF00762842. [DOI] [PubMed] [Google Scholar]
- Hoek J. B. Tyrosine kinase activation and signal transduction mediated by phospholipase D. Lab Invest. 1993 Jul;69(1):1–4. [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Huang C. K., Bonak V., Laramee G. R., Casnellie J. E. Protein tyrosine phosphorylation in rabbit peritoneal neutrophils. Biochem J. 1990 Jul 15;269(2):431–436. doi: 10.1042/bj2690431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. K., Laramee G. R., Casnellie J. E. Chemotactic factor induced tyrosine phosphorylation of membrane associated proteins in rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1988 Mar 15;151(2):794–801. doi: 10.1016/s0006-291x(88)80351-6. [DOI] [PubMed] [Google Scholar]
- Kusunoki T., Higashi H., Hosoi S., Hata D., Sugie K., Mayumi M., Mikawa H. Tyrosine phosphorylation and its possible role in superoxide production by human neutrophils stimulated with FMLP and IgG. Biochem Biophys Res Commun. 1992 Mar 16;183(2):789–796. doi: 10.1016/0006-291x(92)90552-v. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993 Jan 29;72(2):269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- McColl S. R., DiPersio J. F., Caon A. C., Ho P., Naccache P. H. Involvement of tyrosine kinases in the activation of human peripheral blood neutrophils by granulocyte-macrophage colony-stimulating factor. Blood. 1991 Oct 1;78(7):1842–1852. [PubMed] [Google Scholar]
- Mitsuyama T., Takeshige K., Minakami S. Tyrosine phosphorylation is involved in the respiratory burst of electropermeabilized human neutrophils at a step before diacylglycerol formation by phospholipase C. FEBS Lett. 1993 May 17;322(3):280–284. doi: 10.1016/0014-5793(93)81586-o. [DOI] [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Caon A. C., Gilbert C., Gaudry M., Roberge C. J., Poubelle P. E., Bourgoin S. Inhibition of tyrosine phosphorylation by wortmannin in human neutrophils. Dissociation from its inhibitory effects on phospholipase D. Lab Invest. 1993 Jul;69(1):19–23. [PubMed] [Google Scholar]
- Naccache P. H., Gilbert C., Caon A. C., Gaudry M., Huang C. K., Bonak V. A., Umezawa K., McColl S. R. Selective inhibition of human neutrophil functional responsiveness by erbstatin, an inhibitor of tyrosine protein kinase. Blood. 1990 Nov 15;76(10):2098–2104. [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Golde D. W., Daeipour M., Nel A. E. Granulocyte-macrophage colony-stimulating factor activates microtubule-associated protein 2 kinase in neutrophils via a tyrosine kinase-dependent pathway. Blood. 1992 Jun 15;79(12):3350–3354. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge C. J., Gaudry M., de Médicis R., Lussier A., Poubelle P. E., Naccache P. H. Crystal-induced neutrophil activation. IV. Specific inhibition of tyrosine phosphorylation by colchicine. J Clin Invest. 1993 Oct;92(4):1722–1729. doi: 10.1172/JCI116759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Grzeskowiak M., Della Bianca V. Double stimulation with FMLP and Con A restores the activation of the respiratory burst but not of the phosphoinositide turnover in Ca2+-depleted human neutrophils. A further example of dissociation between stimulation of the NADPH oxidase and phosphoinositide turnover. Biochem Biophys Res Commun. 1986 Oct 15;140(1):1–11. doi: 10.1016/0006-291x(86)91050-8. [DOI] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Samuels M. L., Weber M. J., Bishop J. M., McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol. 1993 Oct;13(10):6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993 Feb;18(2):43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- Torres M., Hall F. L., O'Neill K. Stimulation of human neutrophils with formyl-methionyl-leucyl-phenylalanine induces tyrosine phosphorylation and activation of two distinct mitogen-activated protein-kinases. J Immunol. 1993 Feb 15;150(4):1563–1577. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uings I. J., Thompson N. T., Randall R. W., Spacey G. D., Bonser R. W., Hudson A. T., Garland L. G. Tyrosine phosphorylation is involved in receptor coupling to phospholipase D but not phospholipase C in the human neutrophil. Biochem J. 1992 Feb 1;281(Pt 3):597–600. doi: 10.1042/bj2810597. [DOI] [PMC free article] [PubMed] [Google Scholar]