Abstract
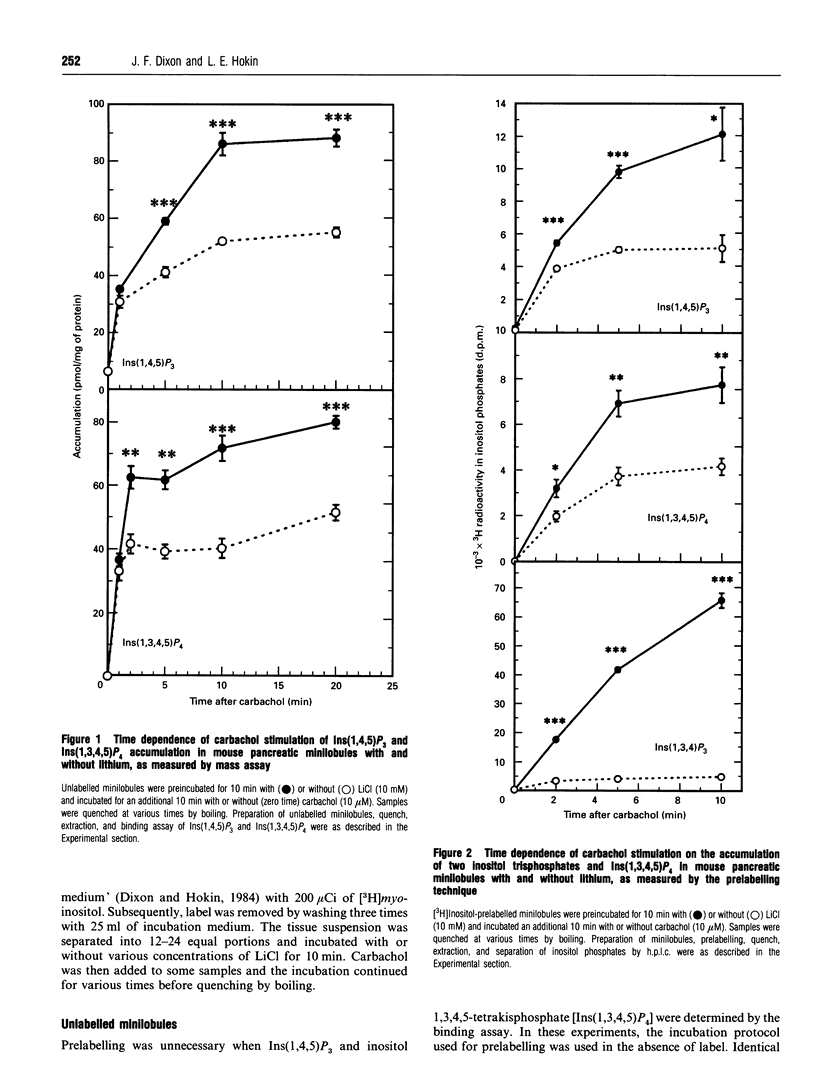
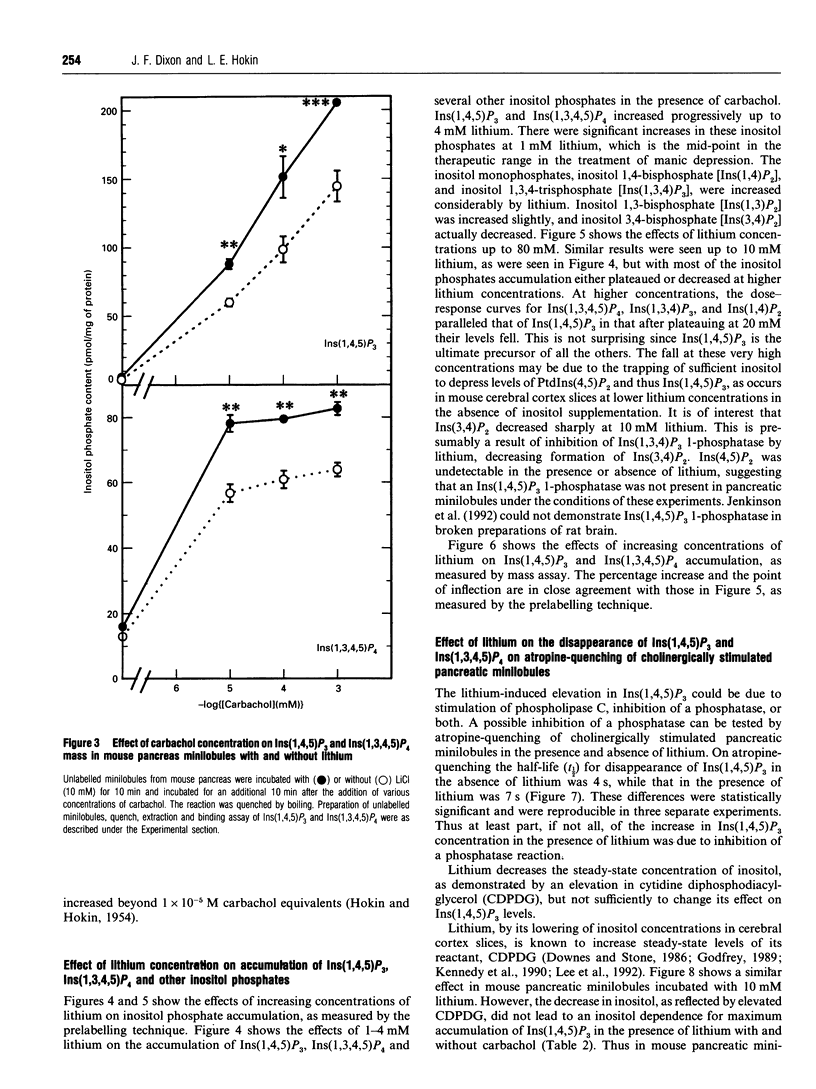
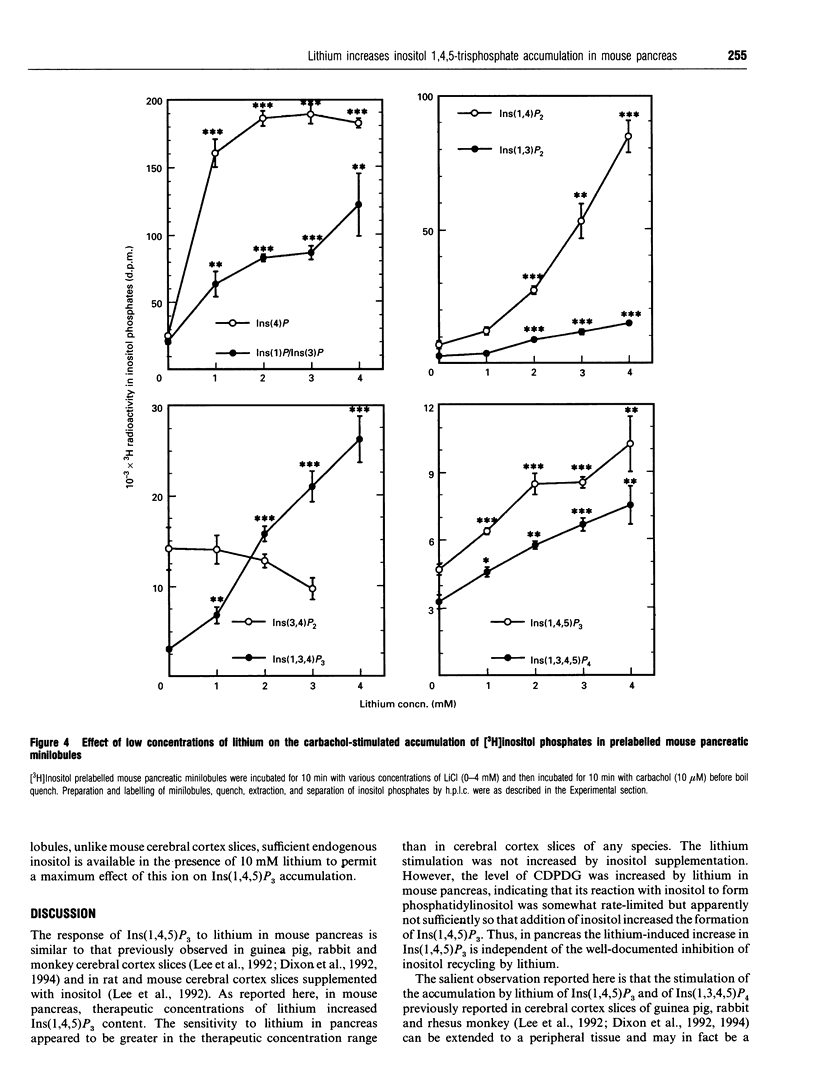
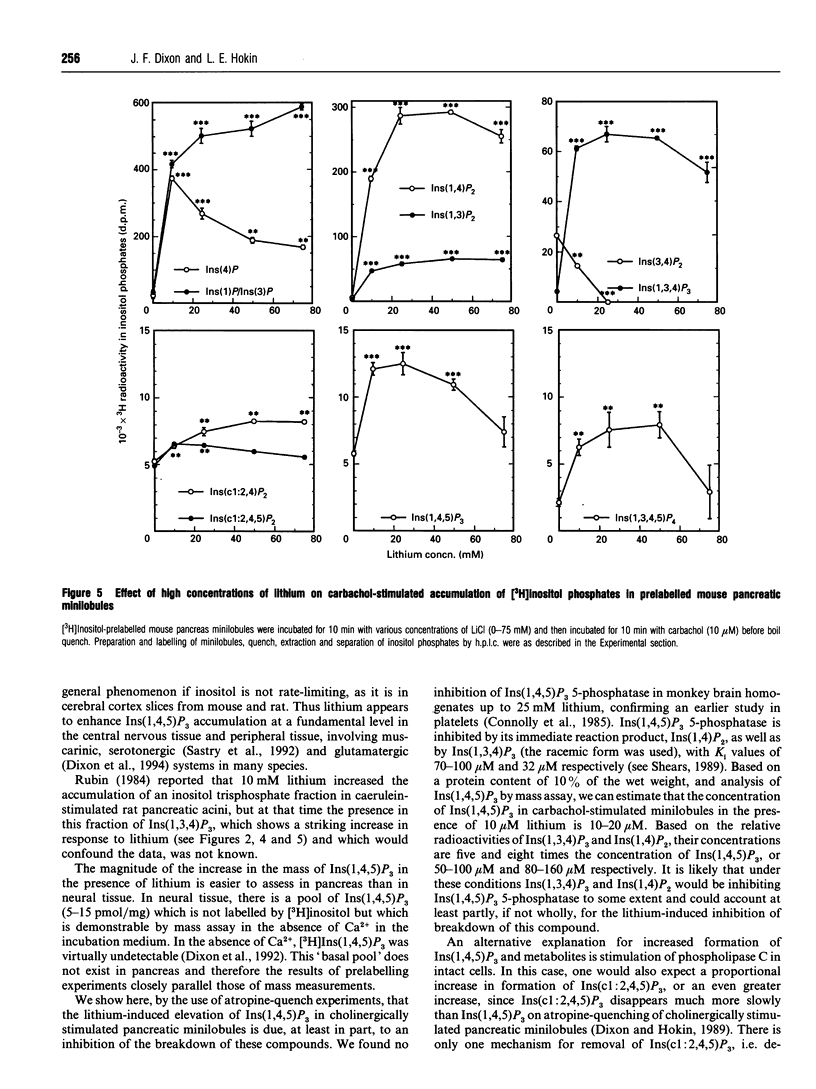
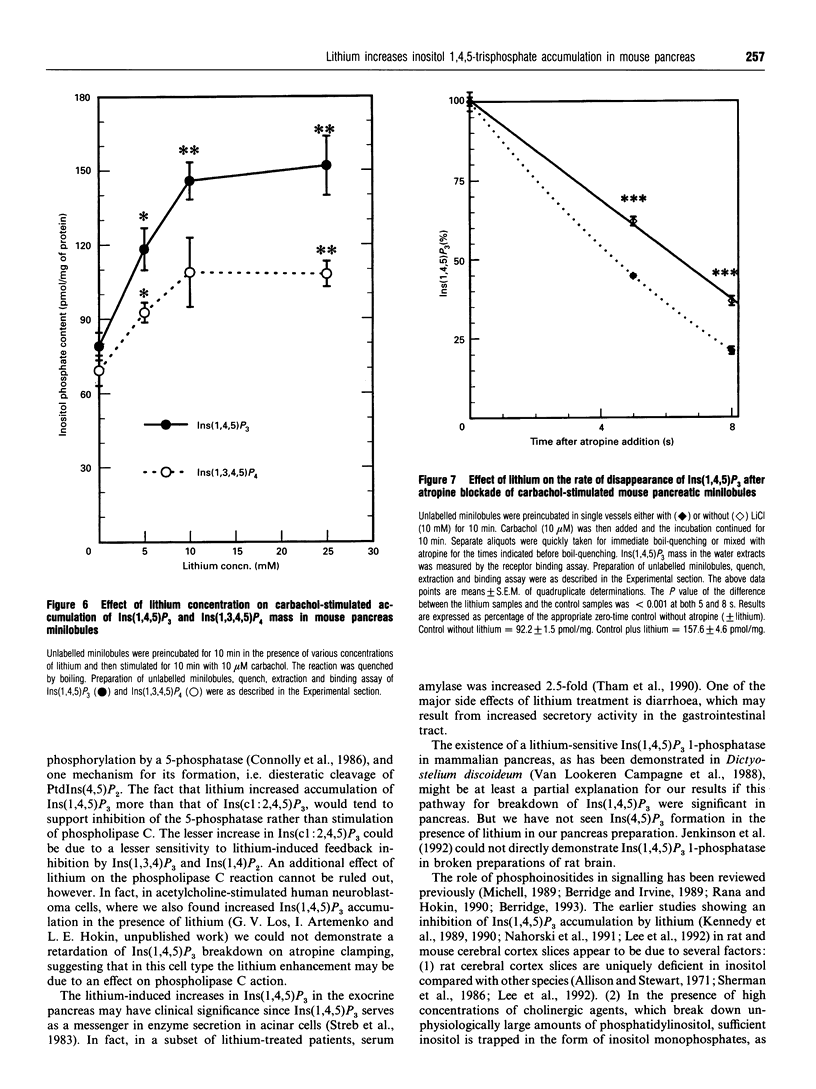
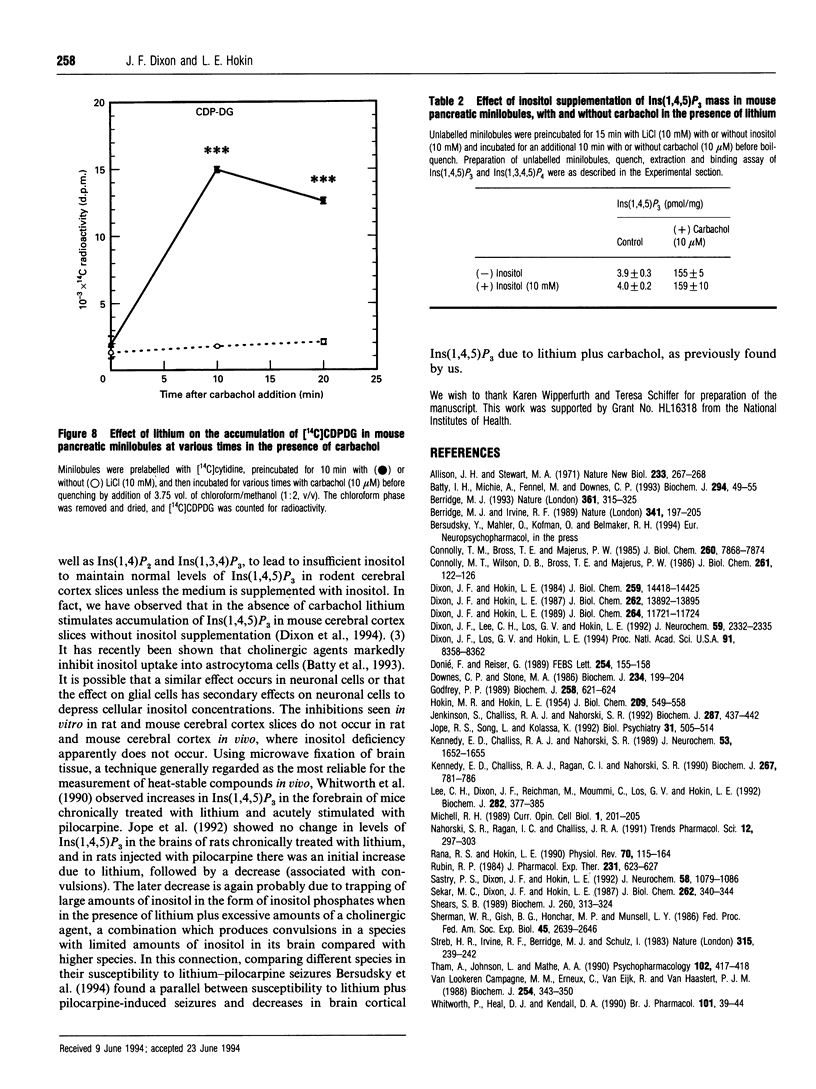
Previous studies showed that lithium, beginning at therapeutic plasma concentrations in the treatment of manic depression, increased the accumulation of second-messenger inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] in cerebral cortex slices of guinea pig and rhesus monkey [Lee, Dixon, Reichman, Moummi, Los and Hokin (1992) Biochem. J. 282, 377-385; Dixon, Lee, Los and Hokin (1992) J. Neurochem. 59, 2332-2335; Dixon, Los and Hokin (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8358-8362]. These studies have now been extended to a peripheral tissue, mouse pancreatic minilobules. In the presence of carbachol, concentrations of lithium from 1 to 20 mM sharply and progressively increased the accumulation of Ins(1,4,5)P3 and inositol 1,3,4,5-tetrakisphosphate, followed by a decrease. Assay of these inositol polyphosphates by either the prelabelling technique or mass assay gave similar results. Atropine quenching of cholinergically stimulated pancreatic minilobules led to a rapid disappearance of Ins(1,4,5)P3. This disappearance was impeded by lithium. This suggested that the lithium-induced elevation in Ins(1,4,5)P3 was due to inhibition of the 5-phosphatase and, on the basis of the markedly elevated concentrations of inositol 1,3,4-trisphosphate [Ins(1,3,4)P3] and inositol 1,4-bisphosphate in the presence of lithium, probably by feedback inhibition by these latter two compounds. An additional mechanism, i.e. a stimulatory effect of lithium on phospholipase C, cannot, however, be ruled out. The other reaction product of phospholipase C, inositol cyclic 1:2,4,5-trisphosphate, also increased in the presence of lithium. This may also be due to inhibition of the 5-phosphatase, which is the exclusive mechanism for removal of this compound. The effects of lithium on the accumulation of other inositol phosphates paralleled that of Ins(1,4,5)P3, with the exception of inositol 3,4-bisphosphate, which decreased. This was presumably due to the inhibition of Ins(1,3,4)P3 1-phosphatase by lithium. Unlike mouse cerebral cortex slices [Lee, Dixon, Reichman, Moummi, Los and Hokin (1992) Biochem. J. 282, 377-385], inositol supplementation was not required to demonstrate lithium-stimulated Ins(1,4,5)P3 accumulation in mouse pancreatic minilobules. This indicates that inositol depletion sufficient to impair lithium-stimulated Ins(1,4,5)P3 accumulation does not occur in mouse pancreatic minilobules, even though an elevation of cytidine diphosphodiacylglycerol occurred, indicating some inositol depletion due to lithium. Elevation of Ins(1,4,5)P3 by lithium may be a general phenomenon in the central nervous system and peripheral tissues under non-rate-limiting concentrations of inositol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. H., Stewart M. A. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971 Oct 27;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- Batty I. H., Michie A., Fennel M., Downes C. P. The characteristics, capacity and receptor regulation of inositol uptake in 1321N1 astrocytoma cells. Biochem J. 1993 Aug 15;294(Pt 1):49–55. doi: 10.1042/bj2940049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Connolly T. M., Wilson D. B., Bross T. E., Majerus P. W. Isolation and characterization of the inositol cyclic phosphate products of phosphoinositide cleavage by phospholipase C. Metabolism in cell-free extracts. J Biol Chem. 1986 Jan 5;261(1):122–126. [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Inositol 1,2-cyclic 4,5-trisphosphate concentration relative to inositol 1,4,5-trisphosphate in pancreatic minilobules on stimulation with carbamylcholine in the absence of lithium. Possible role as a second messenger in long- but not short-term responses. J Biol Chem. 1987 Oct 15;262(29):13892–13895. [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Kinetic analysis of the formation of inositol 1:2-cyclic phosphate in carbachol-stimulated pancreatic minilobules. Half is formed by direct phosphodiesteratic cleavage of phosphatidylinositol. J Biol Chem. 1989 Jul 15;264(20):11721–11724. [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Secretogogue-stimulated phosphatidylinositol breakdown in the exocrine pancreas liberates arachidonic acid, stearic acid, and glycerol by sequential actions of phospholipase C and diglyceride lipase. J Biol Chem. 1984 Dec 10;259(23):14418–14425. [PubMed] [Google Scholar]
- Dixon J. F., Lee C. H., Los G. V., Hokin L. E. Lithium enhances accumulation of [3H]inositol radioactivity and mass of second messenger inositol 1,4,5-trisphosphate in monkey cerebral cortex slices. J Neurochem. 1992 Dec;59(6):2332–2335. doi: 10.1111/j.1471-4159.1992.tb10129.x. [DOI] [PubMed] [Google Scholar]
- Dixon J. F., Los G. V., Hokin L. E. Lithium stimulates glutamate "release" and inositol 1,4,5-trisphosphate accumulation via activation of the N-methyl-D-aspartate receptor in monkey and mouse cerebral cortex slices. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8358–8362. doi: 10.1073/pnas.91.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Stone M. A. Lithium-induced reduction in intracellular inositol supply in cholinergically stimulated parotid gland. Biochem J. 1986 Feb 15;234(1):199–204. doi: 10.1042/bj2340199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P. P. Potentiation by lithium of CMP-phosphatidate formation in carbachol-stimulated rat cerebral-cortical slices and its reversal by myo-inositol. Biochem J. 1989 Mar 1;258(2):621–624. doi: 10.1042/bj2580621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Effects of acetylcholine on phospholipides in the pancreas. J Biol Chem. 1954 Aug;209(2):549–558. [PubMed] [Google Scholar]
- Jenkinson S., Challiss R. A., Nahorski S. R. Evidence for lithium-sensitive inositol 4,5-bisphosphate accumulation in muscarinic cholinoceptor-stimulated cerebral-cortex slices. Biochem J. 1992 Oct 15;287(Pt 2):437–442. doi: 10.1042/bj2870437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S., Song L., Kolasa K. Inositol trisphosphate, cyclic AMP, and cyclic GMP in rat brain regions after lithium and seizures. Biol Psychiatry. 1992 Mar 1;31(5):505–514. doi: 10.1016/0006-3223(92)90261-w. [DOI] [PubMed] [Google Scholar]
- Kennedy E. D., Challiss R. A., Nahorski S. R. Lithium reduces the accumulation of inositol polyphosphate second messengers following cholinergic stimulation of cerebral cortex slices. J Neurochem. 1989 Nov;53(5):1652–1655. doi: 10.1111/j.1471-4159.1989.tb08566.x. [DOI] [PubMed] [Google Scholar]
- Kennedy E. D., Challiss R. A., Ragan C. I., Nahorski S. R. Reduced inositol polyphosphate accumulation and inositol supply induced by lithium in stimulated cerebral cortex slices. Biochem J. 1990 May 1;267(3):781–786. doi: 10.1042/bj2670781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H., Dixon J. F., Reichman M., Moummi C., Los G., Hokin L. E. Li+ increases accumulation of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate in cholinergically stimulated brain cortex slices in guinea pig, mouse and rat. The increases require inositol supplementation in mouse and rat but not in guinea pig. Biochem J. 1992 Mar 1;282(Pt 2):377–385. doi: 10.1042/bj2820377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol lipids and phosphates. Curr Opin Cell Biol. 1989 Apr;1(2):201–205. doi: 10.1016/0955-0674(89)90087-2. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Ragan C. I., Challiss R. A. Lithium and the phosphoinositide cycle: an example of uncompetitive inhibition and its pharmacological consequences. Trends Pharmacol Sci. 1991 Aug;12(8):297–303. doi: 10.1016/0165-6147(91)90581-c. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. Stimulation of inositol trisphosphate accumulation and amylase secretion by caerulein in pancreatic acini. J Pharmacol Exp Ther. 1984 Dec;231(3):623–627. [PubMed] [Google Scholar]
- Sastry P. S., Dixon J. F., Hokin L. E. Agonist-stimulated inositol polyphosphate formation in cerebellum. J Neurochem. 1992 Mar;58(3):1079–1086. doi: 10.1111/j.1471-4159.1992.tb09365.x. [DOI] [PubMed] [Google Scholar]
- Sekar M. C., Dixon J. F., Hokin L. E. The formation of inositol 1,2-cyclic 4,5-trisphosphate and inositol 1,2-cyclic 4-bisphosphate on stimulation of mouse pancreatic minilobules with carbamylcholine. J Biol Chem. 1987 Jan 5;262(1):340–344. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman W. R., Gish B. G., Honchar M. P., Munsell L. Y. Effects of lithium on phosphoinositide metabolism in vivo. Fed Proc. 1986 Oct;45(11):2639–2646. [PubMed] [Google Scholar]
- Tham A., Johnson L., Mathé A. A. Serum amylase in patients treated with lithium. Psychopharmacology (Berl) 1990;102(3):417–418. doi: 10.1007/BF02244114. [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Erneux C., Van Eijk R., Van Haastert P. J. Two dephosphorylation pathways of inositol 1,4,5-trisphosphate in homogenates of the cellular slime mould Dictyostelium discoideum. Biochem J. 1988 Sep 1;254(2):343–350. doi: 10.1042/bj2540343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth P., Heal D. J., Kendall D. A. The effects of acute and chronic lithium treatment on pilocarpine-stimulated phosphoinositide hydrolysis in mouse brain in vivo. Br J Pharmacol. 1990 Sep;101(1):39–44. doi: 10.1111/j.1476-5381.1990.tb12085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


