Abstract
Dyslexia is a specific learning disability that is neurobiological in origin and is characterized by reading and/or spelling problems affecting the development of language-related skills. The aim of this study is to reveal functional markers based on dyslexia by examining the functions of brain regions in resting state and reading tasks and to analyze the effects of special education given during the treatment process of dyslexia. A total of 43 children, aged between 7 and 12, whose native language was Turkish, participated in the study in three groups including those diagnosed with dyslexia for the first time, those receiving special education for dyslexia, and healthy children. Independent component analysis method was employed to analyze functional connectivity variations among three groups both at rest and during the continuous reading task. A whole-brain scanning during task fulfillment and resting states revealed that there were significant differences in the regions including lateral visual, default mode, left frontoparietal, ventral attention, orbitofrontal and lateral motor network. Our results revealed the necessity of adding motor coordination exercises to the training of dyslexic participants and showed that training led to functional connectivity in some brain regions similar to the healthy group. Additionally, our findings confirmed that impulsivity is associated with motor coordination and visuality, and that the dyslexic group has weaknesses in brain connectivity related to these conditions. According to our preliminary results, the differences obtained between children with dyslexia, group of dyslexia with special education and healthy children has revealed the effect of education on brain functions as well as enabling a comprehensive examination of dyslexia.
Keywords: Dyslexia, Special education, Functional connectivity, Brain networks
Introduction
Dyslexia is a specific learning disability that is neurobiological in origin and is characterized by persistent reading and/or spelling problems affecting the development of language-related skills (Miciak and Fletcher 2020; Lyon et al. 2003). This disorder is known to affect approximately 5–17% of children and continue into adulthood (Rüsseler et al. 2018; Shaywitz 1998). In our country, it is stated that approximately 10% of school-age children have dyslexia (Çeliktürk Sezgin and Akyol 2015). While the diagnosis of dyslexia is usually made as a result of the child not being able to learn to read at the desired level in the years when a child typically starts learning to read (8–9 years of age), the age of diagnosis may be earlier or later due to some environmental (Theodoridou et al. 2021) and familial factors (Centanni et al. 2019). Dyslexia is mostly diagnosed at school ages (Yang et al. 2022), and this disorder negatively affects the child’s academic success and self-confidence (Kana et al. 2023; Wajuihian 2011; Wajuihian and Naidoo 2011). Early diagnosis of dyslexia is quite crucial as it will directly affect children’s personal development, academic success, and social life skills (Soğanci and Kulesza 2023; Huang et al. 2020; Nevill and Forsey 2023). Therefore the current study focuses on primary school children aged between 7 and 12 years old.
In recent years, various neuroimaging methods have gained remarkable importance in the diagnosis of neuropsychiatric diseases such as dyslexia (Cainelli et al. 2023; Gallego-Molina et al. 2023). Among neuroimaging methods, functional magnetic resonance imaging (fMRI) has become an extremely important tool thanks to its power in revealing the functional structure of the brain (Prasad et al. 2020). Functional MR imaging can be used to investigate the functional structure of the brain at rest without giving any stimulus, or it can be planned to show the functional structure of the brain during the designed process by giving people visual, auditory or a different stimulus/task. fMRI image analysis methods are focused on exploring functional changes in the brain, which exhibit different superior aspects, primarily emphasizing hypothesis-driven or exploratory approaches. Seed-based analysis, which examines the functional connectivity of any selected region in the brain with the whole brain or another region (Icer et al. 2018; S. İçer et al. 2023), and independent component analysis (ICA), which probes into the independent neurodynamic network structure of the whole brain with an exploratory approach as performed in this study, are among the main methods employed in this field (Icer et al. 2019).
Functional MRI studies performed specifically for dyslexia are mostly aimed at understanding the functional changes the brain shows during a stimulus or task given to individuals with dyslexia. In studies conducting reading analyzes on individuals diagnosed with dyslexia, different task configurations have been designed in the literature, resulting in notable decreases and increases in functional activation in certain brain regions (Li and H.-Y. 2022; Martin et al. 2016; Devoto et al. 2022). Studies aimed at analyzing and understanding the functional connectivity of the dyslexic brain during rest are conducted less frequently than task-based studies. (Seitzman et al. 2019; Schurz et al. 2015; Farris et al. 2011; Koyama et al. 2013; Ye et al. 2014). On the other hand, studies on resting state and task-based research examining both the resting structure of the dyslexic brain and the functional changes during dyslexia-specific neurocognitive tasks are very limited (Turker et al. 2023; Gosse et al. 2022). Ye et al. (2014) applied ICA analysis to examine dynamically modulated functional networks in the processing of incongruent and congruent words in 20 native German-speaking participants. ICA analysis has revealed that in several brain regions, such as the supplementary motor area, were modulated by the incongruous endings of sentences (Ye et al. 2014). Rüsseler et al. (2018) used independent component analysis to identify brain networks involved in the perception of audiovisual speech in a group of adult readers with dyslexia (n = 13) and a group of fluent readers (n = 13). The findings identified several components, including the fusiform gyrus and occipital gyrus, are modulated differently in fluent readers and readers with dyslexia (Rüsseler et al. 2018). Greeley et al. (2021) made an intergroup comparison with a group of readers with reading difficulties (n = 42) and a group of adolescents and children with typical reading abilities (n = 19) using rapid ICA and seed analysis. The results of ICA indicated that the group with reading difficulties exhibited alterations in sensorimotor, salience, and cerebellar networks (Greeley et al. 2021). Mohammadi et al. 2020 performed the ICA approach in adult groups consisting of 20 illiterate and 20 normal readers using resting state fMRI. In addition, literacy training was given to the illiterate group for 7 months and then the groups were evaluated with seed-based correlation analysis. Inter-group analysis revealed changes in connectivity in the left fronto-parietal network, basal ganglia network, and visual network, both before and after training (Mohammadi et al. 2020). In addition to these adult studies, the fact that dyslexia is first noticed in childhood makes it very important to investigate the functional structure and neurobiological basis of dyslexia in childhood.
Although it is not a routine method applied in the psychiatric treatment processes of individuals with dyslexia, special reading education programs are generally applied to children with dyslexia along with their school education. As a result of a comprehensive literature review conducted in this direction, the participant groups, the age range of the participants, the language used in reading tasks, the status of receiving training for dyslexia, and the rest/task studies conducted on the brain networks of interest have given in detail in Table 1.
Table 1.
Studies performing ICA in dyslexia
As compared to recent studies in the literature detailed in the Table 1, superior aspects and unique contributions of this study can be summarized as follows.
In this study, both rs-fMRI data and reading task-fMRI data, specifically designed for our study in which the participants performed continuous reading, were obtained from participants belonging to three groups. With ICA analysis, which is an exploratory method, all brain networks and all functional connections in these networks were examined. In the literature, there are a limited number of ICA studies focusing on dyslexic children within a specific age range. In our study, the participants include children with dyslexia diagnosed for the first time (Dys), dyslexic children who received special education (EDys), and healthy control group children (HC), between the ages of 7 and 12, and ICA analysis was conducted to their fMRI data. This research is the first to employ a continuous reading task focusing on dyslexia with participants who are native Turkish speakers, by making them read a Turkish text selected according to their grade levels. Participants were asked to continuously read a text selected according to grade levels from a specific learning difficulties battery, which would be at eye level and comfortable for them to read, and MRI scans were performed. The implementation of a continuous reading task related to dyslexia, the participants' native language being Turkish, and the reading text being a Turkish text chosen according to grade level are among some of the unique aspects of the study. Participants were asked to continuously read a text from a specific learning difficulties battery, which would be at eye level and comfortable for them to read, and MRI scans were performed. Unlike few studies in the literature focusing on special education conducted at different periods of time, the inclusion of individuals who have been trained for a period of 12–24 months is expected to create a difference in functional connectivity in the dyslexia group who received special education, which forms another unique aspect of the study.
In the light of relavant literature, the aim of this study is to reveal functional markers based on dyslexia by examining the functions of brain regions during resting state and continuous reading tasks and to elaborate on the effects of special education given during the treatment process of dyslexia. In the second part of the study, information about our original data set is given and the methods used in rs-fMRI/task-fMRI analysis are detailed. In the third section, the results obtained from these methods are evaluated and compared with relevant literature findings.
Materials and methods
The current section of the study analyzing the effects of dyslexia and special education involves the following steps: selection of participants, structural and functional data acquisition, experimental design, data preprocess and postprocess.
Participants
A total of 43 children, whose native language is Turkish, were included in this study, comprising 13 children diagnosed with dyslexia for the first time, 15 children who received special education for dyslexia, and 15 healthy control children.
All dyslexic children were diagnosed by an experienced child and adolescent psychiatrist based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) (American Psychiatric Association.2013) and also the specific learning disability (SLD) battery, which comprises subtests that assess literacy and basic arithmetic skills, and tests that assess disorders or problems in visual perception, ranking and sequencing skills, the hand-eye–ear test of the head, lateralization, and fine motor skills (Turgut Turan et al. 2016), was also performed to children with dyslexia. Children with dyslexia who have any central nervous system diseases such as epilepsy, cerebral palsy, developmental delay, and who have any other psychiatric disorders were not included in the study. Children with hearing and vision problems were also excluded.
Children diagnosed with dyslexia for the first time (Dys): 13 children, who are newly diagnosed with dyslexia according to DSM-V (Nevill and Forsey 2023) criteria, who have no additional psychiatric diagnosis, and who had no special education on dyslexia, aged between “7–12”, right-hand dominant, and who had an IQ level of 80 and above, were included in the study. In addition, apart from the 13 children included in the study, two children who underwent MRI were not included in the study due to the high head movement observed as a result of the pre-processing, one child could not be included because the functional MRI scan performed during the reading could not be completed successfully, and one child was not included in the study because a pathological mass was detected in the brain.
Children with dyslexia who received special education (EDys): 15 children, who were diagnosed according to DSM-V criteria, with no additional psychiatric diagnosis, aged between 7 and 12, right-hand dominant, with an IQ level of 80 and above, and who received dyslexia education between 12 and 24 months, were included in the study. An individual education program is implemented for each child (https://orgm.meb.gov.tr/meb_iys_dosyalar/2021_05/21130110_Ogrenme_Guclugu.pdf, 2024).
Healthy Control Group (HC): 16 children, who applied to the child psychiatry outpatient clinics of Erciyes University (ERÜ), Faculty of Medicine, between the ages of “7–12”, right-hand dominant, with an IQ level of 80 and above, and without any psychiatric disorders, were determined for the control group. Afterwards, MRI scans were performed on these children and while 15 children were included in the study, one child was excluded from the study due to high head movement observed during the preprocessing. Demographic information and varios performance values of the participants are given in Table 2.
Table 2.
Demographic informations and performance values
| Dys (n = 13) | EDys (n = 15) | HC (n = 15) | p-values | |
|---|---|---|---|---|
| Gender (F/M) | 3/10 | 6/9 | 10/5 | |
| Age (mean ± SD) | 8.92 ± 1.7 | 9.4 ± 1.5 | 10.06 ± 1.53 | 0.168 |
| Age at diagnosis (mean ± SD) | 8.46 ± 1.56 | 7.92 ± 1.32 | – | |
| Duration of receiving special education (month) (mean ± SD) | – | 18 ± 7.52 | – | |
| Number of words read per minute (mean ± SD) | 28.69 ± 13.81 | 36.46 ± 21.04** | 85.66 ± 19.8* | 0.001 |
| WISC_4 (mean ± SD) | 84.69 ± 6.66 | 92.46 ± 16.34 | 96.6 ± 8.32* | 0.042 |
ANOVA test; post hoc Tukey HSD
*Higher than Dys, **Lower than HC
This study has been approved by the Erciyes University Clinical Research Ethics Committee (Decision No: 2022/504). Written informed consent was obtained from both children and their parents.
Data acquisition
The functional and structural MR images of each participant were acquired using a Siemens Magnetom Aera 1.5 Tesla MRI scanner with a 20-channel head coil at Erciyes University Mustafa Eraslan and Fevzi Mercan Children’s Hospital, Department of Pediatric Radiology (Icer et al. 2018).
Structural MRI data acquisition
Structural MR data were collected with T1-weighted structural MPRAGE (magnetization-prepared rapid gradient-echo). The implemented scan parameters were sagittal orientation, echo time (TE) = 2.670 ms, repetition time (TR) = 1900 ms, 256 × 256 matrix, isotropic resolution = 1.3 mm, flip angle = 15°, and total scan time = 4 min 18 s for 192 slices, respectively. These structural MRI images were used to coregistrate the functional images to the participants’ brain anatomy during the pre-processing stage (Icer et al. 2019).
Functional MRI data acquisition
Functional MR data were collected in an oblique plane (parallel to the anterior commissure–posterior commissure) using a T2*-weighted echo-planar imaging sequence with the following imaging parameters: TR = 2800 ms, TE = 53 ms, flip angle = 90°, field of view = 192 mm, 25 slices covering the whole brain, slice thickness = 5 mm, in-plane resolution = 2 × 2 mm. Two separate screenings were carried out for each participant as resting state and reading tasks, and each scan lasted 6 min and 14 s, and a total of 130 volumes of images were obtained at each scanning.
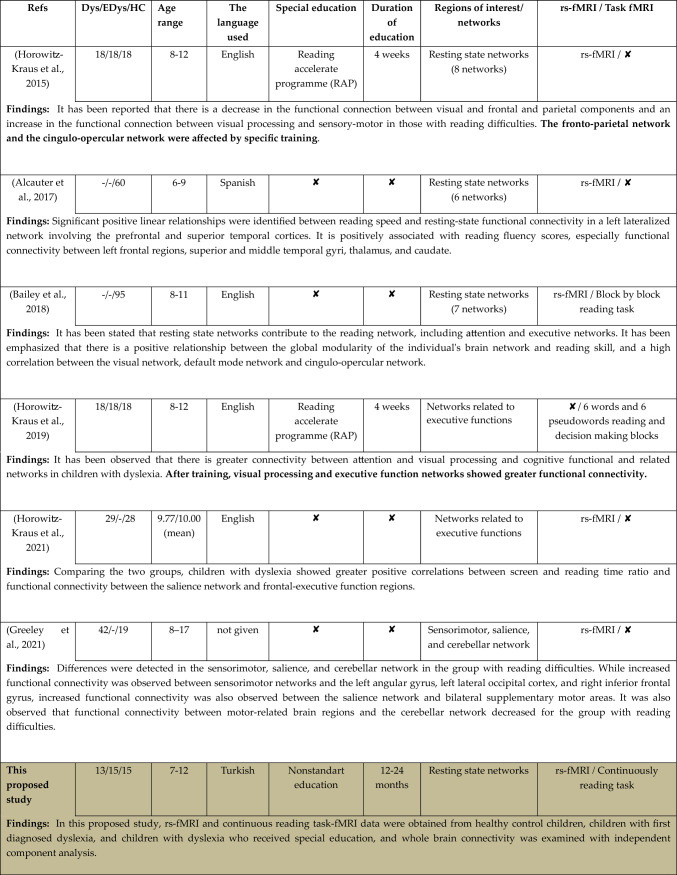
During a resting-state fMRI scan, paticipants were instructed to keep their eyes open and rest during the scan, keeping them relaxed, still, and thinking to a minimum. Under these conditions, brain activities were scanned. After the completion of the resting-state fMRI scan, a specific text determined according to the child’s grade level based on the specific learning difficulty battery (Karakaş et al. 2017) was placed in the upper inner region of the MRI device at the child’s eye level. Texts in different font styles and font sizes were arranged for children to read. These texts were written in children’s native language (Turkish). Participants were primary school 2, 3 and 4th grade students. Therefore, three different texts were separately prepared for students at different grades. All texts were selected from textbooks issued by the Ministry of National Education, Republic of Turkey. Relevant texts were selected in a way that the child could comfortably read based on their academic level, and the task given to the child was to silently read through the text and then start reading it again from the beginning after completing it. This reading action was continued throughout the scanning period, and brain activity was examined. Unlike the stop-and-go tasks commonly seen in the literature, a continuous task condition was implemented. Figure 1 illustrates the methodological framework of the study.
Fig. 1.
Flowchart of the whole data acquisition process
Data preprocessing
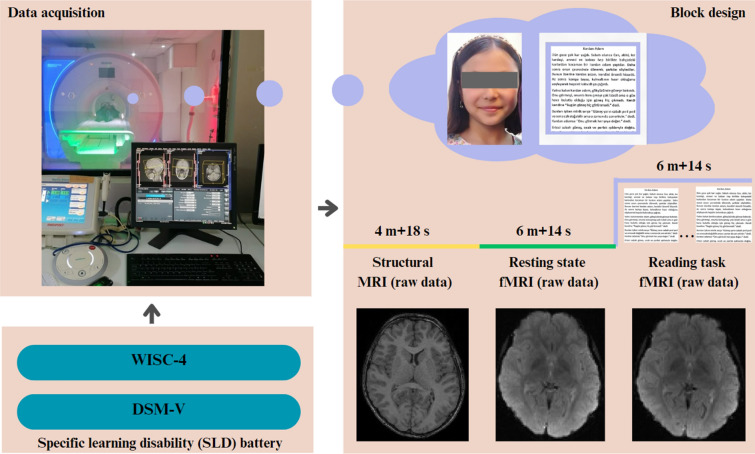
Pre-processing steps for functional MR images are practically carried out with auxiliary software. In this study, the data preprocessing process was carried out with the FMRIB Software Library (FSL 6.0.4) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The same preprocessing steps were performed for the resting state and the continuous reading task. Relavant preprocessing steps used in the study are as follows; brain extraction, slice timing correction, motion correction, spatial smoothing, ICA AROMA, temporal filtering and linear domain registration.
Initially, non-brain structures were extracted from anatomical and functional images using FSL FMRIB's brain extraction tool (BET) (Smith 2002). Next, slice timing correction was performed by shifting all slices to be aligned as if they were acquired at the same point and time using voxel time series interpolation relative to a reference slice. Motion correction was carried out using the middle volume as the initial template and applying 6 degrees of freedom (DOF)—3 rotations and 3 translations—to each volume with FLIRT optimization method to remove motion artifacts (Jenkinson et al. 2002). Children with estimated maximum absolute head motion > 2 (mm or °) or mean motion > 0.5 (mm or °) were excluded from the study to minimize the effects of unwanted motion (Winkler et al. 2014; Sörös et al. 2019). Spatial smoothing involves obtaining the intensity value of each voxel by taking the mean of the values of neighboring voxels, thereby reducing high-frequency fluctuations between adjacent voxels (Chen and Calhoun 2018).
In the study, a low-pass Gaussian filter was applied to the images, eliminating high-frequency signals and preserving low-frequency information. The smoothing process was performed using a Gaussian kernel with FWHM = 5 mm in each fMR image volume separately. After spatial smoothing, FSL’s ICA-AROMA tool (version 0.3-beta), an automatic motion artifact removal tool based on independent component analysis, was used to detect artifacts and then remove these components from the data (Pruim et al. 2015). The ICA AROMA process was carried out after smoothing and before filtering. By means of AROMA, temporal filtering was applied to functional images to eliminate motion artifacts. Through temporal filtering, low-frequency artifacts were removed with high-pass temporal filtering using the local form of a straight line with cut-off = 100 s = 0.01 Hz, which is the recommended value for fMRI data (Smith et al. 2004). The functional data were registered to the high-resolution anatomical images using a 6-degree-of-freedom linear transformation (DOF) applied in the FLIRT linear registration tool available in FSL (Jenkinson et al. 2002).
To overcome some of the shortcomings of FLIRT, registration of structural images to the 2-mm MNI standard space template was performed using a 12-degree-of-freedom linear transformation through the nonlinear registration tool FNIRT (Andersson et al. 2008). This ensures better alignment of the structures. Finally, the low-resolution fMRI image was registered to the standard space, and the two transformations were merged. The preprocessing steps applied to anatomical and functional images are illustrated in Fig. 2.
Fig. 2.
Flowchart of fMRI data preprocessing
Functional connectivity analysis
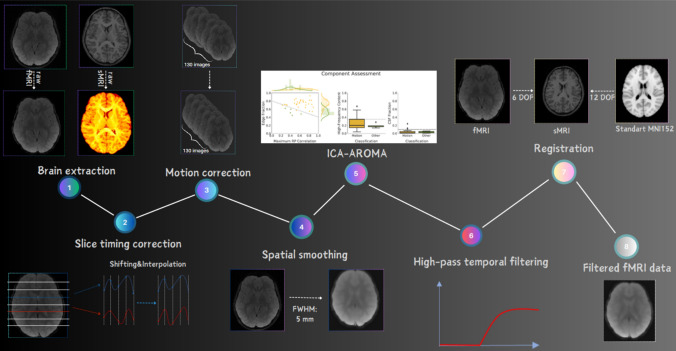
In this study, independent component analysis (ICA) was applied to fMRI data obtained during the resting and reading states. We performed ICA analysis on group data using FSL’s MELODIC software (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC/), which implements probabilistic ICA. The methodology used to make group inferences when performing group ICA analysis in the MELODIC tool is multi-session temporal concatenation. This method is most suitable for resting-state data as there is no common time course for subjects, unlike the task-oriented design. Temporal ICA combines single-subject data sets over time and obtains independent components on the combined data matrix (Vos et al. 2018). Since the reading task in our study was continuous, an analysis method equivalent to resting state ICA analysis was applied. In this study, the number of independent components determined for both the resting state and the reading task was 20. As a result of group ICA analysis, 20-component ICA maps were obtained (Smith et al. 2009; Biswal et al. 2010; Wang and Li 2015).
In this study, after multi-subject group ICA analysis were completed, dual regression was performed on FSL to evaluate the functional connectivity differences between Dys, EDys and HC groups (Andersson et al. 2008). Dual regression allows the definition of a set of network maps and corresponding time series to be compared between groups associated with group ICA components within each individual’s spatial space (Vos et al. 2018). ICA maps obtained as a result of group ICA were utilized as input in dual regression, in which multivariate temporal regression was performed to evaluate individual spatial maps using time courses. Group analysis was then performed by entering participants’ individual independent component (IC) spatial maps into a generalized linear model (GLM) framework using an appropriate design matrix and corresponding contrasts. Independent component analysis and dual regression analysis are summarized in Fig. 3.
Fig. 3.
Flowchart of independent component analysis and dual regression analysis
In the general linear model, three explanatory variables are defined as follows: Dys, EDys and HC groups. Then, 2 sample t-test contrasts corresponding to all possible combinations were defined to evaluate the differences between groups (6 contrasts) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM).
Binary regression with variance normalization was performed to demonstrate the activity and spatial extent of resting-state networks (RSN). Regarding statistical analysis, different component maps were collected in 4D files across participants and tested voxel-wise for statistically significant differences between groups using FSL’s randomized tool performing non-parametric permutation testing. In order to check through multiple comparisons, 5000 permutations were applied for each indicated contrast using the Threshold free cluster enhancement (TFCE) technique. Finally, family wise error (FWE) correction was performed for multiple comparisons applying TFCE using the significance threshold of p < 0.05. Regions with differences between groups were used to extract mean z values from each spatial map (FWE-corrected p < 0.05) (Winkler et al. 2014; Rytty et al. 2013).
Results and discussion
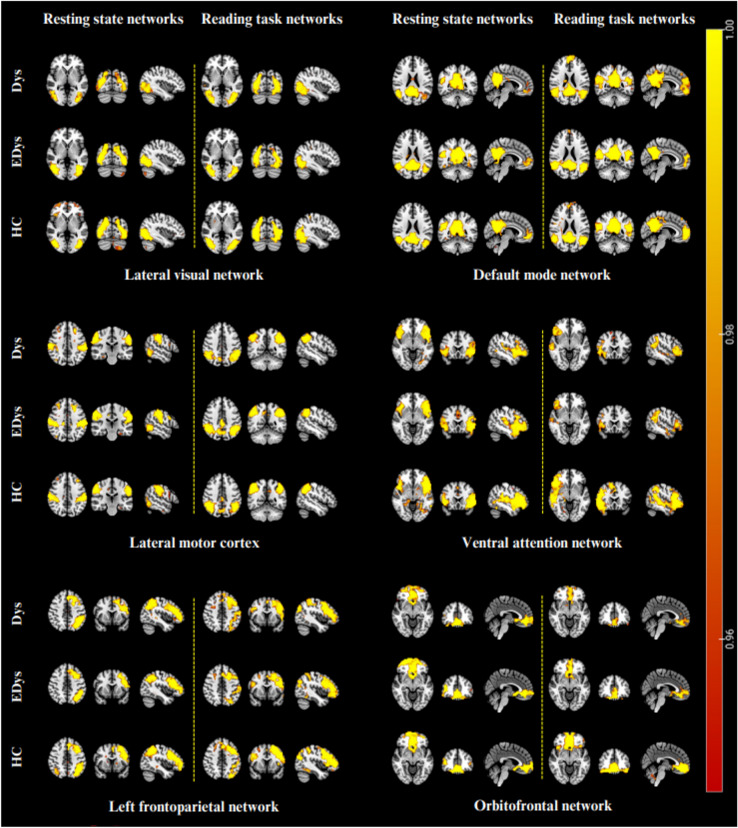
In this study, 20-component ICA analysis was applied to fMRI data obtained from children during both resting-state and reading task fMRI scans, and the results obtained for networks and regions specifically relevant to our study are presented. Figure 4 provides group means of brain networks corresponding to 6 independent components for reading task and resting-state conditions. Table 3 indicates voxel-wise activations in regions of interest within the relevant brain networks for both sessions for each group.
Fig. 4.
Mean networks of each group after dual regression
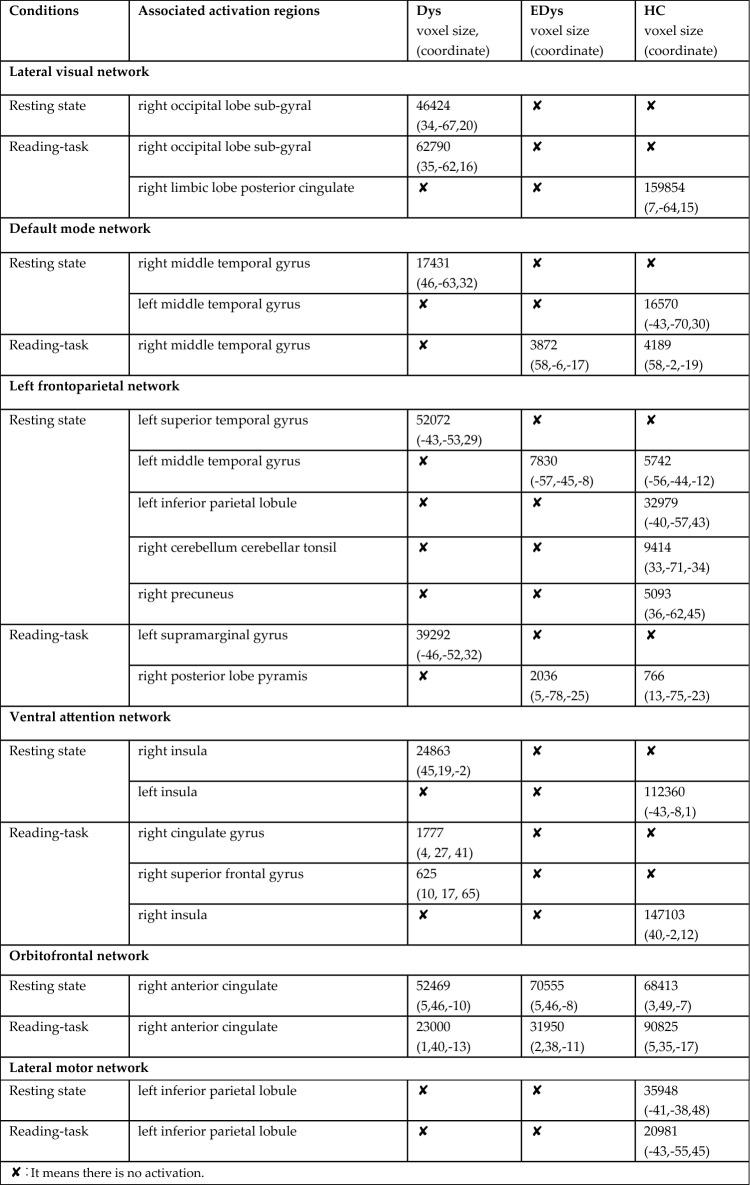
Table 3.
Reading task and resting state mean group networks and activation regions
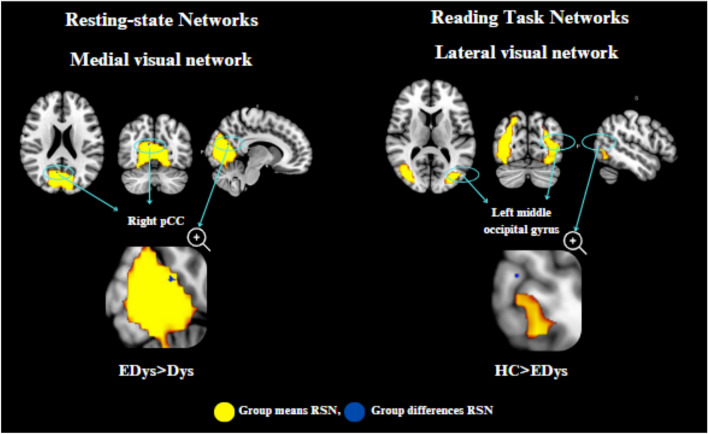
As a result of the ICA analysis, dual regression analysis was applied to the networks formed in both the resting state and the reading task in order to see the differences between the groups. With the dual regression analysis (comparison between groups) performed for resting and reading task, the regions showing significant differences are presented numerically and visually in Table 4 and Fig. 5.
Table 4.
Resting state and reading task dual regression analysis results
| Group contrast | EDys > Dys medial visual network (rs-fMRI) | HC > EDys lateral visual network (task-fMRI) |
|---|---|---|
| Associated activation region | Right posterior cingulate | Left middle occipital gyrus |
| MNI coordinate | 9, 6, 21 | 51, 75, 11 |
| Voxel size | 30 | 7 |
| p-value | 0.9648 | 0.9816 |
Fig. 5.
Significant connectivity differences between groups in resting state and reading task
The findings obtained for the networks of interest within the scope of this study were evaluated by taking into account Table 3, which was obtained based on group means.
Visual network
During the resting state, activation was observed only in the Dys group in the sub-gyral region of the right occipital lobe in the lateral visual network, while during the reading task, activation was again observed only in the Dys group in the same region. However, it is noteworthy that there is increased activation during the reading task compared to resting state. Visual processing difficulties could be attributed to the right occipital lobe sub-gyral.
According to the results of the second-level analysis, during the reading task in the lateral visual network, it was found that there was greater activation in the left middle occipital gyrus region in HC compared to EDys (HC > EDys). Again, the difference in activation in the left middle occipital gyrus observed in healthy controls during the reading task (Table 4; Fig. 5) can be considered as a finding that details such as distance and depth in these children with dyslexia, they have not yet developed as much as healthy children, despite receiving training. According to the second level analysis results, it was found that there was more activation in the right PCC region in Edys than in Dys (EDys > Dys) during rest in the medial visual network.
Considering the relationship between PCC and anxiety (Gorka et al. 2023), the increased connectivity at the right PCC observed in dyslexics who received education during rest (Table 4) can be considered a finding of the effect of special education on both anxiety and episodic memory-related (Alsulami 2019; Stoitsis et al. 2008) differences in these children. This situation may be a sign of improvement in anxiety. As seen in Gorka’s study, PCC has been reported to be associated with increased task performance of anxiety, supporting our results (Stoitsis et al. 2008). In a study featuring an audiovisual task, increased activation in the right cingulate gyrus region was observed in the HC group compared to the Dys group (Kronschnabel et al. 2014). Barquero et al. (2014) conducted a systematic review of the literature on reading intervention in children and adults with studies using fMRI and MEG imaging methods. According to the findings obtained from the meta-analysis, it was stated that participants with reading difficulties manifested increased functional activation following the reading intervention in the right posterior cingulate and left middle occipital gyrus. As a result of their studies, it was found that these regions are probably active in processes that will improve reading ability (Barquero et al. 2014).
Default mode network
In the default mode network, the right middle temporal gyrus region was activated only in the Dys group at rest, in which activation increased in both the Dys and HC groups during reading. Considering the role of MTG in language (Briggs et al. 2021; Zhang et al. 2017), semantic memory (Xu et al. 2015), visual perception (Stein 2014), and multimodal sensory integration (Mesulam 1998), the activation of the right middle temporal gyrus (MTG) in the EDys and HC groups during the reading task can be considered a benefit of the training. When the default mode network was evaluated at rest, functional activation was observed only in Dys group in the right middle temporal gyrus region and only in HC group in the left middle temporal gyrus. However, unlike the right MTG which does not activate during rest in educated individuals, it is observed that the left MTG activates during rest in HC group. This situation can also be considered as one of the connectivity differences between healthy individuals and dyslexics.
In a study by Gosse et al., involving dyslexic children (n = 16) and healthy control (n = 16) group with a mean age of 9.3, it was emphasized that there was a decrease in functional connectivity in the left middle temporal gyrus regions during resting-state in dyslexic children. The function of the middle temporal gyrus is critical for reading as it is responsible for the retrieval of visually presented items (Gosse et al. 2022). In a study by Schurz et al., examining brain areas related to reading in dyslexic readers (n = 15) and typical readers (n = 14) aged 16–20 years, both task-based and resting-state functional connectivity analyses were conducted. They revealed a decreased functional connectivity between the left middle temporal gyrus and inferior frontal gyrus in dyslexic readers (Schurz et al. 2015). This finding was consistently obtained for two different reading tasks and resting-state conditions. However, a meta-analysis study involving Chinese dyslexic children reported hyperactivation in the right middle temporal gyrus region (Li and H.-Y. 2022).
Frontoparietal network
In the left frontoparietal network, during reading, activation was observed only in Dys in the left supramarginal gyrus, while activation was observed in EDys and HC in the right posterior lobe pyramid. This situation may indicate that EDys approaches HC with training. However, activation in the right posterior lobe pyramidis is observed during reading in both HC and Edys, but not in Dys. During resting state, activation is observed in the left inferior parietal, right cerebellum, and right precuneus in HC, with no differences found in EDys and Dys. However, activation is seen in both Edys and HC in the left middle temporal gyrus, while only Dys lacks activation. In the left superior temporal gyrus, it is seen that there is activation only in Dys. In this context, it is seen that EDys group begins to resemble HC group in some regions where the connectivity of Dys group is very different from HC.
In another study involving 16 dyslexic children and 15 children with typical readers, aged between 8 and 16, all participants performed three tasks (phonological, picture naming and semantic (3 types) tasks) during fMRI acquisition. During the complex sentence reading task, activation in the bilateral superior temporal gyrus was observed in the dyslexia group. When the dyslexic group was compared to typical readers during the same task, functional activation was observed in the right superior temporal gyrus (Prasad et al. 2020). Additionally, in a study involving dyslexic readers (n = 13, mean age = 24) and typical reader (n = 13, mean age = 25.3) where auditory-visual stimuli were presented during a reading task, it was shown that typical readers exhibited greater superior temporal activations in scenarios with combined auditory-visual stimuli compared to auditory/visual stimuli alone. On the other hand, such an increase effect is not found in dyslexic readers. In one study, the superior temporal gyrus plays a critical role in the integration of acoustic and visual speech, thus highlighting the potential of superior temporal dysfunction to underlie weak auditory-visual integration in dyslexia (Ye et al. 2017). In another study involving auditory and visual stimuli, Rüsseler et al. designed a task-based study to examine audio-visual speech perception in dyslexic individuals, comparing them with 13 dyslexic and 13 typical readers. The study findings revealed that, when comparing consistent stimuli with inconsistent stimuli, the typical reader group showed increased activation in the bilateral superior temporal gyrus, while in the dyslexic group, this pattern was reversed (Rüsseler et al. 2018).
Unlike the foregoing studies in which audio-visual stimulation was employed, this study only incorporates the continuously text reading task. Given relavant findings in the literature, this indicates that the results in our study may vary depending on the experimental paradigm. In addition, the fact that there are differences in the bilateral superior temporal gyrus between the dyslexic group and the healthy group underpins the functional significance of this region in connection with the dyslexia disorder and the specific stimulus given.
Ventral attention network
It is known that there are morphological differences in the insula in the dyslexia group (Black 2004). However, the lack of activation in the left insula in the dyslexia group in functional studies draws attention as a finding similar to our study. It is known that the insula region is associated with emotional regulation (Jang et al. 2018). Since the left insula is affected in dyslexia, the activation of the right insula, which is thought to be due to compensation, has been supported by other functional imaging studies (Paulesu et al. 1996).
In addition, it is suggested that the left insula, which is not active in dyslexics unlike healthy controls in PET scans (Paulesu et al. 1996) of both different tasks, has an important role in connecting different phonological codes. If these results are reproducible, the value of studying pathological groups to determine the functional anatomy of the normal brain system will be confirmed (Paulesu et al. 1996). In a task-based study conducted by Łuniewska et al., involving typical readers (n = 90) or individuals with dyslexia (n = 20), with children at familial risk for dyslexia (n = 55) and those without any familial risks (n = 35), fMRI scans were performed at the beginning of primary school and repeated 2 years later. In dyslexic children, low activation was observed in the right insula during the initial scan, while over the 2 year period, brain activation during phonological processing increased in the right insula. In typical readers, a decrease in brain activation was observed in the left hemisphere’s language areas, including the insula, after 2 years (Łuniewska et al. 2019).
In our study, in the ventral attention network, the right insula region was activated in Dys group in the resting state, while the left insula region was activated in HC group. However, it was observed that only the right insula was activated in the healthy group during reading. In addition, no activation was observed in the right cingulate gyrus and right superior frontal gyrus regions in EDys and HC groups during the reading task, while activation was observed only in Dys group.
Orbitofrontal network
It was maintained in some studies that there is a potential relationship between the right anterior cingulate gyrus and vision within the orbitofrontal network (Shinoura et al. 2013). Also, the dorsal ACC modulates the tracking of visible targets, visual attention, and the interface between cognition and emotion, thereby affecting emotional self-control and problem-solving capacity. In a study conducted on Brazilian children, a mixed experiment related to the event was carried out using a meaningful word and pseudoword reading test. Participants were asked to choose “Yes” or “No” options when asked about the meaningfulness of the word. As a finding of the reading task in the study, it was revealed that in typical readers, there was more activation in the left anterior cingulate cortex (ACC) dorsal part compared to dyslexic readers (Buchweitz et al. 2019).
In our study, in the orbitofrontal network, activation was observed in the right anterior cingulate gyrus during both rest and reading state in all three groups. However, during reading, there was an increase in connectivity compared to resting state in the healthy group, while a decrease was observed in Dys and EDys groups. The increase in activation seen during reading in HC was not found in the Dys and EDys groups; on the contrary, there was a decrease in activation, which was thought to be related to visual processing. Considering the visual function of this region, the anterior cingulate cortex should be evaluated in addition to focusing only on the temporooccipital region regarding visual processing (Huda et al. 2020). Also, regarding the relationship of this area with impulsivity (Baker and Ireland 2007), it could be said that healthy individuals may able to control their impulsivity better than dyslexia.
Lateral motor network
In the literature, it was reported that the connection between the inferior parietal lobule (IPL) and certain cortical areas is decreased in dyslexic readers both during tasks and at rest (Schurz et al. 2015). Additionally, dysfunction of the IPL in dyslexic children has been reported in numerous studies (Maisog et al. 2008; Richlan et al. 2009, 2011). In our study, in the lateral motor network, activation is observed in the left inferior parietal lobule of the lateral motor cortex during both rest and reading in HC group, while activation is not observed in either EDys or Dys groups. The significant differences observed between healthy and dyslexic groups in both reading and resting conditions may highlight the presence of impairments in motor coordination and phonological awareness (Martin et al. 2016; Devoto et al. 2022; Pellegrino et al. 2023) in the clinical presentation of neurodevelopmental disorders. Considering this aspect, the necessity arises to integrate existing interventions aimed at improving motor coordination into special education programs. It also draws attention to the need to review the impact of existing phonological awareness studies.
A potential limitation of functional connectivity/activation studies based on reading tasks is that different reading tasks may produce different functional connectivity patterns. As noted by Koyama et al., there is still no consensus on the “optimal” task for characterizing the neural networks underlying reading and dyslexia through reading-based functional connectivity research. It is emphasized that one possible optimal solution is to examine functional connectivity during both reading tasks and resting state conditions (Koyama et al. 2010).
Conclusions
We can summarize main findings in our study, together with the results and comments we highlighted above, as follows.
- It has been revealed that there is a need to integrate phonological awareness and motor coordination activities into dyslexia education in accordance with the findings of lateral motor networks.
- In dyslexic groups, it has been observed that dyslexia oriented educational activities contribute to the improvement in functional connectivity of certain brain regions making them closer to that of healthy groups.
- Most brain regions that yield significant results in our study appear to be in common with the literature. Considering the current results, the functional connectivity differences in our study support the need to analyze many factors such as motor skills, phonological awareness, impulsivity and anxiety in dyslexia.
The unique aspects of this study are that all participants were asked to perform both resting and continuous reading tasks, and that it consisted of three compatible data groups, such as MRI protocols and pre-processing processes as well as demographic characteristics of participants. The study’s other distinctive aspects are that the participants read the Turkish text selected according to their grade levels and that all participants were Turkish and their mother tongue was Turkish, ensuring nationality and language compatibility. In addition, it was ensured that participants with dyslexia did not have any other psychiatric diseases that could be confused with dyslexia, and children with pure dyslexia were included in the study.
One limitation of our study is the small sample size to reach a generalizable interpretation over results. The preliminary results presented in this study can be studied in a more comprehensive way by increasing the number of data and applying more diverse functional analysis methods.
Acknowledgements
We would like to thank Erciyes University Pediatric Radiology Department, MR Technician Tuğba Özalp, and Ünal Şahinci for data acquisition.
Author contributions
Conception and design of the study: Ş.G.B., S.İ., and E.D., Data acquisition: E.D., Ş.G.B., B.S, and Z.F.K. Data analysis: S.İ., Ş.G.B., Z.A., İ.A., G.R.S. and E.A, Manuscript writing: Ş.G.B., S.İ., E.D., Z.A., İ.A., G.R.S., and E.A, Final approval of manuscript: All authors.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study has been supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) under project number 122E131.
Data availability
This study has been carried out within the scope of the TÜBİTAK project and data sharing cannot be performed yet.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethics approval statement
This study has been approved by the Erciyes University Clinical Research Ethics Committee (Decision No: 2022/504).
Patient consent statement
Written informed consent was obtained from both children and their parents.
Permission to reproduce material from other sources
The figures and materials used were specifically prepared for this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alcauter S, García-Mondragón L, Gracia-Tabuenca Z, Moreno MB, Ortiz JJ, Barrios FA (2017) Resting state functional connectivity of the anterior striatum and prefrontal cortex predicts reading performance in school-age children. Brain Lang 174:94–102. 10.1016/j.bandl.2017.07.007 10.1016/j.bandl.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Alsulami S (2019) The role of memory in dyslexia. Int J Educ Lit Stud 7:1. 10.7575/aiac.ijels.v.7n.4p.1 10.7575/aiac.ijels.v.7n.4p.1 [DOI] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5TM, 5th edn. American Psychiatric Publishing Inc, Arlington. 10.1176/appi.books.9780890425596 [Google Scholar]
- Andersson J, Smith S, Jenkinson M, 2008 FNIRT-FMRIB’s non-linear image registration tool, 14th Annu. Meet Organ Hum Brain Mapp
- Bailey SK, Aboud KS, Nguyen TQ, Cutting LE (2018) Applying a network framework to the neurobiology of reading and dyslexia. J Neurodev Disord 10:37. 10.1186/s11689-018-9251-z 10.1186/s11689-018-9251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SF, Ireland JL (2007) The link between dyslexic traits, executive functioning, impulsivity and social self-esteem among an offender and non-offender sample. Int J Law Psychiatry 30(6):492–503. 10.1016/j.ijlp.2007.09.010 10.1016/j.ijlp.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Barquero LA, Davis N, Cutting LE (2014) Neuroimaging of reading intervention: a systematic review and activation likelihood estimate meta-analysis. PLoS ONE 9(1):e83668. 10.1371/journal.pone.0083668 10.1371/journal.pone.0083668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski A-M, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li S-J, Lin C-P, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng G-J, Veijola J, Villringer A, Walter M, Wang L, Weng X-C, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang Y-F, Zhang H-Y, Castellanos FX, Milham MP (2010) Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739. 10.1073/pnas.0911855107 10.1073/pnas.0911855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KC 2004 Dyslexia and insula morphology: implications for phonological processing, University of Georgia
- Briggs RG, Tanglay O, Dadario NB, Young IM, Fonseka RD, Hormovas J, Dhanaraj V, Lin Y-H, Kim SJ, Bouvette A, Chakraborty AR, Milligan TM, Abraham CJ, Anderson CD, O’Donoghue DL, Sughrue ME (2021) The unique fiber anatomy of middle temporal gyrus default mode connectivity. Oper Neurosurg 21(1):E8–E14. 10.1093/ons/opab109 10.1093/ons/opab109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchweitz A, Costa AC, Toazza R, de Moraes AB, Cara VM, Esper NB, Aguzzoli C, Gregolim B, Dresch LF, Soldatelli MD, da Costa JC, Portuguez MW, Franco AR (2019) Decoupling of the occipitotemporal cortex and the brain’s default-mode network in dyslexia and a role for the cingulate cortex in good readers: a brain imaging study of brazilian children. Dev Neuropsychol 44:146–157. 10.1080/87565641.2017.1292516 10.1080/87565641.2017.1292516 [DOI] [PubMed] [Google Scholar]
- Cainelli E, Vedovelli L, Carretti B, Bisiacchi P (2023) EEG correlates of developmental dyslexia: a systematic review. Ann Dyslexia 73:184–213. 10.1007/s11881-022-00273-1 10.1007/s11881-022-00273-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çeliktürk Sezgin Z, Akyol H (2015) Improving reading skills of fourth grade elemantary student who has reading disability. Turkish J Educ 4(2):4–16. 10.19128/turje.181115 10.19128/turje.181115 [DOI] [Google Scholar]
- Centanni TM, Norton ES, Ozernov-Palchik O, Park A, Beach SD, Halverson K, Gaab N, Gabrieli JDE (2019) Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. NeuroImage Clin 22:101715. 10.1016/j.nicl.2019.101715 10.1016/j.nicl.2019.101715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Calhoun V (2018) Effect of spatial smoothing on task fMRI ICA and functional connectivity. Front Neurosci. 10.3389/fnins.2018.00015 10.3389/fnins.2018.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos F, Koini M, Schouten TM, Seiler S, van der Grond J, Lechner A, Schmidt R, de Rooij M, Rombouts SARB (2018) A comprehensive analysis of resting state fMRI measures to classify individual patients with Alzheimer’s disease. Neuroimage 167:62–72. 10.1016/j.neuroimage.2017.11.025 10.1016/j.neuroimage.2017.11.025 [DOI] [PubMed] [Google Scholar]
- Devoto F, Carioti D, Danelli L, Berlingeri M (2022) A meta-analysis of functional neuroimaging studies on developmental dyslexia across European orthographies: the ADOD model. Lang Cogn Neurosci 37:285–314. 10.1080/23273798.2021.1970200 10.1080/23273798.2021.1970200 [DOI] [Google Scholar]
- Farris EA, Odegard TN, Miller HL, Ring J, Allen G, Black J (2011) Functional connectivity between the left and right inferior frontal lobes in a small sample of children with and without reading difficulties. Neurocase 17:425–439. 10.1080/13554794.2010.532141 10.1080/13554794.2010.532141 [DOI] [PubMed] [Google Scholar]
- Gallego-Molina NJ, Ortiz A, Martínez-Murcia FJ, Rodríguez-Rodríguez I, Luque JL (2023) Assessing functional brain network dynamics in dyslexia from fnırs data. Int J Neural Syst 33(4):2350017. 10.1142/S012906572350017X 10.1142/S012906572350017X [DOI] [PubMed] [Google Scholar]
- Gorka AX, Philips RT, Torrisi S, Claudino L, Foray K, Grillon C, Ernst M (2023) The posterior cingulate cortex reflects the ımpact of anxiety on drift rates during cognitive processing. Biol Psychiatry Cogn Neurosci Neuroimaging 8:445–451. 10.1016/j.bpsc.2022.03.010 10.1016/j.bpsc.2022.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosse C, Dricot L, Van Reybroeck M (2022) Evidence of altered functional connectivity at rest in the writing network of children with dyslexia. Brain Sci. 10.3390/brainsci12020243 10.3390/brainsci12020243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeley B, Weber RC, Denyer R, Ferris JK, Rubino C, White K, Boyd LA (2021) Aberrant cerebellar resting-state functional connectivity related to reading performance in struggling readers. Dev Sci. 10.1111/desc.13022 10.1111/desc.13022 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, DiFrancesco M, Kay B, Wang Y, Holland SK (2015) Increased resting-state functional connectivity of visual- and cognitive-control brain networks after training in children with reading difficulties. NeuroImage Clin 8:619–630. 10.1016/j.nicl.2015.06.010 10.1016/j.nicl.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Hershey A, Kay B, DiFrancesco M (2019) Differential effect of reading training on functional connectivity in children with reading difficulties with and without ADHD comorbidity. J Neurolinguistics 49:93–108. 10.1016/j.jneuroling.2018.09.002 10.1016/j.jneuroling.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, DiFrancesco M, Greenwood P, Scott E, Vannest J, Hutton J, Dudley J, Altaye M, Farah R (2021) Longer screen vs. reading time is related to greater functional connections between the salience network and executive functions regions in children with reading difficulties vs. typical readers. Child Psychiatry Hum Dev 52:681–692. 10.1007/s10578-020-01053-x [DOI] [PMC free article] [PubMed]
- Huang Y, He M, Li A, Lin Y, Zhang X, Wu K (2020) Personality, behavior characteristics, and life quality impact of children with dyslexia. Int J Environ Res Public Health. 10.3390/ijerph17041415 10.3390/ijerph17041415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda R, Sipe GO, Breton-Provencher V, Cruz KG, Pho GN, Adam E, Gunter LM, Sullins A, Wickersham IR, Sur M (2020) Distinct prefrontal top-down circuits differentially modulate sensorimotor behavior. Nat Commun 11:6007. 10.1038/s41467-020-19772-z 10.1038/s41467-020-19772-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icer S, Benli SG, Gumus K, Demirci E, Ozmen S, Doganay S (2018) Can functional connectivity at resting brain in ADHD indicate the impairments in sensory-motor functions and face/emotion recognition? J Med Biol Eng 38:138–149. 10.1007/s40846-017-0289-2 10.1007/s40846-017-0289-2 [DOI] [Google Scholar]
- Icer S, Benli S, Ozmen S (2019) Differences in brain networks of children with ADHD: whole-brain analysis of resting-state fMRI. Int J Imaging Syst Technol. 10.1002/ima.22348 10.1002/ima.22348 [DOI] [Google Scholar]
- Içer S, Sağir Disleksi GR (2023) Çocukların okuma sırasında beyin aktivitelerinin fonksiyonel Mr Psikolojik etkileşim analizi ile incelenmesi mühendislik bilim. Ve Tasarım Derg 11(4):1310–1327. 10.21923/jesd.1222428 10.21923/jesd.1222428 [DOI] [Google Scholar]
- Jang JH, Kim J-H, Yun J-Y, Choi S-H, An SC, Kang D-H (2018) Differences in functional connectivity of the insula between brain wave vibration in meditators and non-meditators. Mindfulness 9(6):1857–1866. 10.1007/s12671-018-0928-x 10.1007/s12671-018-0928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. 10.1016/s1053-8119(02)91132-8 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Kana F, Geçgel H, Bayraktar. P (2023) Turkish teachers awareness about dyslexia and the problems experienced by students with dyslexia in turkish education. Hacettepe Univ J Educ. 10.1698/HUJE.2023.495 10.1698/HUJE.2023.495 [DOI] [Google Scholar]
- Karakaş S, Erden G, Bakar EE, Doğutepe E (2017) Özgül Öğrenme Bozukluğu Genişletilmiş Nöropsikometri Bataryası. Eğitim Yayınevi, Konya [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP (2010) Reading networks at rest. Cereb Cortex 20:2549–2559. 10.1093/cercor/bhq005 10.1093/cercor/bhq005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, Castellanos FX, Milham MP, Cortical, (2013) signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PLoS ONE. 10.1371/journal.pone.0055454 10.1371/journal.pone.0055454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronschnabel J, Brem S, Maurer U, Brandeis D (2014) The level of audiovisual print-speech integration deficits in dyslexia. Neuropsychologia 62:245–261. 10.1016/j.neuropsychologia.2014.07.024 10.1016/j.neuropsychologia.2014.07.024 [DOI] [PubMed] [Google Scholar]
- Li Y, Bi H-Y (2022) Comparative research on neural dysfunction in children with dyslexia under different writing systems: a meta-analysis study. Neurosci Biobehav Rev. 10.1016/j.neubiorev.2022.104650 10.1016/j.neubiorev.2022.104650 [DOI] [PubMed] [Google Scholar]
- Łuniewska M, Chyl K, Dębska A, Banaszkiewicz A, Żelechowska A, Marchewka A, Grabowska A, Jednoróg K (2019) Children with dyslexia and familial risk for dyslexia present atypical development of the neuronal phonological network. Front Neurosci 13:1287. 10.3389/fnins.2019.01287 10.3389/fnins.2019.01287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA (2003) A definition of dyslexia. Ann Dyslexia 53:1–14. 10.1007/s11881-003-0001-9 10.1007/s11881-003-0001-9 [DOI] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF (2008) A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci 1145:237–259. 10.1196/annals.1416.024 10.1196/annals.1416.024 [DOI] [PubMed] [Google Scholar]
- Martin A, Kronbichler M, Richlan F (2016) Dyslexic brain activation abnormalities in deep and shallow orthographies: a meta-analysis of 28 functional neuroimaging studies. Hum Brain Mapp 37:2676–2699. 10.1002/hbm.23202 10.1002/hbm.23202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1998) From sensation to cognition. Brain 121(6):1013–1052. 10.1093/brain/121.6.1013 10.1093/brain/121.6.1013 [DOI] [PubMed] [Google Scholar]
- Miciak J, Fletcher JM (2020) The critical role of instructional response for identifying dyslexia and other learning disabilities. J Learn Disabil 53:343–353. 10.1177/0022219420906801 10.1177/0022219420906801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi B, Münte TF, Cole DM, Sami A, Boltzmann M, Rüsseler J (2020) Changed functional connectivity at rest in functional illiterates after extensive literacy training. Neurol Res Pract 2:12. 10.1186/s42466-020-00058-0 10.1186/s42466-020-00058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevill T, Forsey M (2023) The social impact of schooling on students with dyslexia: a systematic review of the qualitative research on the primary and secondary education of dyslexic students. Educ Res Rev. 10.1016/j.edurev.2022.100507 10.1016/j.edurev.2022.100507 [DOI] [Google Scholar]
- Öğrenme güçlüğü olan bireyler için destek eğitim programı, Türkiye Cumhuriyeti Milli Eğtim Bakanl. (n.d.). https://orgm.meb.gov.tr/meb_iys_dosyalar/2021_05/21130110_Ogrenme_Guclugu.pdf. Accessed 5 May 2024
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD (1996) Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain 119(1):143–157. 10.1093/brain/119.1.143 10.1093/brain/119.1.143 [DOI] [PubMed] [Google Scholar]
- Pellegrino M, Ben-Soussan TD, Paoletti P (2023) A scoping review on movement, neurobiology and functional deficits in dyslexia: suggestions for a three-fold integrated perspective. Int J Environ Res Public Health. 10.3390/ijerph20043315 10.3390/ijerph20043315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Sagar R, Kumaran SS, Mehta M (2020) Study of functional magnetic resonance imaging (fMRI) in children and adolescents with specific learning disorder (dyslexia). Asian J Psychiatr 50:101945. 10.1016/j.ajp.2020.101945 10.1016/j.ajp.2020.101945 [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF (2015) ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112:267–277. 10.1016/j.neuroimage.2015.02.064 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H (2009) Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp 30:3299–3308. 10.1002/hbm.20752 10.1002/hbm.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H (2011) Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 56:1735–1742. 10.1016/j.neuroimage.2011.02.040 10.1016/j.neuroimage.2011.02.040 [DOI] [PubMed] [Google Scholar]
- Rüsseler J, Ye Z, Gerth I, Szycik GR, Münte TF (2018) Audio-visual speech perception in adult readers with dyslexia: an fMRI study. Brain Imaging Behav 12:357–368. 10.1007/s11682-017-9694-y 10.1007/s11682-017-9694-y [DOI] [PubMed] [Google Scholar]
- Rytty R, Nikkinen J, Paavola L, Abou Elseoud A, Moilanen V, Visuri A, Tervonen O, Renton AE, Traynor BJ, Kiviniemi V, Remes AM (2013) GroupICA dual regression analysis of resting state networks in a behavioral variant of frontotemporal dementia. Front Hum Neurosci 7:461. 10.3389/fnhum.2013.00461 10.3389/fnhum.2013.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Wimmer H, Richlan F, Ludersdorfer P, Klackl J, Kronbichler M (2015) Resting-state and task-based functional brain connectivity in developmental dyslexia. Cereb Cortex 25:3502–3514. 10.1093/cercor/bhu184 10.1093/cercor/bhu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzman BA, Snyder AZ, Leuthardt EC, Shimony JS (2019) The state of resting state networks. Top Magn Reson Imaging 28:189–196. 10.1097/RMR.0000000000000214 10.1097/RMR.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE (1998) Dyslexia. N Engl J Med 338:307–312. 10.1056/NEJM199801293380507 10.1056/NEJM199801293380507 [DOI] [PubMed] [Google Scholar]
- Shinoura N, Yamada R, Tabei Y, Shiode T, Itoi C, Saito S, Midorikawa A (2013) The right dorsal anterior cingulate cortex may play a role in anxiety disorder and visual function. Neurol Res 35:65–70. 10.1179/1743132812Y.0000000101 10.1179/1743132812Y.0000000101 [DOI] [PubMed] [Google Scholar]
- Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. 10.1002/hbm.10062 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. 10.1016/j.neuroimage.2004.07.051 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045. 10.1073/pnas.0905267106 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soğanci S, Kulesza EM (2023) Psychosocial effects of dyslexia in terms of students, parents, and school community– research review. Turkish J Spec Educ Res Pract 5(1):1–17. 10.37233/TRSPED.2023.0134 10.37233/TRSPED.2023.0134 [DOI] [Google Scholar]
- Sörös P, Hoxhaj E, Borel P, Sadohara C, Feige B, Matthies S, Müller HHO, Bachmann K, Schulze M, Philipsen A (2019) Hyperactivity / restlessness is associated with increased functional connectivity in adults with ADHD : a dimensional analysis of resting state fMRI. BMC Psychiatry 19:1–11 10.1186/s12888-019-2031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J (2014) Dyslexia: the role of vision and visual attention. Curr Dev Disord Reports 1:267–280. 10.1007/s40474-014-0030-6 10.1007/s40474-014-0030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitsis J, Giannakakis GA, Papageorgiou C, Nikita KS, Rabavilas A, D. (2008) Anagnostopoulos, evidence of a posterior cingulate involvement (brodmann area 31) in dyslexia: a study based on source localization algorithm of event-related potentials. Prog Neuro-Psychopharmacol Biol Psychiatr 32:733–738. 10.1016/j.pnpbp.2007.11.022 10.1016/j.pnpbp.2007.11.022 [DOI] [PubMed] [Google Scholar]
- Theodoridou D, Christodoulides P, Zakopoulou V, Syrrou M (2021) Developmental dyslexia: environment matters. Brain Sci. 10.3390/brainsci11060782 10.3390/brainsci11060782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgut Turan S, Erdoğan Bakar E, Erden G, Karakaş S (2016) Using neuropsychometric measurements in the differential diagnosis of specific learning disability. Noro Psikiyatr. 53:144–151. 10.5152/npa.2015.10024 10.5152/npa.2015.10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker S, Kuhnke P, Jiang Z, Hartwigsen G (2023) Disrupted network interactions serve as a neural marker of dyslexia. Commun Biol 6:1114. 10.1038/s42003-023-05499-2 10.1038/s42003-023-05499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajuihian S (2011) Neurobiology of developmental dyslexia: part 1: a review of evidence from autopsy and structural neuro-imaging studies. African Vis Eye Heal. 10.4102/aveh.v70i4.117 10.4102/aveh.v70i4.117 [DOI] [Google Scholar]
- Wajuihian S, Naidoo K (2011) Dyslexia: an overview. African Vis Eye Heal. 10.4102/aveh.v70i2.102 10.4102/aveh.v70i2.102 [DOI] [Google Scholar]
- Wang Y, Li T-Q (2015) Dimensionality of ICA in resting-state fMRI investigated by feature optimized classification of independent components with SVM. Front Hum Neurosci. 10.3389/fnhum.2015.00259 10.3389/fnhum.2015.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92:381–397. 10.1016/j.neuroimage.2014.01.060 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang J, Fan L, Li H, Zhang W, Hu Q, Jiang T (2015) Tractography-based parcellation of the human middle temporal gyrus. Sci Rep 5(1):18883. 10.1038/srep18883 10.1038/srep18883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li C, Li X, Zhai M, An Q, Zhang Y, Zhao J, Weng X (2022) Prevalence of developmental dyslexia in primary school children: a systematic review and meta-analysis. Brain Sci. 10.3390/brainsci12020240 10.3390/brainsci12020240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Doñamayor N, Münte TF (2014) Brain network of semantic integration in sentence reading: insights from independent component analysis and graph theoretical analysis. Hum Brain Mapp 35:367–376. 10.1002/hbm.22182 10.1002/hbm.22182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Rüsseler J, Gerth I, Münte TF (2017) Audiovisual speech integration in the superior temporal region is dysfunctional in dyslexia. Neuroscience 356:1–10. 10.1016/j.neuroscience.2017.05.017 10.1016/j.neuroscience.2017.05.017 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li B, Wang H, Li L, Liao Q, Liu Y, Bao X, Liu W, Yin H, Lu H, Tan Q (2017) Decreased middle temporal gyrus connectivity in the language network in schizophrenia patients with auditory verbal hallucinations. Neurosci Lett 653:177–182. 10.1016/j.neulet.2017.05.042 10.1016/j.neulet.2017.05.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study has been carried out within the scope of the TÜBİTAK project and data sharing cannot be performed yet.