Abstract
The Remote Assessment of Disease and Relapse – Alzheimer’s Disease (RADAR-AD) consortium evaluated remote measurement technologies (RMTs) for assessing functional status in AD. The consortium engaged with the European Medicines Agency (EMA) to obtain feedback on identification of meaningful functional domains, selection of RMTs and clinical study design to assess the feasibility of using RMTs in AD clinical studies. We summarized the feedback and the lessons learned to guide future projects.
Subject terms: Drug development, Alzheimer's disease
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, characterized by cognitive decline impairing daily function1,2. Cognitive and functional decline in AD clinical studies is traditionally measured through standard neuropsychological assessments, including questionnaires and interviews with patients and caregivers. The use of remote measurement technologies (RMTs), such as apps and wearables, can provide a broader, more objective, more frequent, or even continuous assessment of function and has the potential to reduce assessment burden on study participants3,4. An increasing number of clinical research programs are exploring the use of RMTs in clinical studies in other disease areas5. Their potential has been recognized by regulators6,7 and insights into regulatory recommendations may stimulate the use of novel RMTs8. The recent qualification of the stride velocity 95th centile as a primary endpoint by the European Medicines Agency (EMA) for ambulatory Duchenne Muscular Dystrophy studies, demonstrates the role that RMT-based assessments can play in drug development and approval9,10.
Regulatory endorsement of novel assessments is essential for their use in clinical studies. However, this process is long and requires leveraging results from multiple studies10,11. Public-private partnerships offer excellent opportunities to support this procedure, bringing together pharmaceutical companies, academic and technical experts, along with input from Patient Advisory Boards12–15.
The project aimed to identify and evaluate RMTs for remote assessment of functional impairment in participants within the complete AD spectrum16–20.
One of the objectives of the RADAR-AD consortium was to discuss the approach taken to identify RMT-based assessments with regulators, to obtain guidance on how to develop a path for formal qualification for their use in AD studies. The consortium met with the EMA through the Innovation Task Force and Qualification Advice procedures and discussed the Concept of Interest (CoI), the Context of Use (CoU), the RMT selection process, RADAR-AD clinical study design, and the results of the interim analysis. Here, we outline the identification of CoI(s), selection of RMTs and feasibility of use assessment in a clinical study, and discuss the feedback received from the Agency and the lessons learned to guide future projects aiming to qualify RMT-based outcome assessments for use in AD.
Identification of concept of interest (CoI)
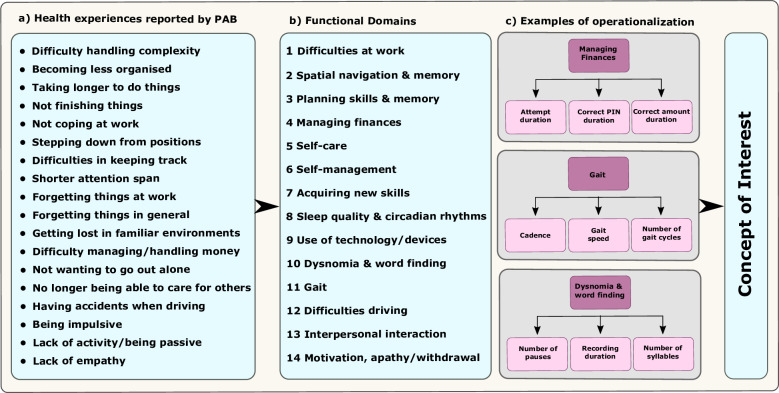
In AD, the meaningful aspects of health are activities of daily living (ADLs) that are strongly associated with declining quality of life, rate of disease progression, and loss of independence21. In RADAR-AD, the meaningful aspects of health were defined with the support from patients and caregivers within the framework of patient and caregiver involvement work with the patient advisory board22. The meaningful aspects of health were then narrowed down to measurable health concepts (functional domains) that correspond to conversion from MCI to AD dementia, early impairment in AD, predictability of decline, and relevance to patients and caregivers. These can ultimately be translated to a final CoI(s), a practically measurable element of an aspect of a disease that is important to a patient (Fig. 1). Thereafter, different digital measures were selected for quantification of each functional domain and included in the study to assess the feasibility of their use in clinical study settings16,23.
Fig. 1. Concept of Interest Derived from a Meaningful Aspect of Health (Activities of Daily Living).
Health experiences (a) reported by the Patient Advisory Board (PAB) were mapped onto the functional domains (b) that were then operationalized (c). For example, the functional domain ‘Managing finances’ was assessed with an App mimicking the withdrawal of money. Operationalizations of this functional domain are then subdivided into measurable elements such as the total duration of an attempt to withdraw money, the duration to enter the correct PIN code, or the duration of entering the correct amount of money to be withdrawn. These ultimately translate into a Concept of Interest (CoI).
Context of use (CoU) of RMT-based assessments
The proposed CoU for RMT-based assessments was to serve as secondary endpoints in clinical studies to complement standard clinical scales for the assessment of ADLs in individuals with preclinical to moderate AD in the home setting to support drug marketing applications. The RMT-based assessments could be used on their own or in combination, as a battery of performance tests, to support efficacy assessments in AD clinical studies.
Selection of RMTs and feasibility assessment
The evaluation of available RMTs was conducted by technical and clinical experts with input from the patient advisory board as described previously16,22–24. A final selection of RMTs was made by mapping them to functional domains identified as relevant by the end users and included three smartphone applications (passive RMT app, the Mezurio app, and Altoida Neuro Motor Index application sofware), a wearable camera (Vicon Autographer) and two wrist-worn activity trackers (Axivity AX3 and Fitbit Charge 3) for at home assessments. The Gait Up device (now Mindmaze) Physilog sensors (worn on shoes and hip), the Banking app (developed by The Center for Research & Technology, Hellas, CERTH), and Altoida were administered in-clinic (see Supplementary Table 1)16,23. Data collection and exchange were realized through the RADAR-base open-source platform (https://radar-base.org).
The main RADAR-AD study was an 8-week, cross-sectional, observational study comparing digital information from selected RMTs with established clinical measures in 237 participants (over 50 years of age) with preclinical AD (PreAD), prodromal AD (ProAD), mild-to-moderate AD dementia (MildAD), and age-matched healthy controls (HC) at 13 sites in 12 European countries16–20. Participants and their study partners gave written informed consent before study start and the study was conducted in accordance with the Declaration of Helsinki. Each local ethics committee approved the study separately: The Netherlands: Medisch Ethische Toetsingscommissie VUmc (2019.518); Spain: Drug Research Ethics Committee (CEIm) of Universitat International de Catalunya (MED-FACE-2020-07); Italy: Comitato Etico IRCCS Centro San Giovanni di Dio Fatebenefratelli di Brescia; Switzerland: Commission cantonale d’éthique de la recherché (2022-00002); Portugal: Comissão de Ética do Centro Académico de Medicina de Lisboa (388/19); United Kingdom: London – West London & GTAC (Gene Therapy Advisory Committee) Research Ethics Committee (20/LO/0183); Germany: Ethics Committee II of the Ruprecht-Karls-University of Heidelberg (Medical Faculty Mannheim) (2020-508 N); Norway: Regionale komiteer for medisinsk og helsefaglig orskningsetikk (98842); Sweden: Swedish Ethical Review Authority (2020-03497); Greece: Ethics Committee of Medical Faculty of Aristotle University of Thessaloniki and Ethics Committee of Alzheimer Hellas (198/2018 AI).
All participants in the AD spectrum were defined by positive positron emission tomography (PET) and/or cerebrospinal fluid amyloid status, and further subdivided by mini-mental state examination (MMSE) and global clinical dementia rating (CDR) scores consistent with the National Institute on Aging and the Alzheimer’s Association (NIA-AA) criteria defining Alzheimer’s disease based on the biology20. ProAD is synonymous to Mild Cognitive Impairment (MCI), a syndromic diagnosis, indicating deficits in a single cognitive domain which does not interfere with daily life independence. Such a syndrome can be caused by other disorders than AD as well. In the context of the RADAR-AD study, however, all participants that functioned at MCI level also had biomarker confirmation of AD pathology. Hence, a definition of ProAD was considered more suited.
The study addressed and examined three research objectives: (1) Compare features from each RMT between the groups (known-groups validity), (2) Identify if there is an association between the features extracted from each RMT and the relevant functional domain scores from standard clinical questionnaires (convergent validity), and (3) the feasibility of RMT use in this setting16.
Interim analysis
An interim analysis of data from 175 participants was performed to explore (1) known-groups validity and (2) convergent validity of features of 6 RMTs. Due to timelines associated with the scientific advice procedure at EMA, the qualification advice meeting took place while the clinical study was still ongoing. EMA’s advice was hence based on interim results (see Supplementary Information). The interim results showed a trend of deteriorating function across the AD spectrum (preclinical to mild AD) across various features of several RMTs. Earliest functional impairment in the PreAD group was captured by the complex cognitive domains as assessed with the Altoida application which has been confirmed in the full dataset25. Evidence of convergent validity for various features across several RMTs was also shown through correlation with established clinical measures where appropriate. The interim analysis results for all RMTs including the table of established clinical measures are shown in Supplementary Tables 2–15 and Figs. 1–15. The consortium concluded that the selected measures were ecologically valid and appropriate to identify clinically meaningful outcomes to detect functional changes even in very early stages of AD. The consortium also proposed using machine learning algorithms to combine several features from each RMT to improve on known group by using stratified cross-validation techniques across multiple devices26,27.
Health authority consultations
The consortium had an EMA Innovation Task Force meeting in 2020 for an informal discussion to obtain feedback on the project. The consortium was advised to use CE marked devices for medical use and to compare information from RMTs with established standard measures in prospective studies in the CoU. This advice was included in the selection of technologies when possible. The consortium included research grade technologies when CE-marked alternatives were not available. The RADAR-base open-source platform, used for data collection and data exchange, was considered acceptable, if it complied with regulatory principles and standards, such as Good Clinical Practice.
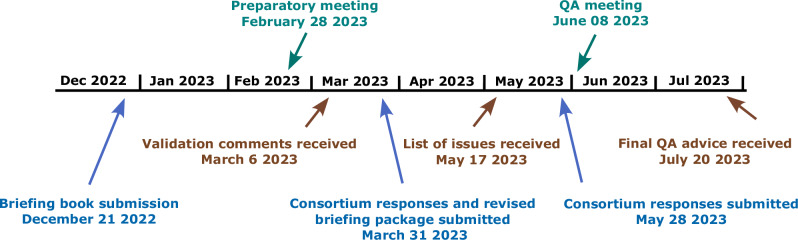
The consortium also initiated a Qualification Advice meeting from the EMA to obtain advice on identification of functional domains, selection of suitable RMTs, their clinical evaluation, data quality and user experience. Additionally, a preliminary protocol proposal was presented, describing a follow-up longitudinal clinical study. The protocol aimed to evaluate the sensitivity of RMT-based assessments to disease progression. For this, participants with amyloid- and tau-confirmed prodromal AD, who are most likely to show disease progression within the study duration of 18 months, were considered as the study population. The Qualification Advice procedure and timelines are outlined in Fig. 2 with the EMA feedback for the current study and recommendations for a future study summarized in Box 1.
Fig. 2. Qualification advice process and timelines.
Overall, the advice period lasted 7 months, which was preceded by the compilation of the briefing book (5 months). Validation comments from the European Medicines Agency (EMA) were received 3 months after submission. After submitting a revised version of the briefing book, the consortium received a list of issues that were addressed in a written response as well as an online meeting. The final Qualification Advice was received 7 months after the briefing book submission. Submissions by the consortium are shown in blue, CHMP responses in brown and meetings in green.
The Committee for Medicinal Products for Human Use (CHMP) agreed with the consortium’s proposed process for item selection and identification of functional domains reflecting meaningful aspects of AD at face value. However, whether these functional domains could distinguish different stages of AD, specifically at the very early stages, predict, or monitor disease progression (e.g., MCI to AD dementia conversion) would need to be studied further using a longitudinal approach. In addition, CHMP agreed to the limitation in assessing convergent validity of certain functional domains such as gait due to the lack of a standard clinical instrument or ground truth. Depending on the AD subpopulation, the combinations of relevant features could differ and functional domains (e.g., difficulties at work) might carry different weight. People’s capabilities to use the RMTs is also anticipated to change over time as the disease progresses. The CHMP recommended to continue the development work for domains relevant for individuals who are still in the workforce and domains that were identified by the patient advisory board, such as interpersonal interactions, motivation, and apathy, but could not be included in the study as suitable RMTs were not available.
The consortium’s approach to select the RMTs based on literature reviews, expert and patient feedback, and available technical information, was considered reasonable. The CHMP expressed concerns regarding the risk of changes in the RMTs introduced by the manufacturers, the use of consumer devices for which the access to the full dataset, including raw data and algorithms may not be possible. The CHMP also highlighted the importance of General Data Protection Regulation (GDPR) compliance, particularly when using consumer devices.
The CHMP emphasized that the convergent and divergent validity of the RMT-based assessments with conventional measures would be key. The selection on the frequency of use of some RMTs (daily, weekly) should be thoroughly justified to decrease patient burden. Furthermore, the learning curve of repeated use of the active RMTs (i.e., RMTs that require user interaction) is important. In the study, participants performed learning exercises during on-site training sessions of the RMTs at the baseline visit, but these were not repeated as it was beyond the scope of this exploratory study. The CHMP recommended that the learning effect of the active RMTs be assessed throughout a future study to understand its impact on the assessment of functional domains. The CHMP also commented that the proposed prospective, longitudinal, observational study with 3 in-clinic visits over a period of 18 months may be too short to establish the relationship between a change in the RMT-based assessments and function outcome as measured by Alzheimer’s Disease Cooperative Study – Activities of Daily Living for Mild Cognitive Impairment (ADCS-ADL-MCI) scale, especially in a prodromal AD population. A future, longitudinal study should be of sufficient duration to allow for conclusions on disease progression or changes in cognition, given the slow progression in AD. Additionally, although most participants would tolerate and be willing to use the devices, particularly wearables, this willingness is likely to decrease over time in longer studies, leading to missing data.
The consortium’s proposed approach to quantify floor and ceiling effects by comparing distributions of the whole population and compare these to distributions known to have floor and ceiling effects, such as ADCS-ADL, was agreed upon. However, it was highlighted that accuracy is only one aspect of validation and the convergent and divergent validity of specific RMTs (or combinations thereof) for the functional domains of interest is also important.
The use of artificial intelligence and machine learning techniques to evaluate RMT-based outcome assessments is accepted as an exploratory exercise, identifying potential promising concepts and tools as alternative assessments of functional domains. Many machine learning algorithms are entirely data driven, generating theoretical constructs. The CHMP agreed with the consortium’s plan to identify different AD stages by combining results from specific RMTs for the functional domains of interest. The Agency also indicated that, if a composite of RMTs features allows an overarching construct, this concept should be made plausible and replicable. However, its content and its convergent and divergent validity will remain to be established against existing scales that measure the same or partially overlapping/related concepts.
The consortium’s approach to investigate known group validity by investigating possible differences between the metrics extracted from each RMT-based assessment and the functional domain scores derived from the conventional questionnaires (convergent validity) was accepted. However, the large overlap in scores between the groups in the RMTs (indicating limited discriminative power of the tests) was noted along with cases of weak correlations (even if significant). For this, the sensitivity to change of a specific (combination of) RMT(s), minimal important difference and sensitivity to show a treatment effect should be part of future validation efforts.
The EU medical device regulation conformity for the technologies not CE marked as medical devices was briefly discussed. The CHMP stated that the qualification of medical devices and algorithms is not within EMA’s remit but falls within the Notified Bodies (organizations authorized by the EU member states to evaluate CE conformity of products prior to marketing). The Scientific Advice Working Party did not agree with the consortium’s position that the proposed CoU of RMTs (only data collection in clinical studies) should not classify them as medical devices. The study participants frequently receive information from the sensors, which could modify their health behavior, and potentially affect the study results. The CHMP also added that when the medical devices are used outside their intended use, setting up a research protocol to test the new CoU is advised. Furthermore, if RMTs investigated were not CE certified, this could impact their long-term scientific reliability, as manufacturers could seek to upgrade features in response to market forces, which could potentially impact the data collection.
In conclusion, in AD drug development, RMT-based assessments have the potential to offer important advantages over conventional methods by allowing continuous and objective assessment of daily function, and can complement current practices, provide new ways to capture existing measures or enable novel measures. Successful implementation of RMT-based assessments in clinical studies is a complex matter and Health Authority consultations early in the process are essential for regulatory acceptance. Herein, we focused on implementation strategies and considerations for longitudinal follow-up studies to validate RMT-based measures in clinical trials. Additionally, it is noted that the received feedback presented aims to provide an overall roadmap for interacting with Health Authorities rather than to address specific RMTs.
Key learnings from the RADAR-AD project (see Box 2) include the identification of a CoI that is relevant and meaningful to the target population. The definition of the CoU, along with a detailed description of how the outcome measure is to be used in the target study population is imperative for regulatory assessment. To increase the probability of success, iterative approaches may be considered, e.g., initial qualification for secondary endpoint followed by considerations for use as primary endpoint10. In addition, a process on how to expand to additional CoUs or target diseases should be agreed upon. The RADAR-AD data calls for a longitudinal follow-up study to further investigate and validate the most promising tools. Designing a larger and longer validation study at the time of EMA qualification advice procedure, however, was beyond the scope of the RADAR-AD project and the available resources. Designing such a study could be a follow-up activity (post consortium) for which qualification advice from EMA and other Health Authorities can be obtained and incorporated in the design prior to implementation.
Considering the complexity of the qualification process28,29, it is advisable to engage with Health Authorities utilizing available advice procedures (e.g., Innovation Task Force and EMA Qualification Advice meetings), as appropriate30, and plan for multiple Health Authority engagements to obtain feedback prior to initiation of the studies. In this way, implementation of the feedback received into the study design is possible, along with follow-up meetings to discuss results and next steps. The interactions with Health Authorities are resource intensive and require full engagement of all partners relevant to the discussion topics. Hence, resource allocations and timelines for these interactions should be built into the program plans and objectives. Considering that these technologies will support global projects, engagement with other major Health Authorities (e.g., US Food and Drug Administration, UK Medicines and Healthcare Products Regulatory Agency, or Japan Pharmaceuticals and Medical Devices Agency) to obtain broader acceptance is essential.
Box 1 EMA feedback and recommendations.
EMA feedback and recommendations
RADAR-AD Exploratory Study
The process for identifying functional domains and RMTs was acceptable
The use of CE-marked RMTs is desirable
Quantification of floor and ceiling effects of RMT parameters is desirable
Future Study
Additional prospective longitudinal study of identifying functional domains indicative of different AD stages is needed
A multi-year study would be needed to adequately measure disease changes
Ensure that learning curves for RMT use are assessed
Include relevant assessments that capture changes from very early stages of AD
Box 2 Guidance for researchers seeking advice from health authorities.
Guidance for Researchers Seeking Advice from Health Authorities
Establish a regulatory strategy, including planning for Health Authority meetings aligned with the project goals
Identification of CoI that is meaningful to the patients
CoU is an essential component of Health Authority review
Prioritize multiple CoU cases and adopt a staged approach for qualification
The preparation for successful Health Authority meetings is time and resource intensive
Supplementary information
Acknowledgements
The authors thank all RADAR-AD participants and clinical sites for their contribution. The RADAR-AD project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 806999. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA and Software AG. See https://www.imi.europa.eu for more details. This communication reflects the views of the RADAR-AD consortium and neither IMI nor the European Union and EFPIA are liable for any use that may be made of the information contained herein. The consortium would like to thank Anna Tavridou, Senior Scientific Officer, EMA for reviewing the manuscript. Research of Alzheimer center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting Steun Alzheimercentrum Amsterdam. We thank all past and present RADAR-AD consortium members for their contribution to the project (in alphabetical order): Dag Aarsland, Halil Agin, Vasilis Alepopoulos, Alankar Atreya, Sudipta Bhattacharya, Virginie Biou, Joris Borgdorff, Anna-Katharine Brem, Neva Coello, Pauline Conde, Nick Cummins, Jelena Curcic, Casper de Boer, Yoanna de Geus, Paul de Vries, Ana Diaz, Richard Dobson, Aidan Doherty, Andre Durudas, Gul Erdemli, Amos Folarin, Suzanne Foy, Holger Froehlich, Jean Georges, Dianne Gove, Margarita Grammatikopoulou, Kristin Hannesdottir, Robbert Harms, Mohammad Hattab, Keyvan Hedayati, Chris Hinds, Adam Huffman, Dzmitry Kaliukhovich, Irene Kanter-Schlifke, Ioannis Kompatsiaris, Ivan Koychev, Rouba Kozak, Julia Kurps, Sajini Kuruppu, Claire Lancaster, Robert Latzman, Ioulietta Lazarou, Manuel Lentzen, Federica Lucivero, Florencia Lulita, Nivethika Mahasivam, Nikolay Manyakov, Emilio Merlo Pich, Peyman Mohtashami, Marijn Muurling, Vaibhav Narayan, Vera Nies, Spiros Nikolopoulos, Andrew Owens, Marjon Pasmooij, Dorota Religa, Gaetano Scebba, Emilia Schwertner, Rohini Sen, Niraj Shanbhag, Laura Smith, Meemansa Sood, Thanos Stavropoulos, Pieter Stolk, Ioannis Tarnanas, Srinivasan Vairavan, Nick van Damme, Natasja van Velthogen, Herman Verheij, Pieter Jelle Visser, Bert Wagner, Gayle Wittenberg, and Yuhao Wu.
Author contributions
G.E. provided regulatory oversight to the project, was the main contact for the EMA Qualification Advice meeting and a major contributor in writing the manuscript. MG Performed data analysis, EMA Qualification Advice Briefing package, and was a major contributor in writing the manuscript. B.W. contributed to the EMA Qualification Advice Briefing package and was a major contributor in writing the manuscript. S.V. performed data and statistical analyses, contributor to EMA Qualification Advice Briefing package, and reviewed the manuscript. J.C. performed data analysis, contributed to the EMA Qualification Advice Briefing package, and reviewed the manuscript. D.A. designed the study, advised on the data analyses, and reviewed the manuscript. G.W. reviewed the manuscript. S.N. designed the study, advised on the data analyses, and reviewed the manuscript. M.M. recruited participants and collected data, performed the data analyses, and reviewed the manuscript. H.F. advised on the data analysis and reviewed the manuscript. C.d.B. designed the study, advised on the data analyses, and reviewed the manuscript. N.M.S. contributed to the EMA Qualification Advice Briefing package and reviewed the manuscript. V.J.M.N. Foundation Lygature acted as formal requester of the Qualification Advice on behalf of the Consortium; Project management and reviewed the manuscript. N.C. reviewed the statistical methodology and analysis and reviewed the manuscript. D.G. contributed to the EMA Qualification Advice Briefing package, coordinated the work of the RADAR-AD Patient Advisory Board, and reviewed the manuscript. A.D. contributed to the EMA Qualification Advice Briefing package, coordinated the work of the RADAR-AD Patient Advisory Board, and reviewed the manuscript. S.F. contributed to the EMA Qualification Advice Briefing package and reviewed the manuscript. W.D. contributed to the EMA Qualification Advice Briefing package and reviewed the manuscript. A.K.B. designed the study, advised on the analyses, contributed to the EMA Qualification Advice Briefing package, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Competing interests
G.E., N.C., J.C., and W.D. are employees of Novartis and may hold shares in the company but have no non-financial competing interests. B.W., S.V., G.W., and S.F. are employees of Janssen Research & Development, LLC, a Johnson & Johnson company, and may hold stock options or shares in the company but have no non-financial competing interests. N.M.S. is an employee of Takeda and holds shares in the company but has no non-financial competing interests. D.A. has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, Biogen, and GE Health, and served as a paid consultant for H. Lundbeck, Eisai, Heptares, Mentis Cura, and Roche Diagnostics, but declares no non-financial competing interests. H.F. has received research support from UCB Pharma and AbbVie GmbH & Co KG. All other authors declare no financial or non-financial competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-024-01211-8.
References
- 1.DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener.14, 32 (2019). 10.1186/s13024-019-0333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois, B. et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement.12, 292–323 (2016). 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piau, A., Wild, K., Mattek, N. & Kaye, J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: systematic review. J. Med. Internet Res.21, e12785 (2019). 10.2196/12785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brem, A. K. et al. Digital endpoints in clinical trials of Alzheimer’s disease and other neurodegenerative diseases: challenges and opportunities. Front Neurol.14, 1210974 (2023). 10.3389/fneur.2023.1210974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaye, J. et al. Using digital tools to advance Alzheimer’s drug trials during a pandemic: The EU/US CTAD Task Force. J. Prev. Alzheimers Dis.8, 513–519 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Medicines Agency Questions and answers: Qualification of digital technology-based methodologies to support approval of medicinal products (EMA/219860/2020). (2020). https://www.ema.europa.eu/en/documents/other/questions-answers-qualification-digital-technology-based-methodologies-support-approval-medicinal_en.pdf. Accessed 10 January 2024.
- 7.FDA Framework for the Use of Digital Health Technologies in Drug and Biological Product Development. (2023) https://www.fda.gov/media/166396/download?attachment. Accessed 10 January 2024.
- 8.Dekker, M. J. H. J., Stolk, P. & Pasmooij, A. M. G. The use of remote monitoring technologies: a review of recent regulatory scientific advices, qualification opinions, and qualification advices issued by the European Medicines Agency. Front Med. (Lausanne).8, 619513 (2021). 10.3389/fmed.2021.619513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Servais, L. et al. First regulatory qualification of a novel digital endpoint in Duchenne muscular dystrophy: a multi-stakeholder perspective on the impact for patients and for drug development in neuromuscular diseases. Digit Biomark.5, 183–190 (2021). 10.1159/000517411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Servais, L. et al. First regulatory qualification of a digital primary endpoint to measure treatment efficacy in DMD. Nat. Med.29, 2391–2392 (2023). 10.1038/s41591-023-02459-5 [DOI] [PubMed] [Google Scholar]
- 11.Bertha, A. et al. Incorporating digitally derived endpoints within clinical development programs by leveraging prior work. NPJ Digit Med.6, 139 (2023). 10.1038/s41746-023-00886-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viceconti, M. et al. Toward a regulatory qualification of real-world mobility performance biomarkers in Parkinson’s patients using digital mobility outcomes. Sensors20, 5920 (2020). 10.3390/s20205920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viceconti, M. et al. On the use of wearable sensors as mobility biomarkers in the marketing authorization of new drugs: a regulatory perspective. Front Med.9, 996903 (2022). 10.3389/fmed.2022.996903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, L. et al. Fatigue and sleep assessment using digital sleep trackers: insights from a multi-device pilot study. Annu. Int. Conf. IEEE Eng. Med Biol. Soc.2022, 1133–1136 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Rochester, L. et al. A roadmap to inform development, validation and approval of digital mobility outcomes: the mobilise-D approach. Digit Biomark.4, 13–27 (2020). 10.1159/000512513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muurling, M. et al. Remote monitoring technologies in Alzheimer’s disease: design of the RADAR-AD study. Alzheimers Res. Ther.13, 89 (2021). 10.1186/s13195-021-00825-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease. (2018) CPMP/EWP/553/95 Rev.2. https://www.ema.europa.eu/en/clinical-investigation-medicines-treatment-alzheimers-disease-scientific-guideline. Accessed 2 June 2024.
- 18.Weintraub, S. et al. Measuring cognition and function in the preclinical stage of Alzheimer’s disease. Alzheimers Dement (N.Y).4, 64–75 (2018). 10.1016/j.trci.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertens, D. et al. The effect of diagnostic criteria on outcome measures in preclinical and prodromal Alzheimer’s disease: implications for trial design. Alzheimers Dement(N. Y.)3(4), 513–523 (2017). [DOI] [PMC free article] [PubMed]
- 20.Petersen, R. C. et al. NIA-AA Alzheimer’s disease framework: clinical characterization of stages. Ann. Neurol.89(6), 1145–1156 (2021). 10.1002/ana.26071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manta, C., Patrick-Lake, B. & Goldsack, J. C. Digital measures that matter to patients: a framework to guide the selection and development of digital measures of health. Digit Biomark.4, 69–77 (2020). 10.1159/000509725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stavropoulos, T. G. et al. Wearable devices for assessing function in Alzheimer’s Disease: a European public involvement activity about the features and preferences of patients and caregivers. Front Aging Neurosci.13, 643135 (2021). 10.3389/fnagi.2021.643135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens, A. P. et al. Selecting remote measurement technologies to optimize assessment of function in early Alzheimer’s disease: a case study. Front Psychiatry11, 582207 (2020). 10.3389/fpsyt.2020.582207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stavropoulos, T. G., Papastergiou, A., Mpaltadoros, L., Nikolopoulos, S. & Kompatsiaris, I. IoT wearable sensors and devices in elderly care: a literature review. Sensors20, 2826 (2020). 10.3390/s20102826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muurling, M. et al. Augmented reality versus standard tests to assess cognition and function in early Alzheimer’s disease. NJP Digit. Med.6, 234 (2023). 10.1038/s41746-023-00978-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentzen, M. et al. Assessment of RMTs for Discriminating Stages of Alzheimer’s Disease. Poster presented at the Alzheimer’s Association International Conference, 16-20 July 2023, Amsterdam, The Netherlands. https://alz.confex.com/alz/2023/meetingapp.cgi/Paper/76856. Accessed 10 January 2024.
- 27.Vairavan, S. et al. A multimodal digital biomarker of functional deficits in early-stage Alzheimer’s disease: Results of the RADAR-AD study. Poster presented at the Alzheimer’s Association International Conference, 16-20 July 2023, Amsterdam, The Netherlands. https://alz.confex.com/alz/2023/meetingapp.cgi/Paper/71136. Accessed 10 January 2024.
- 28.Gelis, L. et al. Digital tools-regulatory considerations for application in clinical trials. Ther. Innov. Regul. Sci.57, 769–782 (2023). 10.1007/s43441-023-00535-z [DOI] [PubMed] [Google Scholar]
- 29.Colloud, S. et al. Evolving regulatory perspectives on digital health technologies for medicinal product development. NJP Digit. Med.6, 56 (2023). 10.1038/s41746-023-00790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.EMA Regulatory Science to 2025: strategic reflection. EMA/110706/2020. (2020) https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ema-regulatory-science-2025-strategic-reflection_en.pdf. Accessed 10 January 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.