Abstract
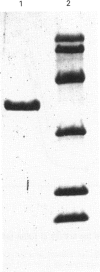
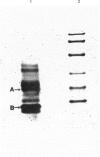
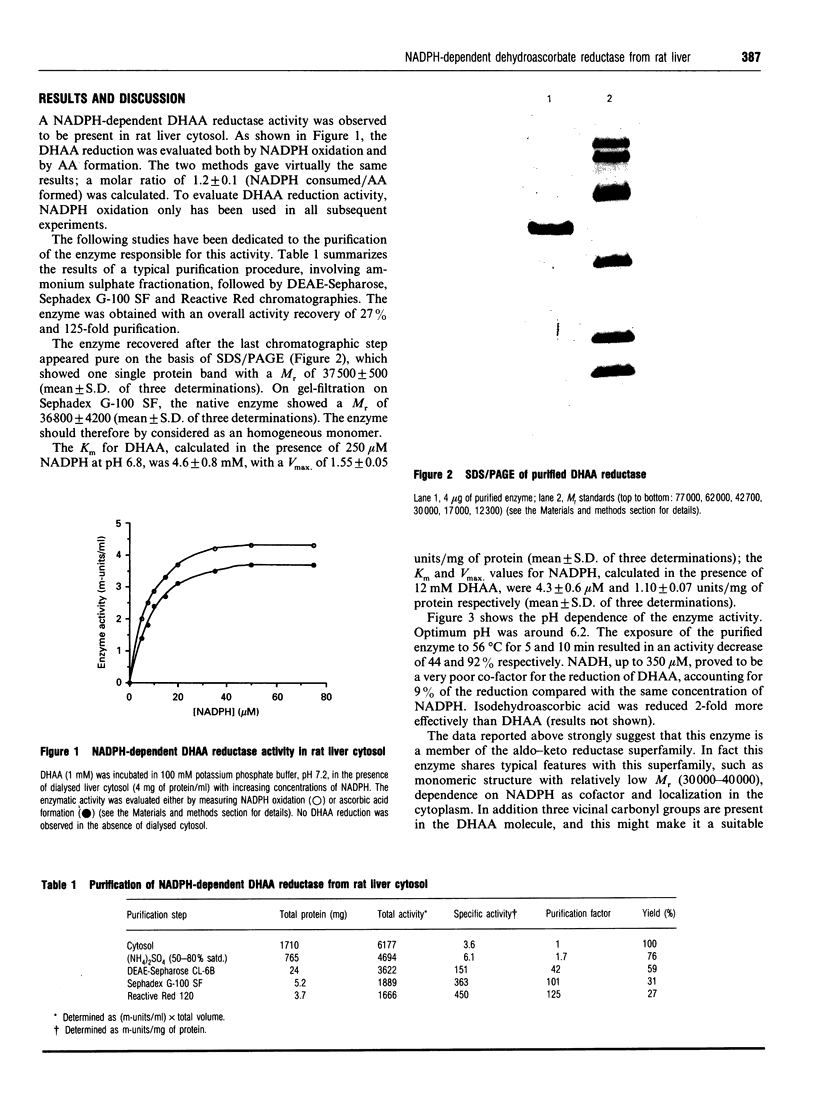
Rat liver cytosol has been found to reduce dehydroascorbic acid (DHAA) to ascorbic acid in the presence of NADPH. The enzyme responsible for such activity has been purified by ammonium sulphate fractionation, DEAE-Sepharose, Sephadex G-100 SF and Reactive Red column chromatography, with an overall recovery of 27%. SDS/PAGE of the purified enzyme showed one single protein band with an M(r) of 37,500. A similar value (36,800) was found by gel filtration on a Sephadex G-100 SF column. The results indicate that the enzyme is a homogeneous monomer. The Km for DHAA was 4.6 mM and the Vmax. was 1.55 units/mg of protein; for NADPH Km and Vmax. were 4.3 microM and 1.10 units/mg of protein respectively. The optimum pH was around 6.2. Several typical substrates and inhibitors of the aldo-keto reductase superfamily have been tested. The strong inhibition of DHAA reductase effected by steroidal and non-steroidal anti-inflammatory drugs, together with the ability to reduce 5 alpha-androstane-3,17-dione strongly, suggest the possibility that DHAA reductase corresponds to 3 alpha-hydroxysteroid dehydrogenase. Microsequence analysis performed on the electro-transferred enzyme band shows that the N-terminus is blocked. Internal primary structure data were obtained from CNBr-derived fragments and definitely proved the identity of NADPH-dependent DHAA reductase with 3 alpha-hydroxysteroid dehydrogenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S., Som S., Deb S., Mukherjee D., Chatterjee I. B. Dehydroascorbic acid reduction in human erythrocytes. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1335–1340. doi: 10.1016/0006-291x(79)91182-3. [DOI] [PubMed] [Google Scholar]
- Bigley R., Riddle M., Layman D., Stankova L. Human cell dehydroascorbate reductase. Kinetic and functional properties. Biochim Biophys Acta. 1981 May 14;659(1):15–22. doi: 10.1016/0005-2744(81)90266-7. [DOI] [PubMed] [Google Scholar]
- Bodannes R. S., Chan P. C. Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 1979 Sep 15;105(2):195–196. doi: 10.1016/0014-5793(79)80609-2. [DOI] [PubMed] [Google Scholar]
- Choi J. L., Rose R. C. Regeneration of ascorbic acid by rat colon. Proc Soc Exp Biol Med. 1989 Apr;190(4):369–374. doi: 10.3181/00379727-190-42874. [DOI] [PubMed] [Google Scholar]
- Coassin M., Tomasi A., Vannini V., Ursini F. Enzymatic recycling of oxidized ascorbate in pig heart: one-electron vs two-electron pathway. Arch Biochem Biophys. 1991 Nov 1;290(2):458–462. doi: 10.1016/0003-9861(91)90566-2. [DOI] [PubMed] [Google Scholar]
- Dennison D. B., Brawley T. G., Hunter G. L. Rapid high-performance liquid chromatographic determination of ascorbic acid and combined ascorbic acid-dehydroascorbic acid in beverages. J Agric Food Chem. 1981 Sep-Oct;29(5):927–929. doi: 10.1021/jf00107a009. [DOI] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Dean G., Carter C., Allen P. L. Tissue, subcellular, and submitochondrial distributions of semidehydroascorbate reductase: possible role of semidehydroascorbate reductase in cofactor regeneration. J Neurochem. 1982 Aug;39(2):563–568. doi: 10.1111/j.1471-4159.1982.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Flynn T. G., Shires J., Walton D. J. Properties of the nicotinamide adenine dinucleotide phosphate-dependent aldehyde reductase from pig kidney. Amino acid composition, reactivity of cysteinyl residues, and stereochemistry of D-glyceraldehyde reduction. J Biol Chem. 1975 Apr 25;250(8):2933–2940. [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES R. E. REDUCTION OF DEHYDROASORBIC ACID BY ANIMAL TISSUES. Nature. 1964 Sep 5;203:1068–1069. doi: 10.1038/2031068a0. [DOI] [PubMed] [Google Scholar]
- Halder A. B., Crabbe M. J. Bovine lens aldehyde reductase (aldose reductase). Purification, kinetics and mechanism. Biochem J. 1984 Apr 1;219(1):33–39. doi: 10.1042/bj2190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Wasil M., Grootveld M. Biologically significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by ascorbic acid. Implications for antioxidant protection in the inflamed rheumatoid joint. FEBS Lett. 1987 Mar 9;213(1):15–17. doi: 10.1016/0014-5793(87)81456-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986 Apr 3;314(14):892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- MAERKI F., MARTIUS C. [Vitamin K reductase, preparation and properties]. Biochem Z. 1960;333:111–135. [PubMed] [Google Scholar]
- Maellaro E., Del Bello B., Sugherini L., Santucci A., Comporti M., Casini A. F. Purification and characterization of glutathione-dependent dehydroascorbate reductase from rat liver. Biochem J. 1994 Jul 15;301(Pt 2):471–476. doi: 10.1042/bj3010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975 Mar 17;63(2):463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Packer J. E., Slater T. F., Willson R. L. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979 Apr 19;278(5706):737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- Pawlowski J. E., Huizinga M., Penning T. M. Cloning and sequencing of the cDNA for rat liver 3 alpha-hydroxysteroid/dihydrodiol dehydrogenase. J Biol Chem. 1991 May 15;266(14):8820–8825. [PubMed] [Google Scholar]
- Penning T. M., Mukharji I., Barrows S., Talalay P. Purification and properties of a 3 alpha-hydroxysteroid dehydrogenase of rat liver cytosol and its inhibition by anti-inflammatory drugs. Biochem J. 1984 Sep 15;222(3):601–611. doi: 10.1042/bj2220601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning T. M., Sharp R. B. Prostaglandin dehydrogenase activity of purified rat liver 3 alpha-hydroxysteroid dehydrogenase. Biochem Biophys Res Commun. 1987 Oct 29;148(2):646–652. doi: 10.1016/0006-291x(87)90925-9. [DOI] [PubMed] [Google Scholar]
- Penning T. M., Talalay P. Inhibition of a major NAD(P)-linked oxidoreductase from rat liver cytosol by steroidal and nonsteroidal anti-inflammatory agents and by prostaglandins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4504–4508. doi: 10.1073/pnas.80.14.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. C. Renal metabolism of the oxidized form of ascorbic acid (dehydro-L-ascorbic acid). Am J Physiol. 1989 Jan;256(1 Pt 2):F52–F56. doi: 10.1152/ajprenal.1989.256.1.F52. [DOI] [PubMed] [Google Scholar]
- Scarpa M., Rigo A., Maiorino M., Ursini F., Gregolin C. Formation of alpha-tocopherol radical and recycling of alpha-tocopherol by ascorbate during peroxidation of phosphatidylcholine liposomes. An electron paramagnetic resonance study. Biochim Biophys Acta. 1984 Sep 28;801(2):215–219. doi: 10.1016/0304-4165(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Smithgall T. E., Harvey R. G., Penning T. M. Regio- and stereospecificity of homogeneous 3 alpha-hydroxysteroid-dihydrodiol dehydrogenase for trans-dihydrodiol metabolites of polycyclic aromatic hydrocarbons. J Biol Chem. 1986 May 15;261(14):6184–6191. [PubMed] [Google Scholar]
- Stahl R. L., Liebes L. F., Silber R. A reappraisal of leukocyte dehydroascorbate reductase. Biochim Biophys Acta. 1985 Mar 29;839(1):119–121. doi: 10.1016/0304-4165(85)90189-8. [DOI] [PubMed] [Google Scholar]
- Sun I., Morré D. J., Crane F. L., Safranski K., Croze E. M. Monodehydroascorbate as an electron acceptor for NADH reduction by coated vesicle and Golgi apparatus fractions of rat liver. Biochim Biophys Acta. 1984 Feb 14;797(2):266–275. doi: 10.1016/0304-4165(84)90130-2. [DOI] [PubMed] [Google Scholar]
- Vogel K., Bentley P., Platt K. L., Oesch F. Rat liver cytoplasmic dihydrodiol dehydrogenase. Purification to apparent homogeneity and properties. J Biol Chem. 1980 Oct 25;255(20):9621–9625. [PubMed] [Google Scholar]
- Wells W. W., Xu D. P., Yang Y. F., Rocque P. A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361–15364. [PubMed] [Google Scholar]